Abstract
Functional neuroimaging techniques including magnetoencephalography (MEG) have demonstrated that the brain is organized into networks displaying correlated activity. Group connectivity differences between healthy controls and participants with major depressive disorder (MDD) can be detected using temporal independent components analysis (ICA) on beta-bandpass filtered Hilbert envelope MEG data. However, the response of these networks to treatment is unknown. Ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, exerts rapid antidepressant effects. We obtained MEG recordings before and after open-label infusion of 0.5mg/kg ketamine in MDD subjects (N=13) and examined networks previously shown to differ between healthy individuals and those with MDD. Connectivity between the amygdala and an insulo-temporal component decreased post-ketamine in MDD subjects towards that observed in control subjects at baseline. Decreased baseline connectivity of the subgenual anterior cingulate cortex (sgACC) with a bilateral precentral network had previously been observed in MDD compared to healthy controls, and the change in connectivity post-ketaminewas proportional to the change in sgACC glucose metabolism in a subset (N=8) of subjects receiving [11F]FDG-PET imaging. Ketamine appeared to reduce connectivity, regardless of whether connectivity was abnormally high or low compared to controls at baseline. These preliminary findings suggest that sgACC connectivity may be directly related to glutamate levels.
Keywords: magnetoencephalography (MEG), depression, ketamine, resting state, connectivity, independent components analysis (ICA)
1. Introduction
Major depressive disorder (MDD) is a highly prevalent and frequently debilitating disease. Current FDA-approved treatments for MDD are frequently inadequate and require weeks of administration before maximal antidepressant effects appear. In contrast, the N-methyl-D-aspartate (NMDA) antagonist ketamine exerts rapid antidepressant effects that appear within 240 minutes post-IV infusion, even in treatment-resistant subjects (Zarate et al., 2006). However, little is known of ketamine’s mechanism of action at the molecular level (for a review, see (Abdallah et al., 2015)), and even less is known of ketamine’s effects on brain activity in individuals with MDD.
The mechanisms of action of various antidepressant treatments have been studied by investigating their effects on brain connectivity, particularly using resting state functional magnetic resonance imaging (rs-fMRI). Results of these studies do not converge to a common finding (Dichter et al., 2014). Nevertheless, reports of increased frontal-limbic connectivity following treatment are common (reviewed in (Gudayol-Ferre et al., 2015)); these are hypothesized to represent increased cognitive control over enhanced limbic threat reactivity. In addition, decreased connectivity within the Default Mode Network (DMN)—towards levels seen in healthy controls—has also been reported following treatment with conventional antidepressants (Li et al., 2013; Posner et al., 2013). Resting state fMRI studies of acute ketamine administration in healthy controls generally report widespread enhanced connectivity (Driesen et al., 2013; Khalili-Mahani et al., 2014), although both increased and decreased connectivity with sensory/somatosensory networks have been reported (Niesters et al., 2012). While a substantial literature has investigated the neural correlates of the acute psychotomimetic effects of ketamine administration, relatively few studies have examined brain responses at later time points, such as those associated with antidepressant response after dissociative effects have dissipated. One rs-fMRI study investigated connectivity 24 hours post-infusion in healthy control subjects and reported significantly reduced connectivity between the DMN and the dorsomedial prefrontal cortex (DMPFC)/pregenual anterior cingulate (pgACC), as well as reduced connectivity between the subgenual anterior cingulate cortex (sgACC) and the DMPFC (Scheidegger et al., 2012). The etiology of these alterations in connectivity is unknown; however, it has been hypothesized that glutamate levels may be involved given that prior work found an association between glutamate levels and/or glutamate plus glutamine (Glx) levels and connectivity (Duncan et al., 2013; Horn et al., 2010).
Because of the uptake of glucose into glial cells in response to neuronal glutamate release, 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) may serve as a useful proxy measure of glutamate levels in the brain (Magistretti and Pellerin, 1996). We previously reported alterations in regional glucose metabolism approximately two hours following ketamine infusion in a sample that overlaps those reported here (Carlson et al., 2013). That study found significantly decreased glucose metabolism in the right habenula and right insula post-ketamine compared to post-placebo. Significant increases were also observed in the right amygdala and bilateral parieto-occipital cortex. In more targeted analyses, we also found correlations between reduced suicidal ideation post-ketamine and reduced glucose metabolism in the infralimbic cortex (Ballard et al., 2015), as well as correlations between changes in anhedonia post-ketamine and changes in dorsal cingulate and orbitofrontal cortex glucose metabolism (Lally et al., 2015). To the best of our knowledge, no other groups have reported the effects of ketamine on glucose metabolism at time periods removed from its acute drug effects, so these results await replication.
Although network connectivity during the resting state—that is, in the absence of any imposed task—has been primarily investigated via fMRI, interest has recently grown in using magnetoencephalography (MEG), which is capable of assessing brain function on a time scale that better reflects neural activity (Scholvinck et al., 2013). Recently, a MEG study of acute ketamine administration demonstrated increased gamma power in both motor and visual cortices, a finding that animal studies have linked to disinhibition of pyramidal cells (Shaw et al., 2015). A subsequent study using source localization showed increases in anterior theta and gamma power, reduced posterior theta, delta, and alpha power, and decreased NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated connectivity between frontal and parietal cortices (Muthukumaraswamy et al., 2015). The alterations in NMDA- and AMPA-mediated connectivity may reflect the phenomenon whereby ketamine binds to NMDA receptors in inhibitory gamma-aminobutyric acid (GABA)-ergic interneurons, disinhibiting cortical pyramidal cells and increasing AMPA throughput (Duman, 2014). Despite these important results in acute electrophysiological responses to ketamine infusion, as with fMRI, little is known regarding brain responses on a time scale corresponding to antidepressant response.
A significant recent development in resting state MRI research is the work by Brookes and colleagues (Brookes et al., 2011), who demonstrated an ICA-based model-free method for identifying resting state networks in MEG data. We previously found that this method can be extended to group analyses (Nugent et al., 2015b). Comparing healthy subjects to subjects with MDD at baseline, we found reduced connectivity between the sgACC and a bilateral precentral independent component (IC), as well as increased amygdalar connectivity with insulo-temporal ICs in individuals with MDD (Nugent et al., 2015b).
