Abstract
The present neuroimaging study investigated two aspects of difficulties with emotion associated with Borderline Personality Disorder (BPD1): affective lability and difficulty regulating emotion. While these two characteristics have been previously linked to BPD symptomology, it remains unknown whether individual differences in affective lability and emotion regulation difficulties are subserved by distinct neural substrates within a BPD sample. To address this issue, sixty women diagnosed with BPD were scanned while completing a task that assessed baseline emotional reactivity as well as top-down emotion regulation. More affective instability, as measured by the Affective Lability Scale (ALS2), positively correlated with greater amygdala responses on trials assessing emotional reactivity. Greater difficulties with regulating emotion, as measured by the Difficulties with Emotion Regulation Scale (DERS3), was negatively correlated with left inferior frontal gyrus (IFG4) recruitment on trials assessing regulatory ability. These findings suggest that, within a sample of individuals with BPD, greater bottom-up amygdala activity is associated with heightened affective lability. By contrast, difficulties with emotion regulation are related to reduced IFG recruitment during emotion regulation. These results point to distinct neural mechanisms for different aspects of BPD symptomology.
Keywords: fMRI, Borderline Personality Disorder, emotion regulation, amygdala, prefrontal cortex
1. Introduction
Borderline Personality Disorder (BPD) is characterized by strong, variable emotions and difficulties with self-regulation that impede functioning (Fletcher et al., 2014; Scott et al., 2013; Tragesser et al., 2007). Current theory suggests that emotional problems are central to BPD (Crowell et al., 2009; Jazaieri et al., 2013; Sebastian et al., 2013). Such problems manifest through intense and unstable emotions (i.e., affective lability) as well as through difficulties with top-down (i.e., volitional and cognitively-driven) emotion regulation, both within and across individuals (Linehan, 1993b; Linehan and Dexter-Mazza, 2007; Westen et al., 1997; Zittel Conklin et al., 2006). While some have concluded that affective lability and difficulties with emotion regulation are overlapping constructs (Marwaha et al., 2013), it is also possible that they are distinct, but difficult to discriminate, constructs. Consistent with this, affective instability and difficulty controlling emotions such as anger, are characterized as distinct yet meaningful diagnostic criteria for diagnosing BPD and such constructs map on closely to affective lability and difficulties with emotion regulation. The present study first sought to test whether symptomology related to affective lability and emotion regulation difficulties were related among individuals with BPD, and second, characterized these two dimensions using neuroimaging analyses focused on individual differences (Lenzenweger et al., 2008; Linehan and Dexter-Mazza, 2007).
1.1 Affective lability in BPD
Affective lability, or the tendency to experience strong and variable emotions, disrupts functioning and well-being in BPD (Gunderson and Zanarini, 1989; Linehan, 1993a). Individuals with BPD experience greater affective lability than healthy individuals and individuals with other clinical disorders (Koenigsberg et al., 2002; Reich et al., 2012; Santangelo et al., 2014) and affective lability predicts worse outcomes, such as suicidal ideation and attempts, among individuals with BPD (Links et al., 2007; Wedig et al., 2012). While the amygdala has been linked to affective lability across various forms of psychopathology (Broome et al., 2015), the neural substrates underlying affective lability in BPD are not yet well-characterized.
The amygdala is critical for detecting, encoding and responding to social and emotional stimuli (Cunningham and Brosch, 2012; Kober et al., 2008; Phelps and LeDoux, 2005), particularly those that are ambiguous or unpredictable (Whalen, 2007). Individuals with BPD show reduced amygdala volumes compared to healthy controls (Ruocco et al., 2012; Schulze et al., 2016), and critically, have poorer white matter integrity in tracts connecting the amygdala to prefrontal regions important for regulating emotional responses (Lischke et al., 2015). Such structural alterations may explain at least in part why individuals with BPD amygdala show alterations in amygdala responses (Ruocco et al., 2013; Schulze et al., 2016), as well as amygdala habituation (Hazlett et al., 2012; Kamphausen et al., 2013), compared to healthy controls. While some studies have found that individuals with BPD show exaggerated amygdala responses when passively viewing emotional content (Donegan et al., 2003; Hazlett et al., 2012; Herpertz et al., 2001; Koenigsberg et al., 2009b; Niedtfeld et al., 2010), others have found blunted responses (Koenigsberg et al., 2009a; Smoski et al., 2011). These discrepancies might partially be due to affective lability among individuals with BPD resulting in variable amygdala responses both within and across individuals. Consistent with this, prior work has demonstrated that affective lability correlates with amygdala responses during passive viewing of aversive and neutral stimuli in BPD (Hazlett et al., 2012). This finding is intriguing but warrants follow-up because 1) it is unclear how to interpret amygdala responses to neutral images, and 2) amygdala responses were assessed solely during passive viewing and not during active regulation as well, making it unclear whether affective lability tracks with differences in bottom-up responding or top-down regulation.
In the present study, it was hypothesized that trait affective lability would track with amygdala responses during naturalistic emotional responding. Testing this hypothesis provides a critical check for models of BPD – if differences in affective lability do not correlate with amygdala recruitment in BPD, this would suggest that amygdala differences between BPD and controls are less clinically relevant than currently believed.
1.2 Difficulties with emotion regulation in BPD
Emotion dysregulation is a core feature of BPD (Fletcher et al., 2014; Scott et al., 2013; Stepp et al., 2014). In healthy adults, regulatory strategies such as reappraisal, which involves thinking about emotional events differently so as to alter their emotional import, recruit dorsal and lateral prefrontal (PFC) regions involved in cognitive control and attenuate amygdala responses (Buhle et al., 2013). Multimodal meta-analytic results have revealed something of a paradox with regards to lateral PFC in BPD – while individuals with BPD exhibit larger gray matter volumes in lateral PFC, they also show reduced lateral PFC activation (Schulze et al., 2016). With regards to reappraisal specifically, individuals with BPD report comparable reappraisal-related decreases in negative affect to controls, yet show different PFC and amygdala recruitment when reappraising (Koenigsberg et al., 2009a; Lang et al., 2012; Schulze et al., 2011). However, PFC effects differ across studies – two found that healthy controls recruited the anterior cingulate cortex (ACC) to a greater degree than did individuals with BPD during regulation (Koenigsberg et al., 2009a; Lang et al., 2012), while another found opposing results in the ACC and greater recruitment of dorsolateral and orbitofrontal cortex in healthy controls relative to individuals with BPD (Schulze et al., 2011). One possibility for these conflicting results is that prefrontal recruitment or prefrontal-amygdala functional connectivity – no work to date has examined reappraisal-related functional connectivity in BPD – during reappraisal may vary widely between different individuals with BPD and this variability has led to inconsistent findings across studies. Moreover, this variability in prefrontal recruitment might correspond to individual differences in trait difficulties in emotion regulation.
