Abstract
Activation of TGF-β by dendritic cells (DCs) expressing αvβ8 integrin is essential for the generation of intestinal regulatory T cells (Tregs) that in turn promote tolerance to intestinal antigens. We have recently shown that αvβ8 integrin is preferentially expressed by CD103+ DCs, and confers their ability to activate TGF-β and generate Tregs. However, how these DCs become specialized for this vital function is unknown. Here we show that β8 expression is controlled by a combination of factors that include DC lineage, and signals derived from the tissue microenvironment and microbiota. Specifically, our data demonstrate that TGF-β itself, along with retinoic acid (RA) and Toll-like receptor (TLR) signaling, drive expression of αvβ8 in DCs. However, these signals only result in high levels of β8 expression in cells of the cDC1 lineage, CD8α+ or CD103+CD11b− DCs, and this is associated with epigenetic changes in the Itgb8 locus. Together, these data provide a key illustrative example of how microenvironmental factors and cell lineage drive the generation of regulatory αvβ8-expressing DCs specialized for activation of TGF-β to facilitate Treg generation.
INTRODUCTION
Dendritic Cells (DCs) serve a unique sentinel role in the body, surveying tissues, integrating peripheral cues and instructing the adaptive immune system accordingly. DCs can both orchestrate powerful pathogen-directed immunity or regulate and suppress immune responses to self-associated or innocuous antigens, and the complexity of these roles is reflected in the diverse populations of DCs found in different tissues and under different conditions (1, 2). Determining how DCs differentiate to carry out specialized functions is critical for our understanding of immunity in health and disease.
The intestine provides a particular challenge for the immune system, containing a high local concentration of microbes, including commensals and potential pathogens, as well as diverse dietary and environmental antigens (3). To prevent inappropriate inflammatory responses to these mostly innocuous antigens, the mucosal immune system has robust immunoregulatory mechanisms, which include regulatory lymphocytes, which reside in the mucosa and associated lymphoid organs and actively suppress immune responses to intestinal antigens (4). The best characterized of these are CD4+ Foxp3+ regulatory T cells, which originate either in the thymus (‘thymic’ or ‘natural’ Tregs), or are generated in the periphery from naïve CD4+ T cells (‘peripheral’ or ‘adaptive’ Tregs). In the intestine, peripheral Tregs are generated by DCs that constitutively acquire and present self and foreign antigens (5, 6), resulting in antigen-specific tolerance (7). The generation of peripheral Tregs requires TGF-β, and we and others have shown that an essential characteristic of DCs that generate Tregs is their ability to activate TGF-β from its inactive or ‘latent’ precursor, to an active form that can engage the TGF-β receptor (8, 9). This requires the action of a specific cell surface integrin, αvβ8, and underscoring the importance of this process, deletion of either the αv or β8 subset from DCs results in failure to generate intestinal Tregs and subsequent development of colitis (10, 11).
Recently we have shown that expression of αvβ8 is tightly regulated in DCs. While the αv subunit, the only known partner of β8 (12), is ubiquitously expressed, β8 expression is restricted to specific subsets of cells in the intestine (8). Under homeostatic conditions, αvβ8 is expressed predominantly on DCs from mesenteric lymph nodes (MLN) and intestinal lamina propria that express the mucosal integrin αEβ7 (CD103), conferring on these cells their preferential ability to activate TGF-β and generate Tregs (8, 9). CD103+ DCs have previously been implicated in the generation of intestinal Tregs, which has also been attributed to their ability to synthesize all-trans retinoic acid (RA), which promotes Treg generation in the presence of TGF-β. These data therefore support the concept that subsets of intestinal DCs are specialized for generation of Tregs. However, the precise mechanisms by which this population of DCs acquires this specialized ability to activate TGF-β and how microenvironmental cues and cell lineage are integrated in this process remain to be fully determined.
In this study, we set out to identify the signals that regulate β8 expression in intestinal DCs. We report that αvβ8 is expressed preferentially on the CD103+CD11b− subset of DCs in the MLNs, and that expression is acquired in the LP. We show that signals from the mucosal microenvironment, specifically TGF-β, retinoic acid (RA) and Toll-like receptor (TLR) agonists, together promote expression of β8 integrin in DCs and that inhibition of signaling through these pathways in mice leads to reduction in αvβ8 expression by DCs. Furthermore, we provide evidence that DC lineage is critical in establishing DC subset-specific expression of β8, demonstrating that DCs derived from the CD103/CD8α cDC1 lineage respond more robustly to these conditioning factors to upregulate β8. Together these data show that the combination of cell lineage, immune mediators and both dietary and microbe-derived environmental factors shape intestinal DCs into critical gatekeepers of TGF-β-dependent immune responses through regulation of β8 integrin expression.
MATERIALS AND METHODS
Mice
All animals were housed under specific pathogen free conditions at Plateau de Biologie Expérimentale de la Souris (PBES, ENS Lyon, France), at Benaroya Research Institute (BRI, Seattle, WA) or at Massachusetts General Hospital (MGH, Boston, MA). Female C57BL/6 mice from Charles River Laboratories (L’Arbesle, France) or The Jackson Laboratory (Bar Harbor, ME) were used between 6 and 15 weeks of age. Vitamin A deficient (VAD) mice were generated at MGH. Pregnant C57BL/6 mice were maintained on a vitamin A sufficient (VA+) diet (4 IU/g, TestDiet 58M1) through day 10 of gestation and then switched to a VAD diet (TestDiet 5T2P, both diets Pharmaserv Inc., Framingham, MA) through weaning. Pups were then maintained either on VA+ or VAD diets. Antibiotic-treated mice were generated at PBES. Mice were maintained on drinking water containing 1 g/L metronidazole, 1 g/L ampicillin, 1 g/L neomycin (all from Sigma-Aldrich, St. Quentin Fallavier, France) and 500 mg/L vancomycin (Mylan, St Priest, France) for 4 weeks. Transgenic CD11-Cre (B6.Cg-Tg(Itgax-cre)1-1Reiz/J) and Ccr7−/− (B6.129P2(C)-Ccr7tm1Rfor/J) mice were obtained from The Jackson Laboratory. Myd88−/− and Trif−/− mice were provided by Rodrigo Mora (MGH, Boston, MA). Tgfbr2fl/fl mice (B6.129S6-Tgfbr2tm1Hlm) were obtained from Harold Moses (13) (Vanderbilt-Ingram Cancer Center, Nashville, TN). Foxp3IRES-eGFP reporter mice were obtained from Dominique Kaiserlian (CIRI, Lyon, France). All mice were on C57BL/6 background except for Tgfbr2-flox mice that were on a mixed B6.129S6 background. Animal experiments were performed under appropriate licenses within local and national guidelines for animal care.