Building on this work, in the present study we applied the same ICA methodology to a group of 13 subjects with MDD who received a single open-label dose of ketamine; 11 had been examined at baseline in our prior study (Nugent et al., 2015b). While there are advantages and disadvantages to ICA methods for resting state data analysis, we chose the ICA method to maximize the interpretability of the current paper in the context of our prior publication. MEG scans were acquired both at baseline and six to seven hours following ketamine administration. Recordings from healthy control subjects who did not receive ketamine were also included. The primary aim of this preliminary study was to assess the overall effects of ketamine on connectivity in MDD subjects, as our sample size was too limited to assess responders versus non-responders. Although we already reported the baseline effects of ketamine in a larger cohort that overlapped with this one (Nugent et al., 2015b), we felt that the additional scans acquired post-ketamine infusion justified further analysis, albeit preliminary and hypothesis-generating in nature. The formulation of hypotheses in this area is difficult given the dearth of research using resting state MEG to study MDD, as well as the lack of literature investigating the effects of ketamine on connectivity in subjects with MDD at a time point corresponding to its antidepressant effects. Notably, this analysis is the first to evaluate ketamine induced changes in resting state connectivity in MDD at a time point corresponding to antidepressant response in a depressed cohort. Based upon studies showing that successful antidepressant treatment normalizes sgACC and amygdala function, we hypothesized that ketamine would normalize connectivity to that seen in healthy control subjects. In addition, because the extant literature suggests that connectivity is reduced 24 hours after ketamine, we were particularly confident that the elevated insulo-temporal-amygdala connectivity seen at baseline would be reduced after ketamine. In addition, we present post-hoc correlations between connectivity and glucose metabolism in order to further explore the relationship between connectivity and the glutamatergic system.
2. Methods
2.1 Study participants
Thirteen medically healthy subjects with MDD participated in the study, 11 of whom had participated in a prior baseline analysis (Nugent et al., 2015b). Although it could be argued that re-use of subjects is somewhat problematic, this study is the first to analyze post-ketamine MEG recordings, and use of baseline scans was necessary to provide a reference. For all subjects, diagnosis was confirmed using the Structured Clinical Interview for DSM-IV-TR (SCID) and an unstructured interview with a psychiatrist. In addition, all subjects were required to not have responded to two prior adequate antidepressant trials. Co-morbidities were common, with nine of the 13 subjects presenting with a comorbid anxiety and/or eating disorder. In addition, four subjects had a prior history of substance abuse or dependence, although none in the last three months. These subjects were recruited as part of a much larger cohort (Ibrahim et al., 2012), but of the recruited subjects, many were either not eligible for MEG or had artifacts or movement rendering scans at one or both time points unusable.
Eighteen control subjects with no personal or family history of mood disorders were included as a control group; 17 of these overlapped with the primary analysis from our previously published study (Nugent et al., 2015b). In this present, preliminary study, healthy control subjects did not receive ketamine. Because these healthy subjects were not independent from the prior analysis, we should emphasize that we include them not to demonstrate between-group differences but instead to provide a normative reference. In addition, because the unpaired comparison between the MDD and healthy control groups is underpowered, we did not expect that all results would be replicated between groups; our primary aim was to demonstrate differences in the MDD subjects pre- and post-ketamine.
All subjects were free of drugs expected to affect CNS function for at least two weeks before data collection (four weeks for fluoxetine) and were medically healthy as assessed by medical history, physical exam, blood tests, electrocardiogram, and urinalysis. Exclusion criteria for all subjects included pregnancy, breastfeeding, meeting DSM-IV-TR criteria for substance abuse/dependence in the last three months, and contraindications to magnetic resonance imaging (MRI). Mood symptoms in participants with MDD were assessed using the Montgomery-Asberg Depression Rating Scale (MADRS).
This study was approved by the NIH combined CNS IRB, and all subjects gave written informed consent.
2.2 Pharmacologic intervention
Following data collection at baseline, subjects with MDD were administered a single, open-label infusion of 0.5 mg/kg ketamine hydrochloride (Abbott Laboratories, Abbott Park, IL) over 40 minutes. Mood was assessed at multiple time points following infusion, and we report response at 230 minutes; this was judged to be the ideal time to measure early antidepressant response to ketamine because by 230 minutes, dissociative/psychotomimetic effects have dissipated and because virtually all responders at this time continue to meet response criteria on Day 1 (Zarate et al., 2006). This time point was also chosen to maintain consistency with previously published studies (Carlson et al., 2013; Cornwell et al., 2012; Salvadore et al., 2009; Salvadore et al., 2010). As part of a separate study designed to test a potential strategy for maintaining response to ketamine, patients also received a single oral dose of either 50mg riluzole (an inhibitor of presynaptic glutamate release) or placebo five to six hours post-infusion. Although this occurred before the MEG scan, consistent with previously reported results (Cornwell et al., 2012), and given the pharmacokinetics of orally administered riluzole, we did not expect this to affect the findings.
2.3 Data acquisition
MEG resting state recordings were acquired for four minutes on a 275 channel CTF system at 1200Hz, at baseline before pharmacologic intervention, and approximately six to seven hours post-ketamine infusion. For anatomical localization, T1 weighted MRI scans were acquired on a GE 3T scanner. Further details appear in the Supplementary Methods.
2.4 Data processing
Methodological details of the data analysis have been previously described in detail (Nugent et al., 2015b), and appear in the Supplementary Methods. Tools developed in house as well as AFNI (Analysis of Functional NeuroImages, NIH, Bethesda, MD) were used for all analyses. Briefly, MRI images were preprocessed to obtain localization of MEG datasets, and transformation to standard Talairach space was derived. MEG images were also preprocessed as previously reported (Nugent et al., 2015b) and are further described in the Supplementary Methods. Hilbert power envelope images in anatomical source space were derived from the RAW MEG signal using synthetic aperture magnetometry (SAM) (Robinson and Vrba, 1999) following beta bandpass (14–30 Hz) filtering. Beta was chosen because it is the band in which most resting state networks reach maximum coherence (Brookes et al., 2012). Data from all subjects were entered into a group independent components analysis (ICA) to extract 25 components. Linear regression was used to obtain group and individual IC maps, which were matched to previously reported ICs as outlined in the Supplementary Methods.