Clinical and neuroscientific evidence suggests that affective lability and difficulties with emotion regulation contribute to BPD but less is known about their neural substrates. The present study addressed this issue with a well-validated fMRI paradigm that has been used to study emotion regulation in healthy adults (Buhle et al., 2013) and individuals with BPD (Koenigsberg et al., 2009a; Lang et al., 2012; Schulze et al., 2011). In this paradigm, participants alternately respond to emotional stimuli in an unregulated way, to assess baseline emotional reactivity, or regulate their emotional responses using reappraisal (Buhle et al., 2013). Given that prior work has already compared individuals with BPD and healthy controls using this paradigm (Koenigsberg et al., 2009a; Lang et al., 2012; Schulze et al., 2011), and that the primary interest of the present study was to characterize within-disorder variability, the present study tested a large sample of women with BPD instead of comparing individuals with BPD to healthy controls. This large sample was critical for assessing individual differences (Yarkoni, 2009) and testing whether: (1) affective lability would be associated with heightened amygdala responses during naturalistic emotional responding, and (2) trait difficulties with regulating emotion would be associated with reduced prefrontal recruitment during emotion regulation.
2. Methods
2.1 Subjects
Sixty, medication-free adult females with BPD participated in this study (Table 1). Participants were a subgroup of individuals recruited through advertisements, clinician referrals and referrals from advocacy groups to be a part of a larger treatment study. All participants met DSM-IV criteria for BPD (American Psychiatric Association, 2000), as determined by the Structured Clinical Interview for DSM-IV (SCID), parts I and II (ICC=.86). Exclusion criteria included being male, present organic mental syndromes and past or present bipolar I disorder, psychotic disorder, schizophrenic disorder, or mental retardation. Participants were excluded if they had a condition contraindicated for neuroimaging. Only women were investigated because the larger study sample was overwhelmingly female (92%), due to more women than men seeking treatment in psychiatric centers (Sansone and Sansone, 2011). Participants were not screened for Attention Deficit Hyperactivity Disorder (ADHD). All participants provided informed consent. The Institutional Review Boards at New York State Psychiatric Institute and Columbia University approved this research.
Table 1.
Demographic characteristics of study participants
| n | Mean | SD | |
|---|---|---|---|
| Age | 60 | 28.55 | 8.97 |
| n | % | ||
| Female | 60/60 | 100 | |
| White | 35/60 | 58 | |
| High school graduate or above | 58/60 | 97 | |
| Single (includes separated and divorced) | 47/60 | 78 | |
| Currently employed | 38/60 | 63 | |
| History of psychiatric hospitalization | 43/60 | 72 | |
To assess affective lability, participants completed the Affective Lability Scales (ALS) (Harvey et al., 1989) which is comprised of 54 questions that assess how rapidly moods vary in terms of depression, elation, anxiety, anger, anxiety/depression, and depression/elation on a scale of 0–3. Reliability on the ALS and its subscales is high (0.86–0.92) in patient populations (Aas et al., 2015). To assess emotion regulation, participants completed the Difficulties in Emotion Regulation Scale (DERS) (Gratz and Roemer, 2004) which is comprised of 36 questions that assess problems with emotion regulation in terms of non-acceptance of emotional responses, difficulties engaging in goal-directed behavior, impulse control difficulties, lack of emotional awareness, limited access to emotion regulation strategies, and lack of emotional clarity on a scale of 1–5. The DERS has high internal consistency for both tis total score and subscales (0.80–0.93) (Gratz and Roemer, 2004). The ALS and DERS have been used to assess affective lability and emotion dysregulation in controls (Aas et al., 2015; Gratz and Roemer, 2004) and individuals with BPD (Gratz and Gunderson, 2006; Hazlett et al., 2007; Koenigsberg et al., 2001; Koenigsberg et al., 2002).
2.2 Training procedures
Prior to scanning, participants were trained to use the ‘immerse’ and ‘distance’ strategies using well-validated procedures (Ochsner et al., 2002). On ‘immerse’ trials, participants were told to imagine standing close to the scene depicted in the photograph and to allow themselves to experience any emotions that the photograph evoked. On ‘distance’ trials, participants were told to imagine standing further away from the scene and to focus more on the facts of the photograph than on its emotional details. Participants practiced using both strategies verbally with an experimenter who provided feedback for four images that were not used in the scanning paradigm and subsequently silently implemented the strategies on their own for four images.
2.3 fMRI task
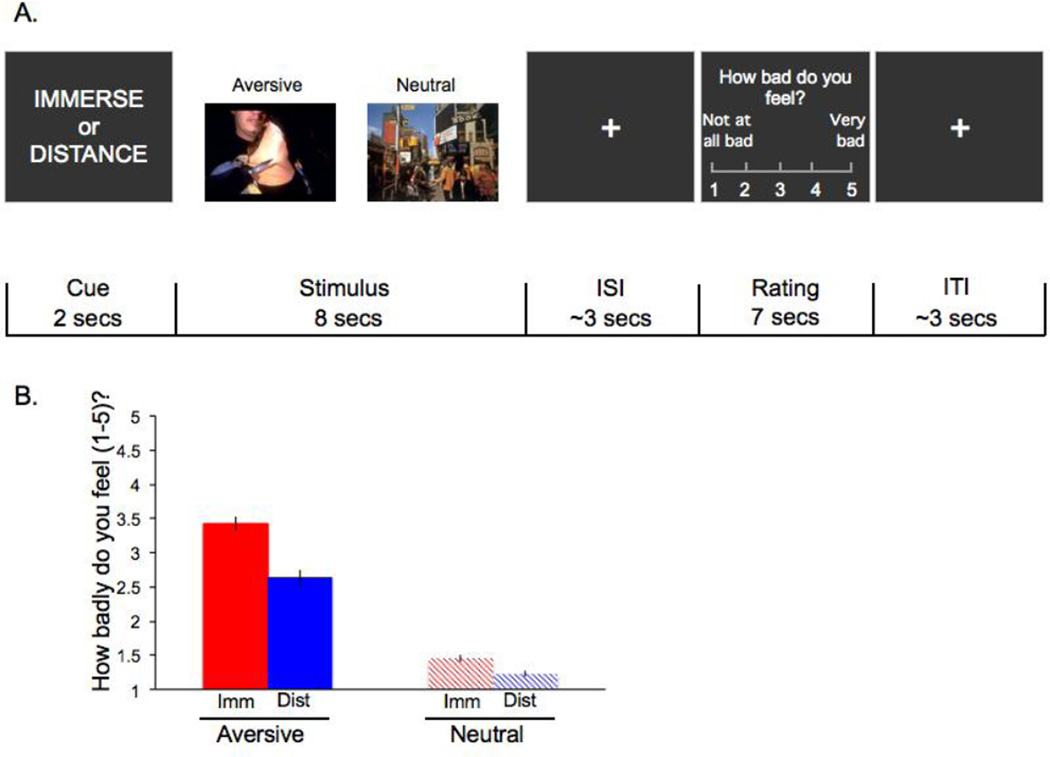
Participants completed a reappraisal task consisting of 90 experimental trials inside the scanner. A unique image was presented on each trial. Sixty trials contained aversive images depicting people, and 30 trials contained neutral images depicting people (e.g., people walking down the street). Half of the aversive stimuli depicted gory scenes (e.g., mutilated bodies) and half depicted interpersonal conflict or rejection (e.g., a couple arguing). Future manuscripts will examine how neural responses differed as a function of aversive stimulus type and thus more aversive trials were included than neutral trials. Stimuli came from the International Affective Picture System (Lang et al., 2001; Lang et al., 1993). The assignment of pictures to instruction was counterbalanced between participants. In total, participants completed 30 immerse/aversive, 30 distance/aversive, 15 immerse/neutral, 15 distance/neutral. On each trial, participants used the strategy indicated by a cue word (‘immerse’ or ‘distance’, shown for 2 seconds) while viewing an image for 8 seconds (Figure 1a). On each trial, participants subsequently rated their negative affect on a five-point scale (1=not feeling badly at all, 5=feeling very badly) via button press for 3 seconds. A variable interval fixation cross was shown for an average of 3 seconds between the image viewing and rating portions of each trial and a variable interval fixation cross was shown for an average of 3 seconds after each trial’s rating screen. At the end of each run (i.e., a block of 18 trials), participants completed an active baseline task comprised of making button presses to indicate the direction of an arrow for 20 seconds.