Dendritic cell isolation
DCs from spleen, MLN and lamina propria were isolated as previously described (8). BMDCs generated in the presence of Flt3-L (FL-DCs) were cultured as previously described (14). Briefly, bone marrow cells were cultured in the presence of 100 ng/ml recombinant Flt3 ligand (Peprotech, Neuilly-sur-Seine, France). Fresh medium was added to the cultures on day 3 and 6, and cells were harvested at day 9. FL-DC subsets were then sorted by flow cytometry as indicated.
DC conditioning in culture
Cells were stimulated in vitro at 37°C in X-vivo 15 medium (Lonza, Levallois-Perret, France) supplemented with MEM non-essential amino acids, 2 mM L-glutamine, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μM 2-β-mercaptoethanol (all, Life Technologies, Saint Aubin, France). Unless stated otherwise, the stimuli were used at the following final concentrations: 0.2 ng/ml TGF-β (R&D biosystems, Lille, France), 0.2 μM all-trans retinoic acid (Sigma-Aldrich), 5 ng/ml TSLP, 5 ng/ml IL-10, 5 ng/ml IL-1β, 5 ng/ml IFN-γ (all, Peprotech), 2% culture supernatant containing GM-CSF (4 ng/ml final), 500 ng/ml Pam3CSK4, 108 cells/ml HKLM, 1 μg/ml poly(I:C), 100 ng/ml LPS-EK, 100 ng/ml ST-FLA, 100 ng/ml FSL1, 1 μg/ml ssRNA40/LyoVec, 2.5 μM ODN1826 (Mouse TLR1-9 agonist kit, InvivoGen, Toulouse, France).
β8 integrin expression analysis
Itgb8 gene expression by quantitative RT-PCR for MLN and spleen DCs was performed as previously described (8). For lamina propria DCs, Itgb8 gene expression was quantified by nested RT-PCR using the following pre-amplification primers, Fw-AGTGAACAATAGATGTGGCTC, Rev-CCGTCATTCGGCACCACTAT, and qPCR primers, Fw-TGGCCCTTTATTCCCGTGAC, Rev-GGGTGGATACTAATGTATGGCGA. β8 integrin protein expression was measured by Western blot as previously described (8) using an anti-β8 antiserum generated in rabbit using the C-terminal tail of the human β8 cytoplasmic region, and provided by Joseph McCarty (15).
In vitro Treg generation assay
In vitro Treg generation assay was performed as previously described (8). For RGD blockade experiments, cRGD or control cRAD peptides (both Enzo Life Sciences, Villeurbanne, France) were added at 2 μg/ml, as previously described (16).
Antibodies
The following antibodies were used: anti-CD11c-PE-Cy7 (HL3), anti-I-A/I-E-FITC (2G9), anti-CD8α-APC (53-6.7), anti-CD45RB/B220-PerCP-Cy5.5 (RA3-6B2), anti-CD103-PE (M290), anti-CD172/Sirpα-PE (P84), anti-CD24-FITC (M1/69), anti-CD11b-PE (M1/70; all from BD Biosciences, Pont de Claix, France), anti-I-A/I-E-APC (M5/114.15.2), anti-CD103-PerCP-Cy5.5 (2E7; Biolegend, Ozyme, Sant-Quentin-Yvelines, France), and anti-CD86-FITC (B7-2), anti-CD11b-APC-eFluor780 (M1/70; eBioscience, Paris, France).
Computational analysis
ChIP-seq datasets of the CD24+ and CD172+ FL-DCs were retrieved from Gene Expression Omnibus (GEO, GSE66899) (17). Raw reads were cleaned using Trimmomatic-0.33 (18) with the following parameters: ILLUMINACLIP:adapters.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:30. The remaining sequences were aligned to the mouse genome (NCBI37/mm9) by bowtie2 version 2.2.5 with default parameters (19). Density tracks were generated by the makeUCSCfile program from the HOMER software suite v4.7 (20). To allow comparison between the two subpopulations density profiles were normalized based on the library size.
Statistical analysis
Data were analyzed using GraphPad Prism Software 6.0a. Statistics were calculated using either unpaired t test when comparing two groups or one-way ANOVA with Dunnett’s post-hoc test when comparing more than two groups. For grouped analysis, statistics were calculated using two-way ANOVA with Tukey’s or Sidak’s post-hoc test, where appropriate.
RESULTS
αvβ8 is preferentially expressed by CD103+CD11b− DCs
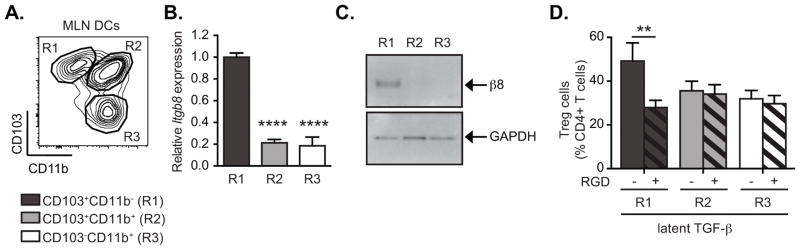
Our previous studies revealed that under homeostatic conditions integrin αvβ8 is expressed preferentially in the CD103+ subpopulation of MLN DCs, and confers on these DCs the ability to generate peripheral Tregs through activation of TGF-β (8, 9). Intestinal CD103+ DCs comprise two distinct populations, distinguished by expression of CD11b, and in further analysis of MLN DCs we found that Itgb8 (the gene encoding β8) was expressed at much higher levels in CD103+CD11b− DCs than in the CD103+CD11b+ subset (Figure 1A–B), while αv integrin gene (Itgav) did not show any significant difference (supplementary figure 1A). Preferential expression of β8 integrin protein by CD103+CD11b− MLN DCs was confirmed by Western Blot analysis (Figure 1C). The expression of high levels of β8 by these DCs was associated with their preferential ability to activate latent TGF-β (l-TGF-β) and generate Tregs (Figure 1D). Furthermore, this difference in TGF-β activation activity was dependent on αvβ8 ligand binding integrins, as treatment with an αv ligand mimetic peptide, cRGD specifically reduced the effects of l-TGF-β on induction of Tregs by CD103+CD11b− DCs. The partial reduction of the effects of l-TGF-β by RGD on Treg generation is likely due to contaminating active TGF-β in the l-TGF-β preparation or to an additional αv-independent TGF-β activation pathway. β8 only pairs with the αv integrin subunit, and deletion of either integrin results in identical effects on TGF-β activation and Treg generation (8, 16) (9, 11), and we therefore feel confident attributing this activity to αvβ8. As these results closely resemble those we had previously reported with pooled CD103+ DCs from MLN or from intestinal LP (8), we concluded that CD103+CD11b− DCs were the cells responsible for the high levels of expression of αvβ8 and activation of TGF-β in the CD103+ fraction.