2.5 Statistical analysis
Our previous study comparing healthy subjects to those with MDD found significantly increased connectivity at baseline between left and right insulo-temporal networks and bilateral amygdala in individuals with MDD (Nugent et al., 2015b). We also found significantly decreased connectivity between a bilateral precentral network and the sgACC in individuals with MDD. Because we had additional post-ketamine recordings on an overlapping group of patients appearing in our prior analysis, we felt that applying the techniques reported in that manuscript to our post-drug group was a worthwhile endeavor. Thus, our a priori comparisons tested the effects of ketamine infusion on connectivity with these ICs, with a priori regions of interest (ROIs) including left and right amygdala and sgACC. Because the data presented herein substantially overlap with those reported previously, these hypotheses are necessarily somewhat biased. We attempt to partly mitigate this in two ways. First, we repeated the ICA for this overlapping sample, including the independent recordings collected post-ketamine infusion. By performing an entirely new ICA on the current cohort, including the previously unanalyzed post-ketamine recordings, we ensured that the recovered IC would be optimized for the current sample. It should also be noted that even though we extracted an IC with a similar spatial pattern to those reported previously, there were likely to be differences in the recovered spatial maps even for the subjects whose data were previously analyzed. Second, we used only anatomically-defined ROIs. The literature on the issue of circular analyses in neuroimaging (Kriegeskorte et al., 2009; Vul et al., 2009) demonstrates that using a functional ROI defined from one comparison (i.e., patients vs. controls) and applying it to a secondary comparison (i.e, patients pre- and post-treatment) is a common statistical error. The functional ROI, defined by selecting voxels that exceed a certain statistical threshold in the first analysis, necessarily “overfits” the baseline comparison and artifactually inflates the difference seen in the secondary comparison. In contrast, an anatomical ROI would mix voxels inside and outside the true “ground truth” region of abnormality, and would not be subject to the same bias as a functional ROI. We thus chose to apply anatomically defined ROIs (which would be equally suboptimal for all comparisons) in all analyses. The a priori hypotheses in the amygdala and sgACC regions, as well as the ICs examined, were chosen from the baseline study, and are therefore biased to at least some degree. However, this bias could not be avoided without collecting a replication dataset, and is why we characterize this study as preliminary and hypothesis-generating in nature.
The mean beta coefficients were extracted for each IC of interest from all voxels within the a priori ROIs. These beta coefficients can be interpreted as representing correlation or connectivity with the IC network, and we will use these terms interchangeably; however, care should be taken not to interpret this as a literal measure of anatomic connectivity. Means for the ROIs were compared between healthy subjects and those with MDD at baseline using independent samples t-tests in SPSS (IBM, Armonk, New York). In subjects with MDD, means were compared between pre- and post-ketamine conditions using paired t-tests.
Taken together, the two ICs examined and the three relevant ROIs yielded a total of five a priori ROI comparisons. We report all results in our a priori ROIs as significant at p<0.05, but highlight those that are below a Bonferroni corrected threshold of p<0.01 (where 0.01 = 0.05/5). Given that we have prior evidence for each of our ROIs, Bonferroni correction may be overly conservative, which is additional justification for reporting all the results in our a priori regions that are significant at p<0.05.
2.6 Post-hoc statistical analysis
We carried out several post-hoc analyses. For a priori ROIs showing significant differences in connectivity pre- vs. post-ketamine, we calculated the correlation between percent change in connectivity pre- vs. post-ketamine with percent change in depression rating score (as assessed by the MADRS) from pre-infusion baseline (−60 minutes) to early response (+230 minutes). Percent change was calculated at post-infusion minus baseline, normalized by the baseline score. Given our small sample size and the likelihood that our data may violate the assumptions required by the Pearson correlation, we calculated the non-parametric Spearman rank correlation. In addition, for the three a priori ICs, we also obtained whole brain group comparisons as described previously ((Nugent et al., 2015b); see Supplementary Methods). Briefly, subject-specific IC maps were derived and compared using paired t-tests (within AFNI) to evaluate differences pre- and post-ketamine; significant clusters (p<0.05 FWE corrected) are reported. Because we examined three separate ICs, we highlight results that would survive a Bonferroni corrected threshold of pFWE<0.017 (0.05/3).
Following these a priori whole brain evaluations, we also carried out additional post-hoc whole brain analyses on the remaining ICs to identify other potential differences. We carried out paired t-tests using AFNI between the pre- and post-ketamine scans in the MDD group for all non-artifactual ICs recovered. We intended this as an additional exploratory analysis that might identify networks particularly sensitive to the effects of ketamine. Clusters significant at pFWE<0.05 corrected are reported.
2.7 Exploratory correlations with glucose metabolism
We previously published an 18F-FDG PET study in a group of patients that overlaps with those presented herein (see Supplementary Methods and (Carlson et al., 2013) for detailed data acquisition and preprocessing methods). The 18F-FDG PET scans were acquired at baseline and approximately 120 minutes post-ketamine infusion, and processed to obtain images of regional metabolic rate of glucose utilization (rMRGlu). Because glucose metabolism is driven by glial uptake of glucose following glutamate release from neurons, rMRGlu can be considered a proxy measure for glutamate levels, albeit an imperfect one (Magistretti and Pellerin, 1996). Our aim in this exploratory analysis was to determine if the connectivity differences we report herein may be at least partly explained by changes in rMRGlu (or glutamate levels) in the same regions. To explore this, mean rMRGlu values were extracted within the previously described amygdala and sgACC ROIs and compared using paired t-tests pre- and post-ketamine. We correlated mean rMRGlu within the left and right amygdala ROIs with mean connectivity to both the left and right insulo-temporal ICs; we also correlated mean rMRGlu within the sgACC ROI with mean connectivity to the bilateral precentral IC. As discussed above, we used non-parametric Spearman rank correlations. This resulted in five comparisons; correlations significant at a Bonferroni corrected value of p<0.01 are reported.
3. Results
3.1 Study participants
Demographic details are presented in Supplementary Table 1. No significant differences in age or gender were noted. MDD subjects’ mood scores placed them in the moderate to severe range. Of the 13 subjects with MDD, four received a single oral dose of riluzole one hour before MEG, and the rest received placebo. As in our prior reports (Ibrahim et al., 2012; Zarate et al., 2006), dissociative and autonomic symptoms during the infusion were common, but all negative symptoms resolved within 80 minutes and were not present at the time of the MEG recording.