Figure 1.
(a) Trial design; (b) Self-reported negative affect as a function of stimulus type and strategy. Main effects of stimulus type and strategy as well as the interaction term between stimulus type and strategy were significant at p<0.001.
Emotional experience was evaluated using self-report for three reasons. First, self-report provides specific information about the valence of one’s emotional state that peripheral physiological measures cannot (e.g., skin conductance reflects gross changes in arousal). Second, self-reported affect on reappraisal paradigms does not relate to an individual’s dispositional need to please others (Ochsner et al., 2002; Silvers et al., 2012). Third, self-reported affective states (e.g., depressed mood) are routinely used for diagnosing and predicting outcomes in clinical populations such as BPD (Bradley et al., 2011; Edelbrock et al., 1985; Lonigan et al., 2003; Silk et al., 2003).
2.4 fMRI acquisition
Whole-brain data were acquired on a GE 1.5 Tesla scanner (General Electric, Milwaukee, Wisconsin). Functional data were acquired with a T2*-sensitive EPI sequence (28 4 mm contiguous axial slices, TR=2000 ms, TE=34 ms, flip angle=84°, FOV=22.4 cm). Anatomical images were acquired with a T1-weighted SPGR scan (1241.5 mm slices, TR=19 ms, TE=5 ms, FOV=22 cm).
2.5 Behavioral data analysis
Self-reported negative affect was analyzed using SPSS 19.0. A repeated-measures ANOVA assessed the effects of strategy (immerse, distance) and stimulus valence (aversive, neutral). To test whether affective lability was associated with baseline emotional reactivity, a reactivity score (percent increase in negative affect on immerse/aversive versus immerse/neutral trials) was calculated for each participant and correlated with ALS scores. To test whether emotion dysregulation was associated with top-down regulatory success, a regulation success score (percent decrease in negative affect on distance/aversive versus immerse/aversive trials) was calculated for each participant and correlated with DERS scores. Standard deviations were assessed for each trial type to assess response variability.
2.6 fMRI data analysis
2.6.1 Preprocessing
The first four volumes of each functional scan were discarded to avoid saturation effects. Preprocessing was conducted using statistical parametric mapping software (SPM8, Wellcome Department of Cognitive Neurology, London, UK) and consisted of slice time correction (using the first slice for reference), realignment and coregistration of the functional and structural data. Coregistered anatomical images were segmented into gray and white matter and normalized (warped) to the standard MNI template brain and warping parameters were applied to all functional images. Normalized functional images were interpolated to 3 × 3 × 3 mm voxels and spatially smoothed with a 6-mm Gaussian filter. A gray-matter mask based on the MNI-standardized Colin-brain was used to constrain the functional data. Motion parameters were estimated during preprocessing and volumes that contained frame-to-frame motion greater than 1.5 mm (translation) or 2 degrees (rotation) were censored.
2.6.2 First-level fMRI analyses
First-level GLM analyses were implemented in NeuroElf (http://neuroelf.net). Strategy cue, picture presentation (coded as four different trial types for the different strategy/picture type combinations), rating period, and active baseline portions of each trial were modeled as boxcar regressors convolved with a canonical hemodynamic response function. For each subject, a robust regression analysis was performed on the conditions of interest. Motion parameters and high-pass temporal filter parameters were included as regressors of no interest.
2.6.3 Second-level fMRI analyses
Random-effects group analyses were thresholded using a peak and extent combination that controlled the family-wise error rate at alpha <0.05 (uncorrected p<0.002 2-tailed, 49 voxels), as calculated by AlphaSim, implemented in NeuroElf. Given a priori hypotheses regarding the amygdala, a targeted analysis was performed using a region of interest (ROI) defined by placing a 6-mm sphere around three peak amygdala coordinates (−20, −6, −18; 20, −4, −20; 30, −2, −28) from a meta-analysis of neuroimaging studies of emotion (Kober et al., 2008). Such ROI approaches are common for the amygdala as it is a relatively small subcortical structure with poorer signal-to-noise properties than cortical regions (LaBar et al., 2001). Small volume correction was completed in four steps in Neuroelf. First, a list of coordinates was created simultaneously within the three amygdala ROIs. Second, a volume was created based on the list of coordinates. Third, each coordinate was expanded according to the local smoothness estimate. Finally, the number of resels (spatial units that have the same smoothness) is summed and the voxelwise threshold within the amygdala mask is adjusted to p<.025 (i.e., p<.05, 2-tailed) divided by the number of resels (in the present study, 73 resels were detected). The adjusted voxelwise r/t and p value combination needed to achieve small volume correction was r=.4403 for correlations, t=3.59 for contrasts and in both cases, p=.0003. As such, all amygdala voxels identified by SVC were identified using a form of Bonferroni correction that took into account spatial smoothness and the number of independent tests performed within the search space. Specific analyses are described below.
2.6.4 Analysis of baseline emotional reactivity
To assess baseline emotional reactivity, a whole-brain robust t-test was computed comparing the immerse/aversive and immerse/neutral conditions.
2.6.5 Analysis of top-down emotional regulation
To assess top-down emotional regulation, a whole-brain robust t-test was computed comparing the distance/aversive and immerse/aversive conditions.
2.6.6 Brain activity related to affective lability
To examine whether affective lability was associated with neural recruitment during baseline emotional reactivity, ALS scores were robustly correlated with the immerse/aversive > immerse/neutral contrast.
2.6.7 Brain activity related to difficulties in emotion regulation
To examine whether emotion dysregulation was associated with neural recruitment during emotional regulation, DERS scores were robustly correlated with the distance/aversive > immerse/aversive contrast. While the amygdala was not hypothesized to be associated with DERS, for completeness, SVC analyses with the amygdala were performed for this analysis as well and no clusters emerged.
3. Results
3.1 Behavioral results
3.1.1 Clinical measures
Descriptive statistics are reported in Table 1. ALS (Mean=94.93; S.D.=29.39; Range=25–152) and DERS (Mean=127.91; S.D.=22.09; Range=74–177) scores varied substantially across participants. Reliability was high for participant responses on both the ALS (Cronbach’s alpha=0.96) and the DERS (Cronbach’s alpha=.91). ALS (Mean difference=68.47, t(55)=17.43, p<.001) and DERS (Mean difference=49.92, t(56)=17.07, p<0.001) scores in this sample were significantly higher than published norms for healthy controls (ALSHealthyControls: Mean=69.66, reported as mean: 0.49, adjusted to sum=26.46, S.D.= 0.41, S.D. adjusted to sum=22.14; DERSHealthyControls: Mean=77.99, S.D.=20.72) (Aas et al., 2015; Gratz and Roemer, 2004). Assuming normal distributions for DERS and ALS published norms in healthy individuals, 8.8% of the present sample fell within 2 standard deviations of the mean for healthy individuals on the DERS and 25% fell within 2 standard deviations of the mean for healthy individuals on the ALS. Thus, BPD participants reported greater difficulties with emotion regulation and affective lability than healthy adults but there was also substantial variability. Three participants did not complete the DERS and four did not complete the ALS. DERS and ALS scores were uncorrelated (N=56; r=0.03, p=0.82). Results from the Kolmogorov-Smirnov test for normality revealed the ALS (D=0.08, p=0.20) and DERS (D=0.08, p=0.20) distributions did not deviate significantly from a normal distribution.