Figure 1. CD103+CD11b− MLN DCs express high levels of αvβ8 expression and activate TGF-β to generate Tregs.
CD11c+ MLN DC subsets were sorted by FACS into three populations based on expression of CD103 and CD11b. (A) Representative FACS plot gated on CD11c+MHC-II+ DCs from MLN shows gating strategy for isolating indicated populations. (B) Quantitative RT-PCR analysis of β8 integrin gene (Itgb8) expression relative to β-Actin (Actb) and presented relative to levels in CD103+CD11b− DCs (R1) in indicated MLN DC subpopulations. Histogram shows mean ± SEM from 3 individual experiments and at least 9 individual mice. (C) Western blot analysis of β8 integrin and GAPDH expression in indicated MLN DC subsets. (D) FACS-sorted MLN DC subsets were cultured with naïve CD4+Foxp3GFP− T cells in serum-free medium with or without addition of latent TGF-β, RGD (+, hatched bars) and/or RAD (−, solid bars) peptides as indicated. After 4 days in culture, Treg generation was assessed by Foxp3GFP expression. Data show mean ± SEM of individual DC:T cell cultures with six independant pools of MLN DCs from two separate experiments. **, p<0.005; ****, p<0.0001; ANOVA with Dunnet’s (B) or Sidak’s (D) post-hoc test.
CD103+ DCs that acquire β8 expression are derived from the intestine
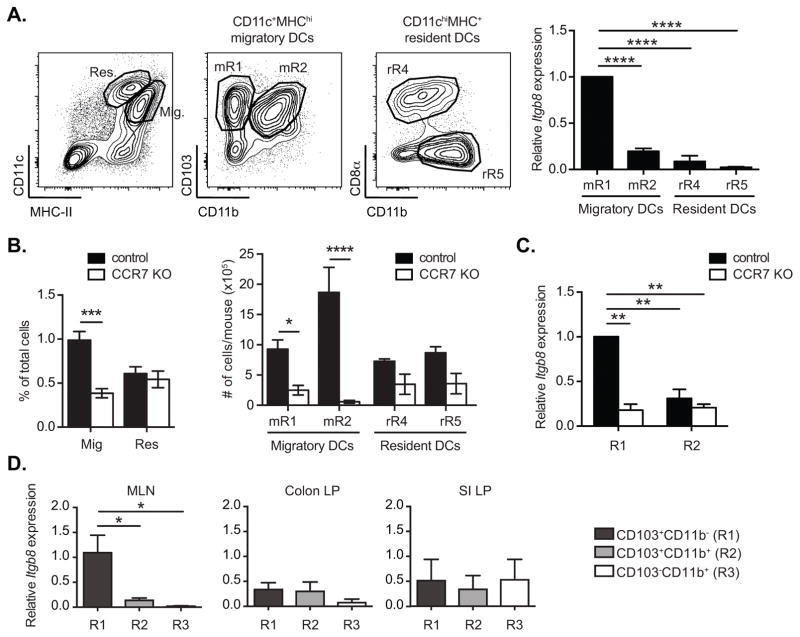
CD103+CD11b− DCs in the MLN are composed of cells that develop in the intestinal LP and then migrate (21), and others that reside in the MLN, and these populations can be distinguished by relative levels of CD11c and MHC-II expression (22). Previous findings by ourselves and others that CD103+ DCs derived from the LP can preferentially activate TGF-β in an αvβ8-dependent mechanism (8, 9) suggested that DCs may acquire expression of αvβ8 in the LP. Supporting this, we found that Itgb8 expression is restricted to LP-derived migratory CD11c+MHC-IIhiCD103+CD11b− MLN DCs (mR1), while neither of the resident DC subsets expressed significant levels of β8 integrin (figure 2A). To investigate this further, we analyzed expression of Itgb8 in DCs from Ccr7−/− mice. CCR7 is required for migration of DCs from the LP to MLN (6, 23, 24) and MLN of Ccr7−/− mice contain predominantly blood-derived DCs with reduced numbers of DCs that have migrated from the intestine ((22) and figure 2B). Notably, the expression of Itgb8 was much lower in CD103+CD11b− DCs from Ccr7−/− cells than in cells from control mice, and was not significantly different from expression on CD103+CD11b+ DCs (Figure 2C). These data therefore confirm that Itgb8 is preferentially expressed on MLN CD103+CD11b− DCs that have migrated from the LP. We also measured expression of Itgb8 in CD11chiMHC-IIhi cells purified directly from the LP, but in contrast to our findings with MLN DCs, we found that none of the major populations of CD11chiMHC-IIhi cells isolated from the LP (CD103+CD11b− DCs, CD103+CD11b+ DCs or CD103−CD11b+ DCs/macrophages) expressed Itgb8 at levels equivalent to those seen in MLN (Figure 2D) when freshly isolated from tissue. This was in agreement with data reported by Atarashi et al., who showed that αvβ8 was not expressed at significant levels by LP CD11c+ cells in the steady state (25).
Figure 2. Migratory CD103+CD11b− DCs acquire β8 integrin expression in the lamina propria.