3.2 A priori ROI analysis
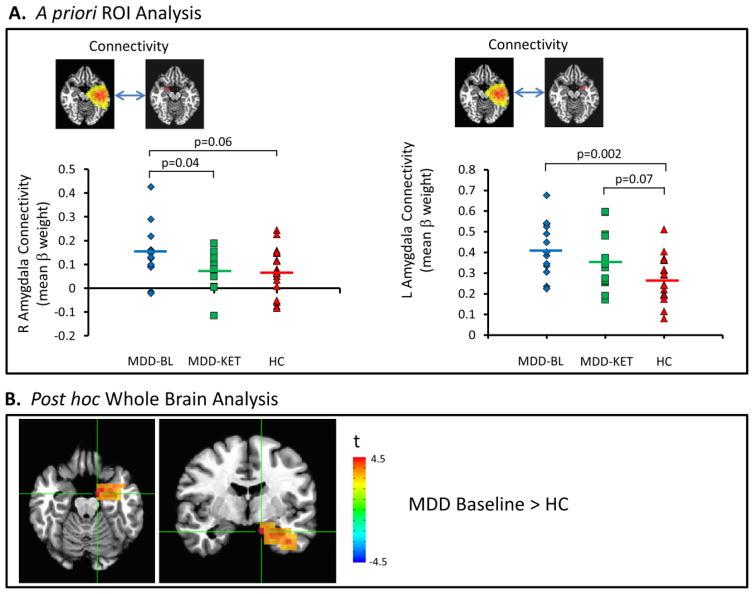
Table 1 summarizes our findings in the ROI analysis. Consistent with our prior study, connectivity between the left insulo-temporal IC (Figure 1A) and right amygdala was significantly elevated at baseline in participants with MDD compared to healthy volunteers (t=3.41, p=0.002). This was significantly reduced (t=2.25, p=0.04) post-ketamine (notated as MDD-KET in all figures) compared to baseline to a level statistically indistinguishable from that in healthy controls (p>0.8). Connectivity between the left insulo-temporal IC and the left amygdala (Figure 1A, right-hand side) was significantly elevated at baseline compared to healthy controls (t=3.41, p=0.002). Post-ketamine, connectivity in the MDD subjects showed only a trend towards elevation compared to the controls (t=1.85, t=0.07).
Table 1.
Mean connectivity values for all a priori ROI analysis. Comparisons between groups are given, note that HC vs. MDD-Baseline and HC vs. MDD-Post ketamine comparisons utilized independent samples t-tests, while the MDD-basline vs. MDD post-ketamine comparison was performed using paired t-tests. Values significant at p<0.05 are highlighted in bright yellow, values that trended towards significance (0.05<p<0.1) are highlighted in pale yellow.
| Independent Component | Mean Connectivity | HC vs. MDD-Baseline | MDD-Baseline vs. MDD-KET | HC vs. MDD-Post | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| ROI | HC | MDD-Pre | MDD-Post | t | p | t | p | t | p | |
| Left Insulo-temporal | Right Amyg | 0.070 | 0.145 | 0.076 | 1.95 | 0.06 | 2.25 | 0.04 | 0.18 | 0.86 |
| Left Amyg | 0.272a | 0.419 | 0.355 | 3.41 | 0.002 | 1.65 | 0.13 | 1.85 | 0.07 | |
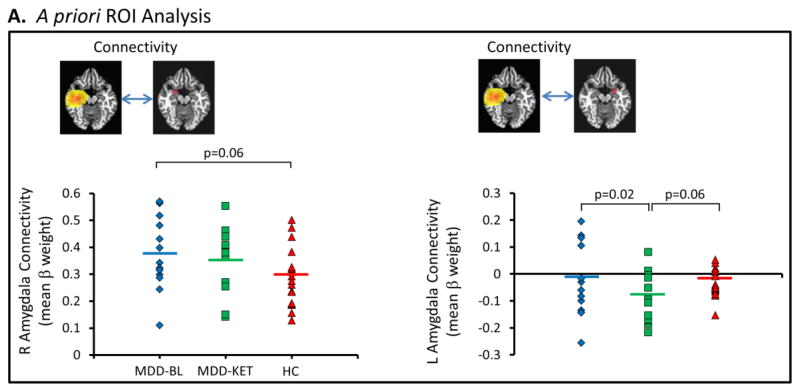
| Right Insulo-temporal | Right Amyg | 0.290a | 0.375 | 0.356 | 1.97 | 0.06 | 0.84 | 0.42 | 1.60 | 0.12 |
| Left Amyg | −0.033a | −0.020 | −0.084 | 0.36 | 0.72 | 2.85 | 0.02 | 1.95 | 0.06 | |
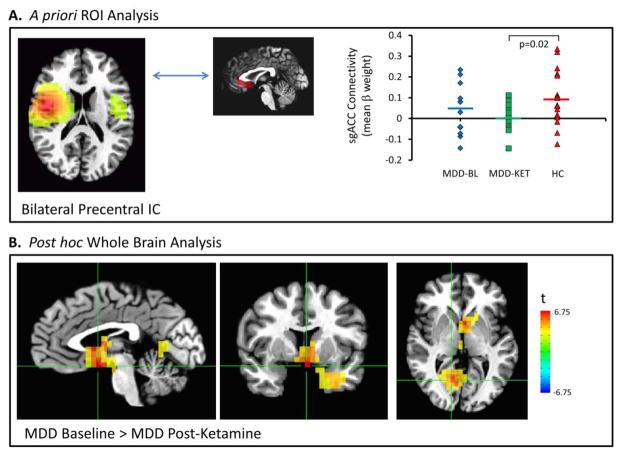
| Bilateral Precentral | sgACC | 0.099b | 0.042 | 0.001 | 1.27 | 0.21 | 1.44 | 0.18 | 2.56 | 0.02 |
Healthy subjects exhibited significantly lower connectivity than patients with MDD at baseline in a larger cohort (Nugent et al., 2015b)
Healthy subjects exhibited significantly greater connectivity than patients with MDD at baseline in a larger cohort (Nugent et al., 2015b)
Figure 1.