Participants also completed measures of: 1) borderline traits (Zanarini et al., 2003); 2) subjective depression (Beck et al., 1961); 3) objective depression (Hamilton, 1960) and anxiety (Hamilton, 1959); 4) suicidal ideation (Beck et al., 1979); 5) global functioning (Endicott et al., 1976); 6) impulsivity (Barratt, 1965); 7) aggression (Brown et al., 1979); 8) hostility (Buss and Durkee, 1957); and 9) suicide attempt history (Oquendo et al., 2003).
Correlations between all clinical questionnaires as well as correlations between clinical and task measures are reported in Table 3.
Table 3.
Correlations between clinical and task data for study participants
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | ||||||||||||
| 2 | 0.521+ | 1 | |||||||||||
| 3 | 0.422 | 0.64+ | 1 | ||||||||||
| 4 | 0.513+ | 0.80+ | 0.49+ | 1 | |||||||||
| 5 | −0.28 | −0.52+ | −0.47+ | −0.33 | 1 | ||||||||
| 6 | 0.30 | 0.32 | 0.43 | 0.24 | −0.41 | 1 | |||||||
| 7 | 0.12 | −0.02 | 0.12 | 0.08 | 0.09 | −0.16 | 1 | ||||||
| 8 | 0.22 | 0.09 | 0.17 | 0.11 | 0.05 | −0.05 | 0.22 | 1 | |||||
| 9 | 0.14 | 0.05 | −0.10 | 0.04 | 0.03 | −0.23 | 0.22 | 0.43 | 1 | ||||
| 10 | 0.21 | 0.28 | 0.35 | 0.22 | 0.02 | −0.09 | 0.25 | 0.59+ | 0.30 | 1 | |||
| 11 | 0.14 | 0.18 | 0.41 | 0.27 | −0.10 | 0.20 | 0.23 | −0.05 | −0.06 | 0.03 | 1 | ||
| 12 | −0.01 | 0.03 | −0.05 | 0.00 | 0.15 | −0.08 | 0.09 | −0.01 | 0.06 | −0.07 | 0.15 | 1 | |
| 13 | 0.23 | 0.33 | 0.20 | 0.23 | −0.24 | 0.24 | −0.21 | −0.26 | −0.08 | −0.14 | 0.07 | 0.01 | 1 |
1Zanarini Rating Scale for Borderline Personality Disorder, 2Hamilton Depression Rating Scale, 3Beck Depression Inventory, 4Hamilton Anxiety Rating Scale, 5Global Affective Functioning, 6Scale for Suicidal Ideation, 7Barratt Impulsiveness Scale, 8Buss-Durkee Hostility Inventory, 9Brown-Goodwin Aggression History Scale, 10Affective Lability Scale, 11Difficulties with emotion regulation Scale, 12Task emotional reactivity (percent increase in negative affect on immerse/negative versus immerse/neutral trials), 13Task regulation success (percent decrease in negative affect on distance/negative versus immerse/negative trials). r values and significance values are reported as
p<0.05, corrected.
3.1.2 Task results
Participants reported more negative affect when viewing aversive than neutral stimuli (Meandifference =1.69, F(1,59)=267.87, p<0.001) and less negative affect (Meandifference=0.50, F(1,59)=117.85, p<0.001) on distance than immerse trials (Figure 1b). Regulatory strategy and stimulus valence interacted such that participants reported greater decreases in negative affect when distancing themselves from negative stimuli (Meandifference=0.78) than neutral stimuli (Mean decrease=.22), F(1,59)=61.40, p<0.001. Participants reported more variable (larger standard deviations) affect for aversive than neutral stimuli (Meandifference=0.49, F(1,59)=149.91, p<0.001), for immerse than distance trials (Meandifference=0.12, F(1,59)=22.94, p<0.001), and a significant valence × strategy interaction (F(1,59)=11.01, p<0.01), such that responses were more variable for immerse/neutral than distance/neutral trials (Meandifference=0.22), but were comparable for immerse/aversive and distance/aversive trials (Meandifference =0.03).
3.2 fMRI results
3.2.1 Analysis of baseline emotional reactivity
Participants recruited numerous prefrontal, subcortical, and midbrain regions when immersing themselves in aversive versus neutral stimuli (Supplemental Table 1). The bilateral amygdala showed significantly greater recruitment when individuals immersed themselves in aversive versus neutral stimuli, as revealed by both whole-brain (see Supplemental Table 1) and small volume correction analyses (Left: MNI coordinates: −15, 0, −21, 114 voxels, maximum t statistic=7.18; Right: 21, −6, −15, 86 voxels, maximum t statistic=5.74).
3.2.3 Analysis of top-down emotional regulation
Participants recruited the inferior parietal lobule when distancing themselves from aversive stimuli (Supplemental Table 2). Small volume correction revealed a two-voxel cluster (MNI coordinates: −15, 0, −21, maximum t statistic=3.74) in the left amygdala that showed significantly less activation on distance/immerse than immerse/aversive trials.