(A) Quantitative RT-PCR analysis of β8 integrin gene (Itgb8) expression in migratory (Mig) CD11c+MHC-IIhi and resident (Res) CD11chiMHC-II+ MLN DC subsets sorted by FACS based on expression of CD103 and CD11b (mR1, mR2) or CD8α and CD11b (rR4, rR5), respectively, using the gating strategy indicated in the representative FACS plots. Histogram shows mean ± SEM of 6 independent pools of mice from 2 separate experiments, presented relative to levels in CD103+CD11b− DCs (mR1). (B) Frequencies and numbers of indicated migratory and resident DC subsets gated as in (A) from MLN of CCR7−/− and control mice. (C–D) Quantitative (C) and nested (D) RT-PCR analysis of Itgb8 expression relative to β-Actin (Actb) in indicated cell subsets sorted as in figure 1 from total CD11c+MHC-II+ DCs from MLN of CCR7−/− and control mice (C) or CD11chiMHC-IIhi cells from MLN, colon and small intestinal (SI) lamina propria (LP) of WT mice (D). (B–D) Histogram shows mean ± SEM from at least 3 individual experiments and in (C) are presented relative to levels in CD103+CD11b− DCs. *, p< 0.05; ***, p<0.0005; two-way ANOVA with Tukey’s (A, D) or Sidak’s (B–C) post-hoc test.
TGF-β and RA contribute to DC β8 expression in vivo
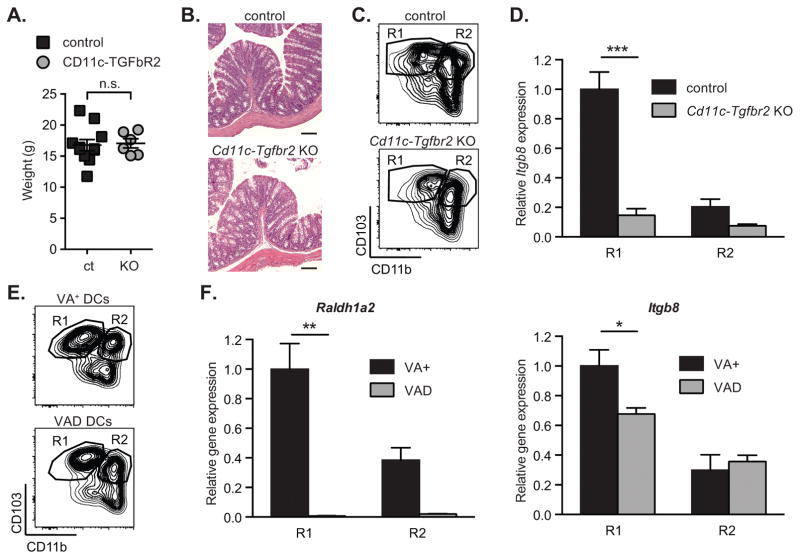
The ability of MLN DCs to generate Tregs and regulate local and systemic immune responses is thought to be due to conditioning events that occur in the intestinal LP, and has been postulated to depend on a number of conditioning factors produced by epithelial cells, including TGF-β and retinoic acid (RA) (26–30). To determine whether these factors regulate expression of β8 integrin in intestinal DCs, we used genetic and dietary manipulation to inhibit potential conditioning factors in mice in vivo. We first investigated TGF-β signaling in DCs, making use of conditional knockout mice in which the TGF-β type II receptor is specifically deleted in DCs (Cd11c-cre; Tgfbr2fl/fl, referred to as Cd11c-Tgfbr2 KO mice). Cd11c-Tgfbr2 KO mice develop normally until 4 weeks of age but then gradually develop severe multi-organ autoimmune inflammation associated with spontaneous colitis (31). In order to exclude the potential contribution of intestinal inflammation on β8 integrin, we used mice less than 5 week old with no clinical signs of colitis or wasting disease (figure 3A–B). CD103+CD11b− and CD103+CD11b+ DCs were both present in MLNs of Cd11c-Tgfbr2-KO mice, at frequencies similar to those seen in control mice, suggesting that development of these cells was largely normal in the absence of TGF-β signaling (Figure 3C). However, CD103+CD11b− DCs from MLNs of Cd11c-Tgfbr2 KO mice expressed much lower levels of Itgb8 than equivalent DCs from control mice; indeed, expression was not significantly different from that seen in CD103+CD11b+ DCs from either control or Cd11c-Tgfbr2 KO mice (Figure 3D). These findings therefore established that TGF-β signaling in DCs is required for preferential expression of β8 in CD103+CD11b− DCs in vivo. We next assessed the role of RA in β8 expression. Mice were depleted of RA by maintenance on a vitamin A-deficient diet (VAD), as previously described (32, 33). Confirming that we had depleted RA signaling in DCs, expression of Raldh1a2, which is induced by RA in immune cells (30, 34), was completely lost in both CD103+CD11b− and CD103+CD11b+ DCs from VAD mice (Figure 3E–F). Itgb8 expression was also significantly reduced in CD103+CD11b− MLN DCs from VAD mice (Figure 3F, right panel), indicating that RA signaling also contributed to the preferential expression of αvβ8 in this DC subset in vivo.
Figure 3. Reduced expression of β8 integrin expression in retinoic acid- and TGF-β signaling-deficient mice.
β8 integrin (Itgb8) expression was assessed in MLN DC subpopulations FACS-sorted from control, CD11c-Tgfbr2 KO mice (A–D) and animals fed with a vitamin A-deficient (VAD) or -sufficient (VA+) diet (E–F). (A) Weight and (B) representative haematoxylin- and eosin-stained sections of colons from CD11c-Tgfbr2 KO and control mice. Scale bar, 100 μm. (C, E) Representative FACS plot of CD11c+MHC-II+ DCs from MLN showing gating strategy for isolating indicated populations. (D, F) Quantitative RT-PCR analysis of Itgb8 (D, F right panel) and Raldh1a2 (F, left panel) expression relative to Actb and presented relative to levels in control CD103+CD11b− DCs (R1) in indicated MLN DC subpopulations. All histograms show mean ± SEM of at least 6 individual mice from 3 separate experiments. *, p< 0.05; **, p<0.005; ***, p<0.0005; two-way ANOVA with Tukey’s post-hoc test.