(A) Mean beta weight within right and left amygdala regions of interest (ROIs) as a measure of connectivity with left lateralized insulo-temporal independent component (IC) at baseline in participants with major depressive disorder (MDD) and healthy controls, and post-ketamine in participants with MDD. (B) Voxel-wise map of significant differences in connectivity between healthy controls and patients with MDD at baseline. BL: baseline; KET: ketamine; HC: healthy controls.
The connectivity between the right insulo-temporal IC and the right amygdala (Figure 2A) showed a trend to be elevated in MDD subjects at baseline compared with healthy control subjects, consistent with our significant finding in the larger sample (Nugent et al., 2015b), while subjects with MDD did not significantly differ from healthy subjects post-ketamine. Connectivity between the right insulo-temporal IC and left amygdala (Figure 2A, right-hand side) was significantly reduced from a negligible baseline correlation to a weak anti-correlation (t=2.85, p=0.02) post-ketamine compared to baseline. Although we had previously shown increased connectivity in MDD subjects at baseline compared to healthy controls (Nugent et al., 2015b), this relationship was not evident in this smaller sample.
Figure 2.
(A) Mean beta weight within right and left amygdala regions of interest (ROIs) as a measure of connectivity with right lateralized insulo-temporal independent component (IC) at baseline in participants with major depressive disorder (MDD) and healthy controls, and post-ketamine in participants with MDD. BL: baseline; KET: ketamine; HC: healthy controls.
Although we previously showed decreased connectivity in MDD subjects at baseline compared to healthy controls (Nugent et al., 2015b), this relationship was not significant in this smaller sample. Post-ketamine, connectivity between the bilateral precentral gyrus IC and the sgACC (Figure 3A), however, was significantly lower than that of healthy control participants (t=2.56, p=0.02).
Figure 3.
(A) Mean beta weight within the subgenual anterior cingulate cortex (sgACC) region of interest (ROI) as a measure of connectivity with the bilateral precentral independent component (IC) at baseline in participants with major depressive disorder (MDD) and healthy controls, and post-ketamine in participants with MDD. (B) Voxel-wise map of significant differences in connectivity between patients with MDD at baseline and post-ketamine infusion. BL: baseline; KET: ketamine; HC: healthy controls.
The results from our a priori ROIs for the pre- vs. post-ketamine comparisons did not survive Bonferroni correction for multiple comparisons, likely due to the small sample size in this preliminary report. However, we did observe increased connectivity between the left insulo-temporal IC and the left amygdala. No significant correlations were observed in any of our ROIs between percent change in connectivity and percent change in mood score post- vs. pre-ketamine infusion. Although subgroups were small, no significant differences (p>0.1) were observed in post-ketamine connectivity in any ROI between those receiving placebo (n=9) and those receiving riluzole (n=4).
3.3 Whole brain analysis in a priori ICs
Connectivity with the left insulo-temporal IC was significantly increased in MDD participants at baseline compared to healthy subjects in clusters in the left amygdala/temporal pole (pFWE<0.05, Figure 1B). No significant differences in connectivity were noted with the right insulo-temporal IC. Post-ketamine, as compared to pre-ketamine, connectivity with the bilateral precentral IC (Figure 3B) was significantly reduced in a cluster encompassing the sgACC, bilateral hippocampus, and right thalamus (pFWE<0.01), and in another cluster within the posterior cingulate (pFWE<0.01). Notably, the finding of reduced sgACC connectivity with the bilateral precentral IC post-ketamine vs. baseline in the whole-brain results met a stringent Bonferroni corrected threshold (pFWE< 0.017) over the three ICs examined, despite the fact that this finding was not evident in our a priori ROI analysis. This is likely due to small variations in the localization of the cluster peak between samples.
3.4 Post-hoc comparisons in other ICs
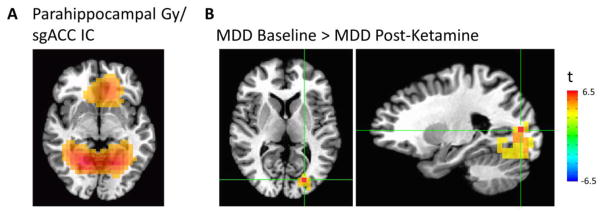
The IC encompassing the bilateral parahippocampal gyrus and sgACC showed differences between pre- and post-ketamine connectivity (Figure 4A). Compared to baseline, participants with MDD exhibited decreased connectivity between this IC and the left middle occipital cortex post-ketamine (pFWE<0.01, Figure 4B). At baseline, pre-treatment connectivity in this region in individuals with MDD was not significantly greater than the connectivity in healthy volunteers.
Figure 4.
(A) Bilateral parahippocampal gyrus/subgenual anterior cingulate cortex (sgACC) independent component (IC). (B) Voxel-wise map of significant differences in connectivity between patients with major depressive disorder (MDD) at baseline and post-ketamine infusion.
3.5 Exploratory correlations with 18F-FDG PET rMRGlu
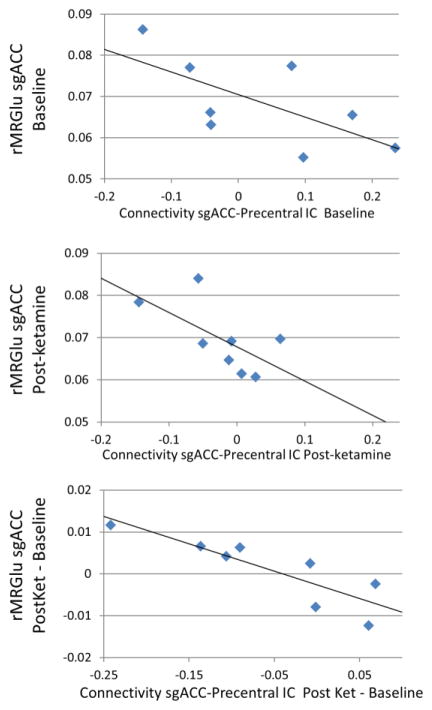
Eight subjects with MDD underwent both MEG and PET scans at both pre- and post-ketamine time points. No differences were observed in rMRGlu pre- and post-ketamine as determined by paired t-tests. No significant correlations were observed between amygdala connectivity and amygdala rMRGlu. There was a significant inverse correlation between rMRGlu in the sgACC and connectivity of the sgACC with the bilateral precentral IC pre- but not post-ketamine (rho=−0.714, p=0.047, and rho=−0.548, p=0.160, respectively); a significant inverse correlation was observed between change in connectivity and change in rMRGlu post- vs. pre-ketamine (rho=−0.905, p=0.002). Correlations are illustrated in Figure 5.