3.2.4 Brain activation related to affective lability
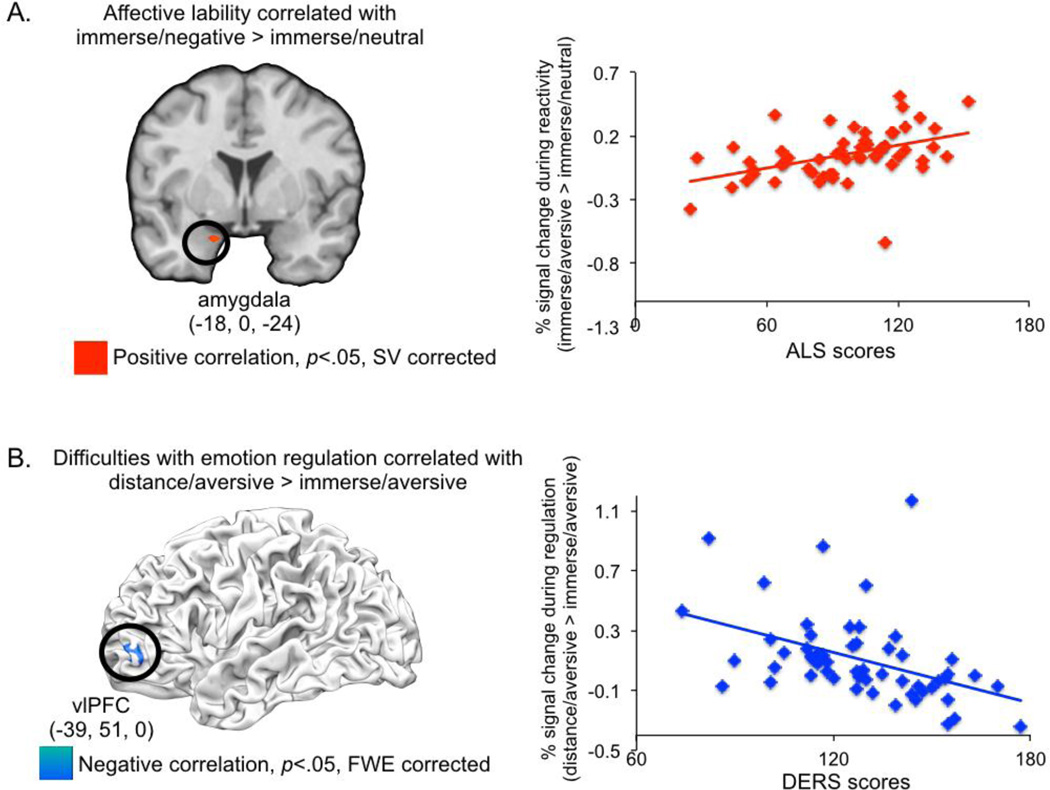
Greater affective lability correlated positively with amygdala activation (MNI Coordinates: −18, 0, −24; maximum r statistic=0.53; 5 voxels, p<0.05 SV corrected) for immerse/aversive versus immerse/neutral trials (Figure 2a). No other brain regions were associated with the ALS in this analysis. When Hamilton Depression, Beck Depression Inventory, Brown-Goodwin, and Buss-Durkee scores (all measures that were correlated with ALS scores) as well as the DERS (despite it not correlating with the ALS) were added as covariates in the whole-brain analysis, a near identical amygdala cluster was observed (MNI coordinates: −18, 0, −27; 5 voxels, maximum r statistic=.47, p<0.05, SV corrected). No brain activation in the immerse/aversive > immerse/neutral contrast was significantly associated with the other scales tested in this multiple regression analysis when examined using whole-brain analyses or small volume correction in the amygdala. The only exception was a single voxel in the caudate – likely an artifact of placing spheres around peak coordinates when constructing ROIs for small volume correction – which was positively associated with the DERS and identified using small volume correction for the amygdala (MNI coordinates: 15, 0, −12; r=.47).
Figure 2.
(a) Affective lability (i.e., ALS scores) was associated with greater amygdala responses for the baseline emotional reactivity contrast (Immerse/aversive > immerse/neutral). SVC=small volume corrected; (b) Emotion dysregulation (i.e., DERS scores) was associated with less left IFG recruitment for the emotional regulation contrast (Distance/aversive > distance/aversive). SVC=small volume corrected.
3.2.5 Brain activation related to difficulties in emotion regulation
Greater difficulties in emotion regulation were associated with less left inferior frontal gyrus (IFG) recruitment (MNI coordinates: −39, 51, 0; maximum r statistic=−0.49; 53 voxels, p<0.05 FWE-corrected) during regulation (distance/aversive > immerse/aversive; Figure 2b). DERS scores did not correlate with any other brain regions for the distance/aversive > immerse/aversive contrast, even when amygdala responses were examined using small volume correction. Follow-up exploratory correlations were conducted in SPSS to determine which subscales of the DERS correlated most strongly with mean signal from IFG. This revealed that that IFG activation significantly was weakly associated with non-acceptance of emotional responses (r=−0.29, p<0.05, uncorrected; non-significant after Bonferroni correction) and more strongly associated with difficulty engaging in goal-directed behavior (r=−0.52, p<.001, uncorrected; significant after Bonferroni correction) and impulse control difficulties (r=−0.36, p<.01, uncorrected; significant after Bonferroni correction). IFG recruitment was not correlated with the emotional awareness, limited access to emotion regulation strategies, and lack of emotional clarity subscales (p’s>.08, uncorrected). Although regulation success on the reappraisal task was not associated with DERS scores, IFG recruitment was associated with greater regulation success (r=0.25, p=0.05). IFG activation during emotion regulation was unrelated to amygdala activation during emotional reactivity (r=−0.05, p=0.73).
When Beck Depression Inventory and Hamilton Anxiety scores (the only measures that were correlated with DERS scores) as well as ALS scores (though they did not correlate with the DERS) were added as covariates in the whole-brain analysis, a slightly smaller IFG cluster was observed that did not survive FWE correction. However, an exploratory analysis revealed that a portion of the initial IFG cluster (MNI coordinates: −42, 48, 0; 28 voxels) was significant at a relaxed threshold (p<.005, uncorrected). No other brain regions were significant at p<.005, k=10 voxels for this whole brain analysis nor did the ALS correlate significantly with any voxels in the distance/aversive > immerse/aversive analysis. No brain activation in the immerse/aversive > immerse/neutral contrast was significantly associated with the other scales tested in this multiple regression analysis when examined using whole-brain analyses or small volume correction in the amygdala.
4. Discussion
Affective lability and difficulties regulating emotion contribute to debilitating outcomes in BPD. In the present study, we found that affective lability correlated with amygdala recruitment, a brain region critical for responding to motivationally salient stimuli (Cunningham and Brosch, 2012; Kober et al., 2008; Phelps and LeDoux, 2005), and that difficulties with emotion regulation were associated with reduced recruitment of left IFG, a brain region that together with neighboring regions in ventrolateral prefrontal cortex supports cognitive (Wager and Smith, 2003; Wager et al., 2005) and emotional control (Buhle et al., 2013), and that these two tendencies were unrelated. Moreover, left IFG recruitment was associated with greater regulatory success on the reappraisal task. These data have implications for models of BPD and for efforts to translate basic emotion research to actionable clinical knowledge.
4.1 Implications for models of BPD
Although clinical data suggests that BPD is a heterogeneous disorder (Lenzenweger et al., 2008; Linehan and Dexter-Mazza, 2007), most prior neuroimaging work in BPD has employed methods that capitalize on homogeneity rather than heterogeneity. Such studies have shown that individuals with BPD exhibit atypical amygdala responsivity and prefrontal recruitment in the context of emotional responding and regulation (Krause-Utz et al., 2014; Lis et al., 2007; Rosenthal et al., 2008; Ruocco et al., 2013), suggesting that on average BPD is associated with atypical emotional processing and difficulties with regulation.
Yet, the present results underscore two benefits of considering individual differences rather than group averages in BPD. First, they suggest that neuroimaging may be a valuable tool for characterizing and discriminating clinical symptomatology. That ALS and DERS scores, as well as amygdala and IFG recruitment, were uncorrelated with one another suggests that different BPD features have dissociable neural substrates. Second, the fact that ALS and DERS scores tracked with neural responses, but not self-reported negative affect, suggests that fMRI may be more sensitive than self-report for probing individual differences in BPD on emotion regulation paradigms. Indeed, numerous fMRI studies have failed to identify differences in self-reported negative affect between individuals with BPD and healthy controls while simultaneously observing differences in objective questionnaire measures and in engagement of prefrontal-amygdala circuitry (Koenigsberg et al., 2009a; Lang et al., 2012; Schulze et al., 2011). This is consistent with findings in other clinical domains, such as in the impulse control literature, where questionnaire and laboratory self-report measures often show no or weak relationships (Stahl et al., 2014). Such discrepancies could be either interpreted in at least three ways. One possibility is that individuals with BPD engaging different reappraisal tactics or to differences in ease or familiarity with reappraising. The fact that IFG activation was specifically associated with the “difficulty engaging in goal-directed behavior” DERS subscale suggests that left IFG variability might be driven by differences in the ability to implement goal-directed regulatory strategies. This is consistent with prior work linking rostral portions of left IFG with response selection and execution (Rowe et al., 2008), as well as work proposing that ventrolateral prefrontal regions including IFG may be particularly important for initiating (but perhaps not executing) emotion regulation (Kohn et al., 2014). Second, individuals with BPD may be fairly accurate about their ability to report on their general affective states (i.e., on questionnaires) but have less reliable “online” ratings. A third possibility is that individuals with BPD show greater discordance between subjective and neural markers of emotion than typical individuals and as such, brain and behavior are less tightly coupled for them than other samples.