Intestinal microbes and TLR signaling contribute to expression of Itgb8 in DCs
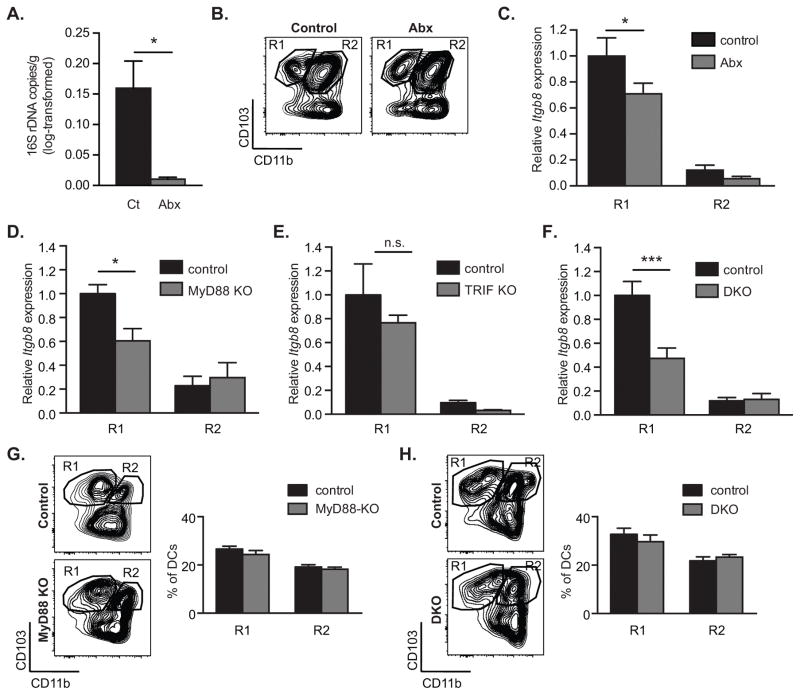
The presence of intestinal microbes has been linked to effective development of LP DCs and establishment of normal mucosal immunoregulatory responses (30, 35, 36). To determine whether the intestinal microbiota contributed to induction of αvβ8 expression in DCs, we treated mice with antibiotics, which reduced the presence of microbes in the intestine by 16-fold, assessed by measurement of 16S rDNA in stool (Figure 4A). As expected based on previous studies (22), intestinal CD103+ CD11b− DCs were present and migrated normally to the MLN of antibiotic-treated animals; however, they expressed significantly lower levels of Itgb8 than MLN DCs from untreated mice (Figure 4B–C), indicating a role for the intestinal microbiota in conditioning of these regulatory DCs. We next analyzed Itgb8 expression in intestinal DCs from mice deficient in Myd88 and Trif, components of the Toll-like receptor (TLR) signaling pathway by which DCs sense microbes. CD103+CD11b− DCs from Myd88−/− mice had significantly reduced expression of Itgb8 compared to DCs from control mice (Figure 4D). In contrast, deletion of TRIF did not significantly affect β8 expression, and showed no additive effect with MyD88 deficiency (Figure 4E–F). Importantly, in both Myd88−/− and in Myd88−/−; Trif−/− double knockout mice (DKO), CD103+CD11b− and CD103+CD11b+ MLN DCs were present in similar proportion than in control mice (figure 4G–H). Notably, the effects of Myd88 deficiency on Itgb8 expression were more pronounced than those of antibiotic treatment, which may be due to the continued presence of microbes in antibiotic treated mice, endogenous TLR ligands, or additional MyD88-dependent signaling pathways such as IL-1β.
Figure 4. Intestinal microbes and MyD88 signaling promote β8 integrin expression in CD103+CD11b− MLN DCs.
β8 integrin (Itgb8) expression was assessed in MLN DC subpopulations FACS-sorted from control, antibiotics-treated (A–C), MyD88- (D, G), TRIF- (E) and MyD88-TRIF-deficient (F, H) mice. (A) Log-transformed 16S rDNA copy numbers per gram of stool in control and antibiotics-treated (Abx) animals, as determined using real-time PCR. (B) Representative FACS plot of CD11c+MHC-II+ DCs from MLN showing gating strategy for isolating indicated populations. (C–F) Quantitative RT-PCR analysis of Itgb8 expression relative to Actb and presented relative to levels in control CD103+CD11b− DCs (R1) in indicated MLN DC subpopulations in control and antibiotics-treated (C), MyD88-deficient (MyD88 KO) (D), TRIF-deficient (TRIF KO) (E) and MyD88-TRIF-deficient mice (DKO) (F). (G–H) Representative FACS plot and frequencies of CD11c+MHC-II+ DCs from MLN of MyD88-KO (G) and Myd88-TRIK DKO mice (H). Histograms show mean ± SEM of n individual mice from 1 (E, n=3), 2 (D, n≥8, F, n≥6) or 3 (C, n≥5) separate experiments. *, p<0.05; ***, p<0.0005; unpaired student t test (A) or two-way ANOVA with Tukey’s post-hoc test (C–H).