Figure 5.
Scatter plot illustrating subgenual anterior cingulate cortex (sgACC) connectivity with the bilateral precentral independent component (IC) shown in Figure 3A, plotted vs. glucose metabolism in the same sgACC region of interest (ROI). Panels from top to bottom depict absolute sgACC rMRGlu at baseline, post-ketamine, and the difference between post-ketamine and baseline sessions, plotted against connectivity at the same time points. Data from eight subjects with both magnetoencephalography (MEG) and positron emission tomography (PET) data are shown.
4. Discussion
This preliminary study investigated how a single open-label infusion of the NMDA antagonist ketamine altered resting state connectivity in 13 subjects with MDD. In MDD participants, connectivity to limbic regions following ketamine administration was reduced compared to baseline, and this occurred regardless of whether or not connectivity was abnormally increased or decreased at baseline relative to controls. Connectivity between the insulo-temporal ICs and amygdala, which was abnormally elevated at baseline, was reduced by ketamine infusion to levels more in line with those seen in healthy subjects. In contrast, connectivity between the precentral IC and the sgACC, which was abnormally reduced at baseline in our previous report, was further reduced by ketamine infusion, exacerbating differences between MDD and control subjects. Although not all results met a Bonferroni-corrected statistical threshold, it should be noted that connectivity between the sgACC and precentral IC was significantly reduced post-ketamine in the whole brain analysis. Furthermore, in a subset of our sample, change in sgACC and precentral connectivity post-ketamine were significantly correlated with change in sgACC glucose metabolism as measured by [18F]FDG-PET.
Multiple lines of research implicate the metabolism (reviewed in (Drevets et al., 2008)) and connectivity (Savitz and Drevets, 2009) of the sgACC in MDD, although the direction of the measured abnormality is far from consistent across studies. Successful antidepressant treatment has also been shown to modify sgACC metabolism (Drevets et al., 2008) and connectivity (McCabe and Mishor, 2011; Olbrich et al., 2014). Furthermore, deep brain stimulation of the sgACC was also found to result in an antidepressant effect (Mayberg et al., 2005), and the antidepressant efficacy of transcranial magnetic stimulation (TMS) stimulation at specific loci within the dorsolateral prefrontal cortex (DLPFC) was strongly correlated with functional connectivity between the DLPFC site and the sgACC (Fox et al., 2012; Fox et al., 2013). Our finding that ketamine further reduced abnormally decreased baseline sgACC connectivity may suggest that ketamine globally reduces connectivity at times removed from its acute effects.
Similar to the sgACC findings, abnormalities in the structure and function of the amygdala have been documented extensively in individuals with MDD (Price and Drevets, 2010). Consistent with our findings, functional (Avery et al., 2013; Ramasubbu et al., 2014; Wu et al., 2011; Zeng et al., 2012) and structural (Arnold et al., 2012; Fang et al., 2012) connectivity of the amygdala with temporal regions is also well documented, although other studies (Lui et al., 2011; Tahmasian et al., 2013; Veer et al., 2010) found decreased pre-treatment connectivity between the amygdala and insula. Studies of the effects of antidepressants on the amygdala are largely inconsistent (Dichter et al., 2014; McCabe and Mishor, 2011; Sripada et al., 2014). Given the discrepancies in the literature, it is difficult to interpret our findings in light of these results; however, our study has the particular strength of extending prior findings (largely obtained with fMRI) into the electrophysiological domain. Given the role of the amygdala in threat and fear response (Grillon, 2008), and the role of the insula in salience determination (Uddin, 2015), we hypothesize that increased connectivity at baseline is at least partially responsible for enhanced vigilance and the bias towards negative stimuli seen in depressed subjects (Roiser et al., 2012); ketamine’s ability to lower connectivity may serve to normalize this circuit.
In our exploration of other ICs, we observed post-ketamine reductions in connectivity between a parahippocampal/sgACC IC and the middle occipital cortex (MOC). We find this result particularly interesting for several reasons. First, the observed IC has not previously been reported in rsfMRI studies that use similar ICA methods (Beckmann et al., 2005; Damoiseaux et al., 2006). Although we previously observed this network (Nugent et al., 2015b), no significant group differences were noted. Thus, the identification of a new network salient to the treatment of MDD that exhibits phase coherence in band limited power but is not hemodynamically active (i.e. not visible in BOLD fMRI) is a significant finding. Further characterization of this network is necessary to determine its function and role in both normal cognition and psychiatric disease. There is precedent for the involvement of visual recognition circuits including the MOC in MDD (reviewed in (Dichter et al., 2014)); in particular, increased connectivity within visual circuits including the MOC in MDD patients relative to healthy controls at baseline (Guo et al., 2013) was reported, and the MOC may be associated with antidepressant response (Furey et al., 2013). Although this line of thought is highly speculative, we can hypothesize that MOC abnormalities may represent lower-level sensory processing deficits underlying depressive pathology such as the bias towards negative stimuli, although further studies would be necessary to confirm or disprove this conjecture.
Taken together, our findings suggest that ketamine reduces connectivity somewhat indiscriminately amongst MDD-relevant regions. Because our data did not include post-ketamine scans for healthy subjects, we cannot determine if connectivity would be similarly reduced regardless of psychiatric diagnosis, although our findings would be consistent with this hypothesis. The mechanism behind these changes, however, is unclear. Evidence links connectivity with the glutamatergic system, at least within some regions. For instance, glutamate levels in the posterior cingulate measured via magnetic resonance spectroscopy (MRS) were shown to strongly correlate with DMN connectivity (Kapogiannis et al., 2013), and glucose metabolism is related to connectivity within both the DMN and the dorsal attention network (Tomasi et al., 2013). Nevertheless, this correlation may be confined to these specific networks rather than reflect a general relationship across the brain within subjects, at least in healthy individuals (Nugent et al., 2015a). The specificity of the relationship between glutamate and connectivity to the DMN is consistent with our finding of a relationship between glutamate and connectivity in the sgACC (sometimes included as a DMN region), but not in the amygdala. It is important to note that it is also not entirely clear what a change in power envelope beta-band connectivity reflects in terms of the function of individual neurons. Although neuronal oscillations were once thought to be functionally irrelevant, mounting evidence suggests that oscillations are crucial to the synchronization of large scale cortical networks (Hipp et al., 2011). Oscillations may serve as a gating mechanism, given that information will be preferentially transferred between neuronal ensembles at the time of maximal depolarization (Fries, 2005; Fries et al., 2007). Beta oscillations may be particularly well suited to maintaining long range connections as they can withstand long neuronal conduction delays (Kopell et al., 2000).