4.2 Implications for translating basic emotion research
The present results exemplify how basic affective neuroscience models may account for clinical symptomatology (Gross and Barrett, 2011; Gross et al., 2011; Henry et al., 2001; Linehan, 1993a; Ochsner et al., 2012). Amygdala responses to emotional images explained a significant portion of ALS variance (r coefficient = 0.53 ^ 2 = 28%) and left IFG responses during emotion regulation explained much of the DERS variance (r coefficient = 0.49 ^ 2 = 24%). It is intriguing, and perhaps even surprising, that these two measures and their neural correlates were unrelated in the present sample given prior work linking atypical amygdala responses to reduced or atypical prefrontal function or connectivity in BPD (Cullen et al., 2011; Kamphausen et al., 2012; Soriano-Mas et al., 2012). As such, future research ought to examine more closely whether these two constructs might be either connected indirectly or may both act on brain regions involved in integrating affective cues to evaluate their significance (e.g., ventromedial prefrontal cortex), thus jointly contributing to emotional problems in BPD.
Translational approaches have been successfully used with other clinical phenomena – for example, translating basic fear extinction models to anxiety disorders and vice versa (Davis et al., 2006; Hofmann, 2008) – but are less common in BPD. The present results are encouraging and suggest that individual differences may be critical to characterizing and treating BPD. For example, perhaps individuals who struggle with affective lability respond better to treatment geared towards stabilizing mood and individuals who struggle with emotion regulation respond best to treatment that promotes regulatory skills.
4.3 Limitations and future directions
Several limitations ought to be considered when interpreting the present findings. First, because the present study was conducted exclusively within individuals with BPD, the present results cannot speak to whether affective lability and difficulties with emotion regulation are useful constructs for characterizing other disorders or healthy individuals. The fact that the link between trait difficulties in emotion regulation and left IFG activation during distancing was attenuated when other symptomology was controlled for suggests that there might be at least partially shared neural substrates for different types of symptomology within BPD, and perhaps more generally. Relatedly, future work would benefit from assessing whether symptomology associated with other disorders like ADHD, which has high comborbidity with BPD (Asherson et al., 2014; Philipsen et al., 2008), relates to the present findings. Second, given that the present sample was all-female, it would be useful for future work to compare trait-related variability between men and women with BPD (Sansone and Sansone, 2011) using the present methods. Third, social aversive stimuli were used in the present study because BPD is characterized by interpersonal instability and thus social stimuli are likely to evoke particularly relevant regulatory changes for individuals with BPD. Future work ought to examine whether the present findings generalize to all aversive stimuli or whether they are unique to aversive social stimuli. Finally, the present study examined activation of the amygdala and IFG, but not functional or structural connectivity between these regions. Given mounting evidence that BPD is characterized not just by dysfunction in individual brain regions but in their connections as well (Krause-Utz and Schmahl, 2016; Lischke et al., 2015; Niedtfeld et al., 2012; Salvador et al., 2014), it is critical for future work to use multimodal methods and connectivity measures to gain a fuller picture of the neural bases of affective lability and difficulties with emotion regulation in BPD.
Supplementary Material
Table 2.
Clinical and task data for study participants
| n | Mean | SD | |
|---|---|---|---|
| Zanarini Rating Scale for BPD | 60 | 15.88 | 5.65 |
| Hamilton Depression Rating Scale | 60 | 25.65 | 9.66 |
| Beck Depression Inventory | 58 | 28.83 | 10.56 |
| Hamilton Anxiety Rating Scale | 60 | 14.95 | 5.70 |
| Global Assessment of Functioning | 59 | 49.88 | 6.78 |
| Scale for Suicidal Ideation | 60 | 8.47 | 8.65 |
| Barratt Impulsivity Scale | 59 | 67.24 | 18.39 |
| Buss-Durkee Hostility Inventory | 57 | 47.63 | 10.64 |
| Brown-Goodwin Aggression History Scale | 60 | 19.8 | 4.98 |
| Lifetime Number of Suicide Attempts | 60 | 1.62 | 1.50 |
| Affective Lability Scale (sum) | 56 | 94.93 | 29.39 |
| Difficulties with Emotion Regulation Scale | 57 | 127.91 | 22.09 |
| n | % | ||
| SCID summary | |||
| Current MDD | 44/60 | 73 | |
| Lifetime MDD | 53/60 | 88 | |
| Current Bipolar II/NOS Disorder | 4/60 | 7 | |
| Lifetime Bipolar II/NOS Disorder | 5/60 | 8 | |
| Current Anxiety Disorder | 38/60 | 63 | |
| Lifetime Anxiety Disorder | 41/60 | 68 | |
| Current Eating Disorder | 5/60 | 8 | |
| Lifetime Eating Disorder | 11/60 | 18 | |
| Current Substance Use Disorder | 5/60 | 8 | |
Highlights.
Borderline Personality Disorder (BPD) is characterized by emotional problems.
Affective lability predicts greater amygdala responses to aversive stimuli in BPD.
Difficulties with emotion regulation predict less prefrontal recruitment in BPD.
These results identify distinct neural signatures of BPD symptomology.