Signals encountered in the mucosal environment induce β8 expression by direct effects on DCs
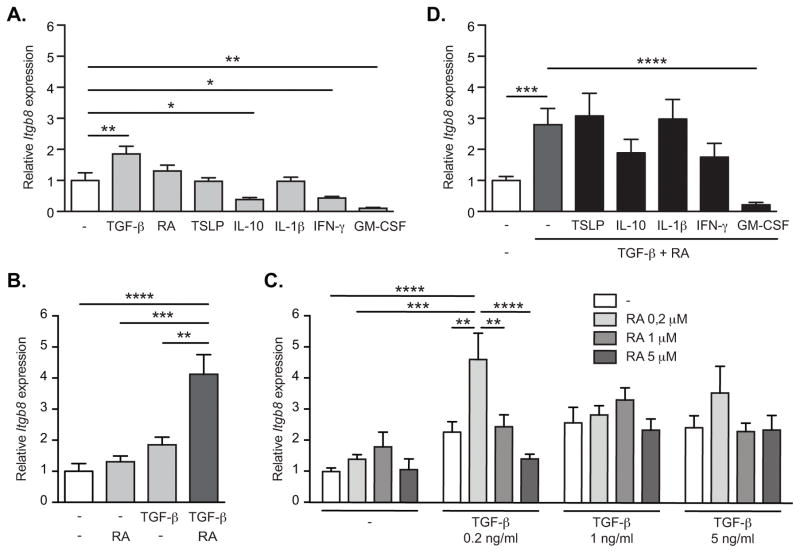
Together, these data indicated that TGF-β, RA and TLR signaling contributed to the expression of αvβ8 in intestinal DCs. To determine whether these stimuli could promote αvβ8 expression in DCs directly, we treated spleen DCs, which do not express β8 when freshly isolated (8), with TGF-β, RA and TLR ligands. Culture of spleen DCs with TGF-β induced a small increase in Itgb8 (Figure 5A), whereas other cytokines associated with the intestinal microenvironment either had no effect on Itgb8 expression (TSLP, IL-1β) or reduced expression (IL-10, IFN-γ, GM-CSF) (Figure 5A). Culture of DCs with RA alone had no effect on Itgb8 expression, but when combined with TGF-β further promoted expression of Itgb8 (Figure 5B–C). Hence, RA synergized with TGF-β signaling to increase gene expression, as has been previously shown for induction of FoxP3+ Tregs in the intestine (28, 29). This synergistic effect did not appear to extend to other cytokines however, as combined treatment with TGF-β/RA with other cytokines or factors did not significantly increase Itgb8 expression (Figure 5D).
Figure 5. TGF-β and RA directly induce Itgb8 expression in spleen DCs.
CD11c+ spleen DCs were sorted with magnetic beads and then cultured in serum free medium for 16h. Cells were either left untreated (−) or stimulated with TGF-β, retinoic acid (RA), or both (A–C). In addition cells were treated with TSLP, IL-10, IL-1β, IFN-γ or GM-CSF, without (B) or with (D) addition of TGF-β and RA. 0.2 ng/ml TGF-β and 0.2 μM RA were used unless indicated otherwise. β8 integrin (Itgb8) gene expression was assessed by quantitative RT-PCR analysis relative to Actb and presented relative to levels in unstimulated spleen DCs. Histograms show mean ± SEM of cultures from 15 (A), 6 (B; D) or 9 (C) independent pools of mice from at least 3 separate experiments. *, p<0.05; **, p<0.005; ***, p<0.0005; ****, p<0.0001; one-way ANOVA with Dunnet’s post-hoc test (A–B; D) or two-way ANOVA with Tukey’s post-hoc test (C).
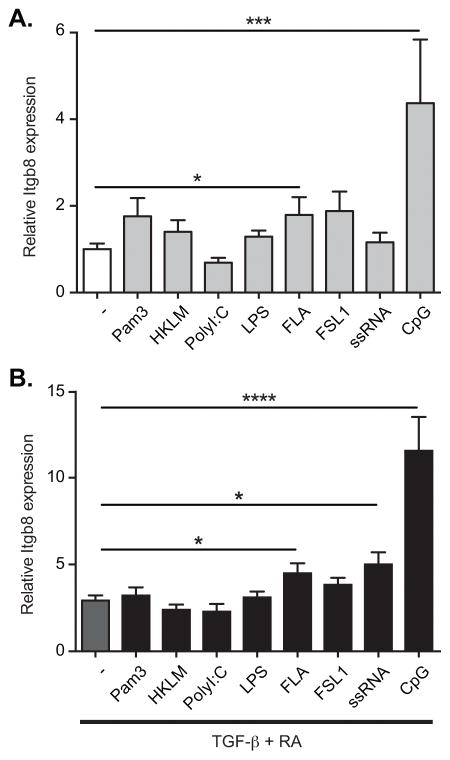
We next tested the role of TLR signaling in DC induction of Itgb8. Stimulation of spleen DCs with the TLR9 ligand CpG oligodeoxynucleotide (ODN), and the TLR5 ligand Flagellin, both stimulated expression of Itgb8 (Figure 6A). This induction of Itgb8 was significantly enhanced by addition of RA/TGF-β which in combination with CpG ODN increased expression by greater than 10-fold over unstimulated DCs (Figure 6B), in line with the preferential expression of Itgb8 that we have seen in MLN DCs. Together these data therefore show that TGF-β, RA and TLR ligands were sufficient to induce expression of Itgb8 in DCs, and in combination induced levels similar to those seen in vivo.
Figure 6. TLR stimulation induces Itgb8 expression in spleen DCs.
CD11c+ spleen DCs were sorted with magnetic beads and then cultured in serum free medium for 16h. Cells were either left untreated (−) or stimulated with agonists for TLR-1 (Pam3), TLR-2 (HKLM), TLR-3 (PolyI:C), TLR-4 (LPS), TLR-5 (FLA), TLR-6 (FSL1), TLR-7 (ssRNA) or TLR-9 (CpG), with (B) or without (A) addition of TGF-β and RA. β8 integrin (Itgb8) gene expression was assessed by quantitative RT-PCR analysis relative to Actb and presented relative to levels in unstimulated spleen DCs. Histograms show mean ± SEM of cultures from 9 independent pools of mice from 3 separate experiments. *, p<0.05; ***, p<0.0005; ****, p<0.0001; one-way ANOVA with Dunnet’s post-hoc test.