Our results can also be discussed in the context of the pharmacology of ketamine. Although ketamine acts as an NMDA antagonist, it is thought to result in an acute glutamate “surge”, due to reduced inhibition of GABAergic interneurons (Duman et al., 2012); this is supported by most (Rowland et al., 2005; Stone et al., 2012), but not all (Taylor et al., 2012), MRS studies examining acute changes in glutamatergic transmission. Furthermore, this surge period appears to correlate with widespread increases in connectivity as measured by BOLD fMRI (Driesen et al., 2013; Khalili-Mahani et al., 2014). At time points removed from this acute surge, however, ketamine’s effects on glutamate levels are not as well understood. A rebound effect may exist, such that a period of widespread reduced connectivity may follow an acute increase in connectivity. Our finding of a negative, rather than positive, correlation between glucose metabolism and sgACC connectivity may simply reflect the fact that the PET scan was performed 120 minutes post-infusion, while the MEG scan took place five to six hours later.
This study has several limitations. First and foremost is the small sample size. While this severely reduced statistical power, we believe that these data may still be informative as hypothesis-generating for future work. Importantly, these data still remain the only published resting state MEG data in MDD patients pre- and post-ketamine. Second, it would have been more desirable to have a completely independent sample from our previously reported larger cohort including healthy controls at baseline (Nugent et al., 2015b), particularly because we considered that larger study to be hypothesis-generating in terms of targeted networks and ROIs. We attempted to ameliorate this in part by using anatomical ROIs, although these preliminary results should be treated with some caution until they are replicated in an independent sample. An additional limitation was the fact that we did not have post-ketamine scans in our healthy subjects, and could thus not perform a more sophisticated mixed model approach at analysis with both group and drug as factors. Although this study recruited only patients, future studies on the topic should include control subjects for all interventions. The choice of the time point post-ketamine at which MEG recordings were obtained may also have been a limitation. Given the complex effects of ketamine on the glutamatergic system, the ideal study would have covered several time points encompassing full response, including from the glutamate surge during the infusion to peak antidepressant response period (four to 24 hours) to the maintenance phase (two to seven days) to depressive relapse. Related to the choice of MEG procedure timing, an additional limitation is the fact that four subjects received a single oral dose of 50mg riluzole approximately one to two hours before MEG. A relatively low dose (50mg) was administered, however, and no differences were observed, either in this study or in prior work (Cornwell et al., 2012), between subjects who received riluzole and those who did not. Nonetheless, this remains an uncertainty in the present study. A final potential limitation is the choice of MEG to investigate resting state functional connectivity as opposed to the better-characterized fMRI. MEG cannot equal the spatial resolution of fMRI, and thus cannot pinpoint our results to small sections of the cortex or subcortical nuclei. Although there are far more rsfMRI studies than MEG studies, interpreting the former is nevertheless challenging due to the convolution with hemodynamic response function. In contrast, our study specifically implicates synchronous beta oscillations of neuronal populations. This, in turn, suggests a further limitation: that we only investigated beta-band connectivity. However, we felt that given the support for maximal coherence of resting state networks in the beta band, along with our prior findings (Nugent et al., 2015b), a more focused investigation in a single band was justified. Limiting the hypotheses tested seemed to be particularly important given the small sample size and preliminary nature of this investigation.
Despite the limitations of the current study, we believe our findings significantly add to the literature concerning the effects of ketamine on the depressed brain. We found significantly reduced connectivity between a bilateral precentral network and the sgACC post-ketamine compared to baseline, and this reduction was related to reduced sgACC glucose metabolism. We also found significantly reduced connectivity between bilateral insulo-temporal nodes and the contralateral amygdala post-ketamine infusion compared with baseline, potentially reflecting a normalization of these circuits and a reduction in the elevated limbic reactivity seen in MDD. Furthermore, we showed reduced connectivity between a limbic network (sgACC/orbitofrontal cortex/hippocampus) and MOC visual areas. Taken together with the results of previous studies, these results support the idea that a period of reduced connectivity follows the glutamate surge, at least in limbic and related cortical areas, with concomitant complex alterations in glucose metabolism. We hypothesize that the reduced connectivity observed here and by others on the same time scale as the peak antidepressant effect may potentially represent a rebound reaction following the initial glutamate surge. Because we found no correlation between magnitude of connectivity reduction and antidepressant response to ketamine, our findings may reflect a neurophysiological reaction to the drug rather than a true mechanism of antidepressant action. Future studies are necessary to further explore this hypothesis as the present study was underpowered to detect correlations with treatment response. Further elucidation of the electrophysiological response to ketamine may help develop more selective agents that have more nuanced effects on the glutamatergic system. By normalizing network function rather than globally reducing connectivity, such agents may potentially enhance efficacy.
Supplementary Material
Highlights.
Magnetoencephalography (MEG) showed correlated networks in patients with depression
Ketamine decreased connectivity in the amygdala and subgenual cingulate (sgACC)
Decreased sgACC connectivity was proportional to change in glucose metabolism
Acknowledgments
Role of Funding Source
Funding for this work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; Clinical Trials Identifier: NCT00024635 (ZIA-MH002927-04)), by a NARSAD Independent Investigator to Dr. Zarate, and by a Brain & Behavior Mood Disorders Research Award to Dr. Zarate. These funding sources had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; Clinical Trials Identifier: NCT00024635 (ZIA-MH002927-04)), by a NARSAD Independent Investigator to Dr. Zarate, and by a Brain & Behavior Mood Disorders Research Award to Dr. Zarate. The authors thank the 7SE research unit and staff for their support. Ioline Henter, MA (NIMH) provided invaluable editorial assistance.