Acknowledgments
Completion of this article was supported by the following grants from the National Institutes of Health: MH061017 (Stanley), MH090964 (Mann), AG043463 (Ochsner), HD069178 (Ochsner) and MH094056 (Silvers).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Borderline Personality Disorder
Affective Lability Scale
Difficulties with Emotion Regulation Scale
Ventrolateral prefrontal cortex
References
- Aas M, Pedersen G, Henry C, Bjella T, Bellivier F, Leboyer M, Kahn J, Cohen RF, Gard S, Aminoff SR, Lagerberg TV, Andreassen OA, Melle I, Etain B. Psychometric properties of the Affective Lability Scale (54 and 18-item version) in patients with bipolar disorder, first-degree relatives, and healthy controls. Journal of Affective Disorders. 2015;172:375–380. doi: 10.1016/j.jad.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Asherson P, Young AH, Eich-Höchli D, Moran P, Porsdal V, Deberdt W. Differential diagnosis, comorbidity, and treatment of attention-deficit/hyperactivity disorder in relation to bipolar disorder or borderline personality disorder in adults. Current Medical Research and Opinion. 2014;30:1657–1672. doi: 10.1185/03007995.2014.915800. [DOI] [PubMed] [Google Scholar]
- Assocation, A.P. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Barratt ES. Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychological reports. 1965;16:547–554. doi: 10.2466/pr0.1965.16.2.547. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of consulting and clinical psychology. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bradley B, DeFife JA, Guarnaccia C, Phifer J, Fani N, Ressler KJ, Westen D. Emotion dysregulation and negative affect: association with psychiatric symptoms. Journal of Clinical Psychiatry. 2011;72:685–691. doi: 10.4088/JCP.10m06409blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome MR, He Z, Iftikhar M, Eyden J, Marwaha S. Neurobiological and behavioural studies of affective instability in clinical populations: A systematic review. Neuroscience & Biobehavioral Reviews. 2015;51:243–254. doi: 10.1016/j.neubiorev.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb Cortex. 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of consulting psychology. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality: Elaborating and extending Linehan's theory. Psychol Bull. 2009;135:495–510. doi: 10.1037/a0015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Vizueta N, Thomas KM, Han GJ, Lim KO, Camchong J, Mueller BA, Bell CH, Heller MD, Schulz SC. Amygdala functional connectivity in young women with borderline personality disorder. Brain Connectivity. 2011;1:61–71. doi: 10.1089/brain.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience: Amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21:54–59. [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Edelbrock C, Costello AJ, Dulcan MK, Kalas R, Conover NC. Age differences in the reliability of the psychiatric interview of the child. Child development. 1985;56:265–275. [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Fletcher K, Parker G, Bayes A, Paterson A, McClure G. Emotion regulation strategies in bipolar II disorder and borderline personality disorder: differences and relationships with perceived parental style. J Affect Disord. 2014;157:52–59. doi: 10.1016/j.jad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Gunderson JG. Preliminary data on an acceptance-based emotion regulation group intervention for deliberate self-harm among women with borderline personality disorder. Behavior therapy. 2006;37:25–35. doi: 10.1016/j.beth.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2004;26:41–54. [Google Scholar]
- Gross JJ, Barrett LF. Emotion generation and emotion regulation: one or two depends on your point of view. Emotion review : journal of the International Society for Research on Emotion. 2011;3:8–16. doi: 10.1177/1754073910380974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Sheppes G, Urry HL. Cognition and emotion lecture at the 2010 SPSP Emotion Preconference: Emotion generation and emotion regulation: A distinction we should make (carefully) Cognition and Emotion. 2011:25. doi: 10.1080/02699931.2011.555753. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Zanarini MC. Pathogenesis in borderline personality, Review of Psychiatry. Washington, D.C.: American Psychiatric Press; 1989. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. The British journal of medical psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. Journal of clinical psychology. 1989;45:786–793. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Speiser LJ, Goodman M, Roy M, Carrizal M, Wynn JK, Williams WC, Romero M, Minzenberg MJ, Siever LJ, New AS. Exaggerated affect-modulated startle during unpleasant stimuli in borderline personality disorder. Biol Psychiatry. 2007;62:250–255. doi: 10.1016/j.biopsych.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Zhang J, New AS, Zelmanova Y, Goldstein KE, Haznedar MM, Meyerson D, Goodman M, Siever LJ, Chu KW. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol Psychiatry. 2012;72:448–456. doi: 10.1016/j.biopsych.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Mitropoulou V, New A, Koenigsberg HW, Silverman JM, Siever LJ. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. Journal of psychiatric research. 2001;35:307–312. doi: 10.1016/s0022-3956(01)00038-3. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clinical psychology review. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaieri H, Urry H, Gross J. Affective disturbance and psychopathology: an emotion regulation perspective. Journal of Experimental Psychopathology. 2013;4:584–599. [Google Scholar]
- Kamphausen S, Schroder P, Maier S, Bader K, Feige B, Kaller CP, Glauche V, Ohlendorf S, Tebartz van Elst L, Kloppel S, Jacob GA, Silbersweig D, Lieb K, Tuscher O. Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2013;14:307–318. S301–S304. doi: 10.3109/15622975.2012.665174. [DOI] [PubMed] [Google Scholar]
- Kamphausen S, Schröder P, Maier S, Bader K, Feige B, Kaller CP, Glauche V, Ohlendorf S, Tebartz van Elst L, Klöppel S, Jacob GA, Silbersweig D, Lieb K, Tüscher O. Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. The World Journal of Biological Psychiatry. 2012;14:307–318. doi: 10.3109/15622975.2012.665174. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, Dorantes C, Guerreri S, Tecuta L, Goodman M, New A, Siever LJ. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol Psychiatry. 2009a;66:854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, New AS, Goodman M, Silverman J, Serby M, Schopick F, Siever LJ. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? Journal of personality disorders. 2001;15:358–370. doi: 10.1521/pedi.15.4.358.19181. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, Schmeidler J, New AS, Goodman M, Silverman JM, Serby M, Schopick F, Siever LJ. Characterizing affective instability in borderline personality disorder. Am J Psychiatry. 2002;159:784–788. doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Siever LJ, Lee H, Pizzarello S, New AS, Goodman M, Cheng H, Flory J, Prohovnik I. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res. 2009b;172:192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation — An ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz A, Schmahl C. A More Global Look at Altered Neural Structure and Resting-State Function in Borderline Personality Disorder. Biol Psychiatry. 2016;79:76–77. doi: 10.1016/j.biopsych.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Current psychiatry reports. 2014;16:438. doi: 10.1007/s11920-014-0438-z. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Mesulam MM, Parrish TB. Impact of signal-to-noise on functional MRI of the human amygdala. Neuroreport. 2001;12:3461–3464. doi: 10.1097/00001756-200111160-00017. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS), Instruction Manual and Affective Ratings, Technical Report. Gainesville, FL: The University of Florida; 2001. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang S, Kotchoubey B, Frick C, Spitzer C, Grabe HJ, Barnow S. Cognitive reappraisal in trauma-exposed women with borderline personality disorder. Neuroimage. 2012;59:1727–1734. doi: 10.1016/j.neuroimage.2011.08.061. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Clarkin JF, Yeomans FE, Kernberg OF, Levy KN. Refining the borderline personality disorder phenotype through finite mixture modeling: implications for classification. Journal of personality disorders. 2008;22:313–331. doi: 10.1521/pedi.2008.22.4.313. [DOI] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-Behavioral Treatment of Borderline Personality Disorder. New York: Guilford Press; 1993a. [Google Scholar]