β8 expression is differentially regulated in distinct DC subsets
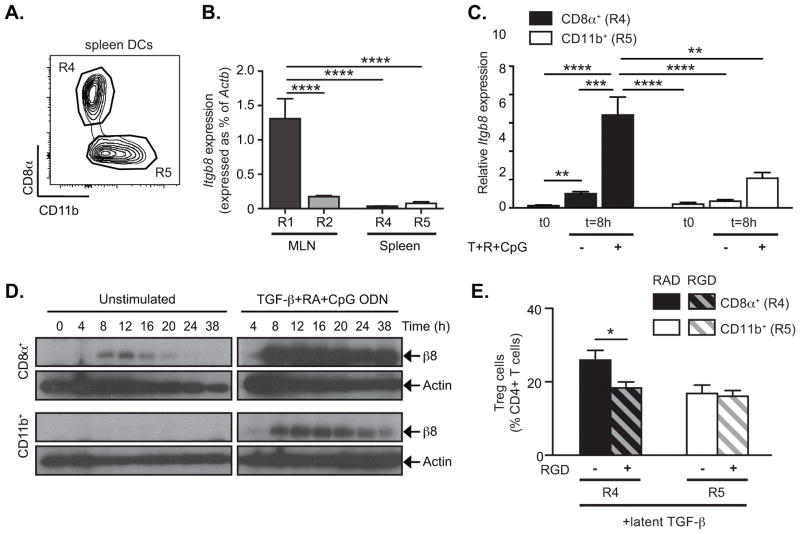
These data therefore support the hypothesis that factors encountered in the intestinal LP induce β8 integrin expression in DCs. However, it is unclear why αvβ8 is expressed preferentially by the CD103+CD11b− subset of intestinal DCs, and not by other DCs exposed to the intestinal microenvironment. One possible explanation is that distinct lineages of DCs show differential responses to Itgb8-inducing signals and we therefore tested the ability of individual subsets of spleen DCs to up-regulate Itgb8 in response to conditioning signals in culture. Spleen DCs consist of two main subsets, CD11b−CD8α+ DCs and CD11b+CD8α − DCs (Figure 7A: referred to here as CD8α+ and CD11b+ DCs respectively). Sorted spleen CD8α+ DCs did not express significant levels of Itgb8 when freshly isolated (Figure 7B), but gene expression could be detected after 8 hours in culture without additional stimulation (Figure 7C). Furthermore, treatment of CD8α+ DCs with the combination of TGF-β, RA and CpG ODN induced significant further increase in Itgb8 expression (Figure 7C), as we had seen for cultures of total spleen DCs. Notably, western blot analysis showed that combined treatment with TGF-β, RA and CpG ODN increased both levels and duration of β8 protein expression (Figure 7D). In contrast, spleen CD11b+ DCs did not express Itgb8 spontaneously in culture (Figure 7C–D). Furthermore, although these cells did express Itgb8 mRNA and β8 protein after stimulation with TGF-β, RA and CpG ODN, expression was significantly lower than seen in CD8α+ DCs, and was not sustained, diminishing to near background levels by 24–36 hours (Figure 7D). This differential induction of Itgb8 in DC subsets was not due to a generalized inability of CD11b+ DCs to respond to the conditioning factors, as these cells expressed similar or higher levels of TLRs and receptors for TGF-β and RA than MLN CD103+CD11b− and spleen CD8α+ DCs (Supplementary Figure 1A and 2) and both spleen subsets showed equivalent levels of TGF-β and TLR signaling when stimulated (Supplementary Figure 3). Furthermore, consistent with their differential ability to express αvβ8 in culture, spleen CD8α+ DCs were able to generate Tregs in the presence of l-TGF-β through an αv-dependent mechanism whereas CD11b+ DCs could not (Figure 7E). To determine whether similar differences in response to inducing stimuli may underlie differential expression of αvβ8 in intestinal DCs, we cultured MLN DCs with TGF-β, RA and CpG ODN and followed Itgb8 expression. As we have shown in previous figures, in freshly isolated cells, Itgb8 was expressed preferentially in CD103+CD11b− DCs (R1) compared with CD103+CD11b+ DCs (R2) from MLN, or either population of spleen DCs (Figure 7B). Culture under Itgb8-inducing conditions for 8 hours increased expression of Itgb8 in both CD103+CD11b− and CD103+CD11b+ MLN DCs. However, the absolute level of Itgb8 remained significantly lower in CD103+CD11b+ cells than CD103+CD11b− (Supplementary figure 1B). Based on these results, we therefore concluded that the difference in αvβ8 expression between CD103+CD11b− and CD103+CD11b+ DCs could not be explained solely by differential exposure to conditioning factors, but was also determined by the ability of those factors to induce Itgb8 expression.
Figure 7. CD8α+ spleen DCs preferentially express αvβ8 and generate Treg in vitro.
DC subsets were sorted by FACS. (A) Representative FACS plot gated on CD11c+MHC-II+ DCs from spleen shows gating strategy for isolating indicated populations. MLN DC subsets were sorted as is Figure 1. (B) β8 integrin gene (Itgb8) expression was analyzed by quantitative RT-PCR in freshly isolated DC subsets from MLN and spleen and expressed as % of Actb. (C–D) Sorted DC subsets were cultured in serum free medium for 8h (C) or indicated times (D). Cells were either left untreated (−) or stimulated with TGF-β, RA and CpG ODN (+) and harvested either in TRIzol (C) or RIPA buffer (D) for analysis of β8 integrin expression. Alternatively for each freshly isolated DC subsets, their ability to induce Treg generation was assessed in vitro (E). (C) Quantitative RT-PCR analysis of Itgb8 expression relative to Actb and presented relative to levels in unstimulated (−) CD8α+ DCs (R4). (D) Western blot analysis of β8 integrin and β-actin. (E) FACS-sorted spleen DC subsets were cultured with naïve CD4+Foxp3GFP− T cells in serum-free medium with or without addition of latent TGF-β, RGD (+, hatched bars) and/or RAD (−, solid bars) peptides as indicated. After 4 days in culture, Treg generation was assessed by Foxp3GFP expression. Data show mean ± SEM of individual DC:T cell cultures with 8 independent pools of spleen DCs from two separate experiments. Histograms show mean ± SEM of n independent pools of mice from 2 (B, n=5) or 4 (C, n=10) separate experiments. *, p<0.05; **, p<0.005; ***, p<0.0005; ****, p<0.0001; one-way ANOVA with Dunnet’s post-hoc test (B) or two-way ANOVA with Tukey’s post-hoc test (C, E).
Furthermore, recent studies establishing that splenic CD8α+ DCs (R4) and intestinal CD103+CD11b− DCs (R1) share a common lineage (cDC1) (37–39), whereas spleen CD11b+ DCs (R5) appear to be more closely related to intestinal CD103+CD11b+ DCs (R2) of the cDC2 lineage (40–42) indicate that the CD8α+/CD103+CD11b− DC lineage may be uniquely specialized for high expression of αvβ8 and activation of TGF-β.