Footnotes
Conflict of Interest
Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression; he has assigned his rights in the patent to the US Government but will share a percentage of any royalties that may be received by the Government. The remaining authors declare no competing financial interests.
Contributors
ACN: helped design the study; carried out all data analyses; wrote and revised the manuscript; approved the final version of the manuscript.
SER: authored some of the software used in the data analysis; helped interpret the data; revised the manuscript for intellectual content; approved the final version of the manuscript.
RC: oversaw collection of the MEG data; helped interpret the data; revised the manuscript for intellectual content; approved the final version of the manuscript.
CAZ: designed the study; oversaw the study; revised the manuscript for intellectual content; approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JF, Zwiers MP, Fitzgerald DA, van Eijndhoven P, Becker ES, Rinck M, Fernandez G, Speckens AE, Tendolkar I. Fronto-limbic microstructure and structural connectivity in remission from major depression. Psychiatry Res. 2012;204(1):40–48. doi: 10.1016/j.pscychresns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2013;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Lally N, Nugent AC, Furey ML, Luckenbaugh DA, Zarate CA. Neural Correlates of Suicidal Ideation and Its Reduction in Depression. International Journal of Neuropsychopharmacology. 2015;18(1) doi: 10.1093/ijnp/pyu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Liddle EB, Hale JR, Woolrich MW, Luckhoo H, Liddle PF, Morris PG. Task induced modulation of neural oscillations in electrophysiological brain networks. Neuroimage. 2012;63(4):1918–1930. doi: 10.1016/j.neuroimage.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Barnes GR, Smith SM, Morris PG. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci U S A. 2011;108(40):16783–16788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Manji HK, Zarate CA, Jr, Drevets WC. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry. 2013;73(12):1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA., Jr Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72(7):555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2014;172C:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety. 2014;31(4):291–296. doi: 10.1002/da.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, Doyon J, Northoff G. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS One. 2013;8(4):e60312. doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Zeng LL, Shen H, Wang L, Li B, Liu L, Hu D. Increased cortical-limbic anatomical network connectivity in major depression revealed by diffusion tensor imaging. PLoS One. 2012;7(9):e45972. doi: 10.1371/journal.pone.0045972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72(7):595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends in Neurosciences. 2007;30(7):309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA., Jr Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70(3):280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199(3):421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudayol-Ferre E, Pero-Cebollero M, Gonzalez-Garrido AA, Guardia-Olmos J. Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front Hum Neurosci. 2015;9:582. doi: 10.3389/fnhum.2015.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Xue Z, Gao K, Liu Z, Xiao C, Chen H, Zhao J. Abnormal resting-state cerebellar-cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:51–57. doi: 10.1016/j.pnpbp.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69(2):387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili-Mahani N, Niesters M, van Osch MJ, Oitzl M, Veer I, de Rooij M, van Gerven J, van Buchem MA, Beckmann CF, Rombouts SA, Dahan A. Ketamine interactions with biomarkers of stress: A randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. Neuroimage. 2014;108:396–409. doi: 10.1016/j.neuroimage.2014.12.050. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97(4):1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA., Jr Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. 2015;29(5):596–607. doi: 10.1177/0269881114568041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, Hu D. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74(1):48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, Huang X, Kemp GJ, Mechelli A, Gong Q. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry. 2011;168(6):642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry. 1996;1(6):445–452. [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 2011;57:1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J Neurosci. 2015;35:11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Khalili-Mahani N, Martini C, Aarts L, van Gerven J, van Buchem MA, Dahan A, Rombouts S. Effect of subanesthetic ketamine on intrinsic functional brain connectivity: a placebo-controlled functional magnetic resonance imaging study in healthy male volunteers. Anesthesiology. 2012;117(4):868–877. doi: 10.1097/ALN.0b013e31826a0db3. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Martinez A, D’Alfonso A, Zarate CA, Theodore WH. The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J Cereb Blood Flow Metab. 2015a;35:583–591. doi: 10.1038/jcbfm.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Robinson SE, Coppola R, Furey ML, Zarate CA., Jr Group differences in MEG-ICA derived resting state networks: Application to major depressive disorder. Neuroimage. 2015b;118:1–12. doi: 10.1016/j.neuroimage.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich S, Trankner A, Chittka T, Hegerl U, Schonknecht P. Functional connectivity in major depression: increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res. 2014;222(1–2):91–99. doi: 10.1016/j.pscychresns.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70(4):373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SE, Vrba J. Biomag. Tohoku University Press; Sendai: 1999. Functional neuroimaging by synthetic aperture magnetometry (SAM) pp. 302–305. [Google Scholar]
- Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37(1):117–136. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162(2):394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65(4):289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35(7):1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164(1):300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7(9):e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Leopold DA, Brookes MJ, Khader PH. The contribution of electrophysiology to functional connectivity mapping. Neuroimage. 2013;80:297–306. doi: 10.1016/j.neuroimage.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AD, Saxena N, LEJ, Hall JE, Singh KD, Muthukumaraswamy SD. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur Neuropsychopharmacol. 2015;25(8):1136–1146. doi: 10.1016/j.euroneuro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Welsh RC, Marx CE, Liberzon I. The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Hum Brain Mapp. 2014;35:3249–3261. doi: 10.1002/hbm.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17(7):664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Knight DC, Manoliu A, Schwerthoffer D, Scherr M, Meng C, Shao J, Peters H, Doll A, Khazaie H, Drzezga A, Bauml J, Zimmer C, Forstl H, Wohlschlager AM, Riedl V, Sorg C. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front Hum Neurosci. 2013;7:639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol. 2012;26(5):733–737. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A. 2013;110(33):13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem MA, van der Wee NJ, Rombouts SA. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:41. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspectives on Psychological Science. 2009;4(3):274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wu QZ, Li DM, Kuang WH, Zhang TJ, Lui S, Huang XQ, Chan RC, Kemp GJ, Gong QY. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum Brain Mapp. 2011;32(8):1290–1299. doi: 10.1002/hbm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, Zhou Z, Li Y, Hu D. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135(Pt 5):1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.