- Linehan MM. Skills-Training Manual for Treatment of Borderline Personality Disorder. New York: Guilford; 1993b. [Google Scholar]
- Linehan MM, Dexter-Mazza ET. Dialectical behavior therapy for borderline personality disorder. In: Barlow D, editor. Clinical Handbook of Psychological Disorders. 4th. New York: Guilford; 2007. pp. 365–420. [Google Scholar]
- Links PS, Eynan R, Heisel MJ, Barr A, Korzekwa M, McMain S, Ball JS. Affective instability and suicidal ideation and behavior in patients with borderline personality disorder. Journal of personality disorders. 2007;21:72–86. doi: 10.1521/pedi.2007.21.1.72. [DOI] [PubMed] [Google Scholar]
- Lis E, Greenfield B, Henry M, Guile JM, Dougherty G. Neuroimaging and genetics of borderline personality disorder: a review. Journal of psychiatry & neuroscience : JPN. 2007;32:162–173. [PMC free article] [PubMed] [Google Scholar]
- Lischke A, Domin M, Freyberger HJ, Grabe HJ, Mentel R, Bernheim D, Lotze M. Structural alterations in white-matter tracts connecting (para-)limbic and prefrontal brain regions in borderline personality disorder. Psychological medicine. 2015;45:3171–3180. doi: 10.1017/S0033291715001142. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Phillips BM, Hooe ES. Relations of positive and negative affectivity to anxiety and depression in children: evidence from a latent variable longitudinal study. Journal of consulting and clinical psychology. 2003;71:465–481. doi: 10.1037/0022-006x.71.3.465. [DOI] [PubMed] [Google Scholar]
- Marwaha S, He Z, Broome M, Singh SP, Scott J, Eyden J, Wolke D. How is affective instability defined and measured? A systematic review. Psychological medicine. 2013;44:1793–1808. doi: 10.1017/S0033291713002407. [DOI] [PubMed] [Google Scholar]
- Niedtfeld I, Kirsch P, Schulze L, Herpertz SC, Bohus M, Schmahl C. Functional connectivity of pain-mediated affect regulation in Borderline Personality Disorder. PLoS One. 2012;7:e33293. doi: 10.1371/journal.pone.0033293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedtfeld I, Schulze L, Kirsch P, Herpertz SC, Bohus M, Schmahl C. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biol Psychiatry. 2010;68:383–391. doi: 10.1016/j.biopsych.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: the utility and limitations of research instruments. In: First MB, editor. Standardized Evaluatin in Clinical Practice. Washington, D.C.: American Psychiatric Publishing, Inc.; 2003. [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Philipsen A, Limberger MF, Lieb K, Feige B, Kleindienst N, Ebner-Priemer U, Barth J, Schmahl C, Bohus M. Attention-deficit hyperactivity disorder as a potentially aggravating factor in borderline personality disorder. The British Journal of Psychiatry. 2008;192:118–123. doi: 10.1192/bjp.bp.107.035782. [DOI] [PubMed] [Google Scholar]
- Reich DB, Zanarini MC, Fitzmaurice G. Affective lability in bipolar disorder and borderline personality disorder. Comprehensive psychiatry. 2012;53:230–237. doi: 10.1016/j.comppsych.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rosenthal MZ, Gratz KL, Kosson DS, Cheavens JS, Lejuez CW, Lynch TR. Borderline personality disorder and emotional responding: a review of the research literature. Clinical psychology review. 2008;28:75–91. doi: 10.1016/j.cpr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Rowe J, Hughes L, Eckstein D, Owen AM. Rule-selection and action-selection have a shared neuroanatomical basis in the human prefrontal and parietal cortex. Cerebral Cortex. 2008;18:2275–2285. doi: 10.1093/cercor/bhm249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF. Neural correlates of negative emotionality in borderline personality disorder: an activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73:153–160. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Zakzanis KK. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: A meta-analysis of magnetic resonance imaging studies. Psychiatry Research: Neuroimaging. 2012;201:245–252. doi: 10.1016/j.pscychresns.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Salvador R, Vega D, Pascual JC, Marco J, Canales-Rodriguez EJ, Aguilar S, Anguera M, Soto A, Ribas J, Soler J, Maristany T, Rodriguez-Fornells A, Pomarol-Clotet E. Converging Medial Frontal Resting State and Diffusion-Based Abnormalities in Borderline Personality Disorder. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Sansone LA. Gender patterns in borderline personality disorder. Innovations in clinical neuroscience. 2011;8:16–20. [PMC free article] [PubMed] [Google Scholar]
- Santangelo P, Bohus M, Ebner-Priemer UW. Ecological momentary assessment in borderline personality disorder: a review of recent findings and methodological challenges. Journal of personality disorders. 2014;28:555–576. doi: 10.1521/pedi_2012_26_067. [DOI] [PubMed] [Google Scholar]
- Schulze L, Domes G, Kruger A, Berger C, Fleischer M, Prehn K, Schmahl C, Grossmann A, Hauenstein K, Herpertz SC. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiatry. 2011;69:564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Schulze L, Schmahl C, Niedtfeld I. Neural correlates of disturbed emotion processing in borderline personality disorder: a multimodal meta-analysis. Biol Psychiatry. 2016;79:97–106. doi: 10.1016/j.biopsych.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Scott LN, Kim Y, Nolf KA, Hallquist MN, Wright AG, Stepp SD, Morse JQ, Pilkonis PA. Preoccupied attachment and emotional dysregulation: specific aspects of borderline personality disorder or general dimensions of personality pathology? Journal of personality disorders. 2013;27:473–495. doi: 10.1521/pedi_2013_27_099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Jacob G, Lieb K, Tuscher O. Impulsivity in borderline personality disorder: a matter of disturbed impulse control or a facet of emotional dysregulation? Current psychiatry reports. 2013;15:339. doi: 10.1007/s11920-012-0339-y. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents' emotion regulation in daily fife: Links to depressive symptoms and problem behavior. Child development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JD, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Salsman N, Wang L, Smith V, Lynch TR, Dager SR, LaBar KS, Linehan MM. Functional imaging of emotion reactivity in opiate-dependent borderline personality disorder. Personality disorders. 2011;2:230–241. doi: 10.1037/a0022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Mas C, Niedtfeld I, Kirsch P, Schulze L, Herpertz SC, Bohus M, Schmahl C. Functional Connectivity of Pain-Mediated Affect Regulation in Borderline Personality Disorder. PLoS ONE. 2012;7:e33293. doi: 10.1371/journal.pone.0033293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C, Voss A, Schmitz F, Nuszbaum M, Tüscher O, Lieb K, Klauer KC. Behavioral components of impulsivity. Journal of Experimental Psychology: General. 2014;143:850–886. doi: 10.1037/a0033981. [DOI] [PubMed] [Google Scholar]
- Stepp SD, Scott LN, Morse JQ, Nolf KA, Hallquist MN, Pilkonis PA. Emotion dysregulation as a maintenance factor of borderline personality disorder features. Comprehensive psychiatry. 2014;55:657–666. doi: 10.1016/j.comppsych.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tragesser SL, Solhan M, Schwartz-Mette R, Trull TJ. The role of affective instability and impulsivity in predicting future BPD features. Journal of personality disorders. 2007;21:603–614. doi: 10.1521/pedi.2007.21.6.603. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wedig MM, Silverman MH, Frankenburg FR, Reich DB, Fitzmaurice G, Zanarini MC. Predictors of suicide attempts in patients with borderline personality disorder over 16 years of prospective follow-up. Psychological medicine. 2012;42:2395–2404. doi: 10.1017/S0033291712000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westen D, Muderrisoglu S, Fowler C, Shedler J, Koren D. Affect regulation and affective experience: Individual differences, group differences, and measurement using a Q-sort procedure. Journal of consulting and clinical psychology. 1997;65:429–439. doi: 10.1037//0022-006x.65.3.429. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Yarkoni T. Big Correlations in Little Studies: Inflated fMRI Correlations Reflect Low Statistical Power - Commentary on Vul et al. (2009) Perspectives on Psychological Science. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. Journal of personality disorders. 2003;17:233–242. doi: 10.1521/pedi.17.3.233.22147. [DOI] [PubMed] [Google Scholar]
- Zittel Conklin C, Bradley R, Westen D. Affect regulation in borderline personality disorder. Journal of Nervous and Mental Disease. 2006;194:69–77. doi: 10.1097/01.nmd.0000198138.41709.4f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.