DC lineage determines the pattern of β8 gene expression
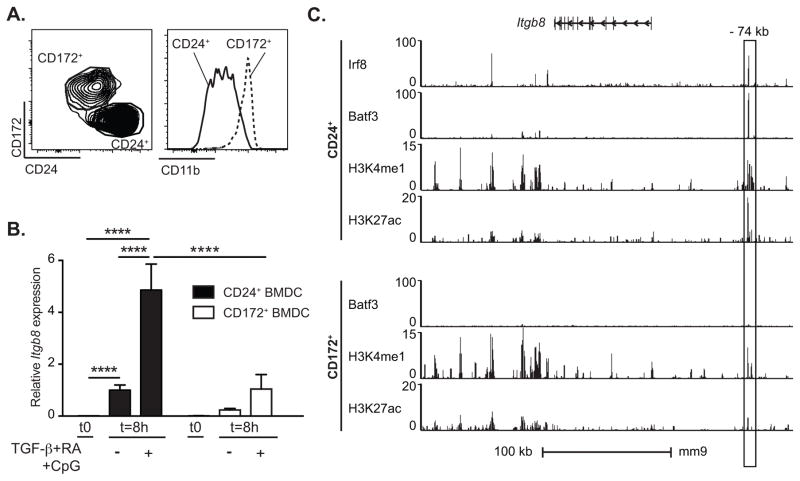
To further investigate whether lineage determines the ability of DCs to express αvβ8 in the intestinal microenvironment, DCs were generated from bone marrow precursors in culture with Flt3 ligand, and their ability to express β8 integrin measured. This method generates 2 main populations of DCs, CD24+CD172−CD11blow DCs (CD24+ FL-DCs), which resemble CD8α+ or CD103+CD11b+ DCs, and CD24−CD172+CD11b+ DCs, (CD172+ FL-DCs), which are more related to CD11b+ DCs (14, 38, 39) (Figure 8A). CD24+ FL-DCs showed consistently higher levels of Itgb8 expression than CD172+ DCs from the same culture. Furthermore, this difference was significantly enhanced after stimulation with TGF-β, RA and CpG ODN (Figure 8B). These findings are consistent with our data from sorted spleen DCs subsets (Figure 7C), and suggest that the preferential ability of CD8α+/CD103+CD11b− DCs to express Itgb8 expression is acquired during development. To determine whether this might be due to epigenetic programming, we compared the epigenetic landscape of the Itgb8 locus in CD24+ and CD172+ FL-DCs, using existing chromatin immunoprecipitation followed by sequencing (ChIP-seq) datasets (17). In CD24+ FL-DCS, we identified an active enhancer marked by both monomethylation of histone 3 lysine 4 (H3K4me1) and acetylation of histone 3 lysine 27 (H3K27ac) 74 kb upstream of the Itgb8 coding region (Figure 8C; upper panel). This active enhancer was also associated with binding to the lineage specific transcription factors Batf3 and IRF8. The same site in CD172+ FL-DCs showed reduced H3K4me1 marks and no H3K27ac (Figure 8C, lower panel), and no other H3K27ac peak could be detected in the vicinity of Itgb8, suggesting that this locus is poised but not active in these cells. The presence of an active enhancer 74 kb upstream of Itgb8 specifically in CD24+ FL-DCs is consistent with the preferential ability of these cells to express β8 integrin. Altogether, these data therefore demonstrate that cell lineage plays a critical role in permitting expression of β8 in response to conditioning factors from the microenvironment, and support the concept that the ability of DCs to express high levels of β8 integrin is determined during development.
Figure 8. CD24+ FL-DCs preferentially express αvβ8 and present an active enhancer in Itgb8 locus.
(A–B) Bone marrow-derived DCs were generated in culture in presence of Flt-3 ligand (FL-DCs). After 9 days in culture, FL-DC subsets were sorted by FACS and cultured in serum free medium for 8h. Cells were either left untreated (−) or stimulated with TGF-β, RA and CpG ODN (+).β8 integrin gene (Itgb8) expression was measured by quantitative RT-PCR relative to Actb and presented relative to levels in unstimulated (−) CD24+ DCs at 8h. (A) Representative FACS plot of CD11c+MHC-II+ FL-DCs showing gating strategy for isolating indicated populations. (B) Histogram shows mean ± SEM of individual cultures from 4 independent experiments. ****, p<0.0001; two-way ANOVA with Tukey’s post-hoc test. (C) ChIP-seq analysis of Batf3, IRF8, H3K4me1 and H3K27ac in CD24+ and CD172+ FL-DCs purified by sorting (ChIP-seq data from (17), GEO accession code GSE66899). Box represents outlined area at −74 kb relative to the Itgb8 TSS. mm9, NCBI37/mm9 assembly of the mouse genome.
DISCUSSION
Despite major advances in determining the origin of DCs subpopulations, our understanding of the factors that determine their functional diversity and tissue specialization remains limited. In this study, we have identified factors that contribute to differentiation of DCs specialized for generation of Tregs in the intestine. We show that expression of integrin αvβ8, which is required for activation of TGF-β by intestinal DCs, is controlled by factors that include DC lineage, tissue microenvironment and the microbiota. Specifically, we show that the combination of TGF-β and RA, factors found at high levels in the intestinal microenvironment, together with TLR signaling, promote expression of β8. However, these signals only result in high levels of αvβ8 in the CD8α+/CD103+CD11b− lineage of DCs. Together, these data provide a key illustrative example of how microenvironmental factors and cell lineage drive the generation of DCs that perform critical specialized functions, in this case the local activation of TGF-β to facilitate Treg generation.
Our data emphasize the critical role of signals derived from the mucosal environment, namely TGF-β, RA and TLR signaling, in promoting expression of αvβ8 both in vitro and in vivo. Furthermore, we find that MLN DCs expressing high levels of αvβ8 have emigrated from the intestine, further supporting the importance of intestinal conditioning in generation of αvβ8+ DCs. However, we find that expression of αvβ8 is consistently lower in CD11chi cells freshly isolated from the LP than those from MLNs, suggesting to us that the process of migration and ‘maturation’ of DCs is also required for high expression of αvβ8. Supporting this, we have previously reported that CD103+ LP DCs develop the ability to activate TGF-β through an αv-dependent mechanism when cultured with T cells (8), conditions that promote activation or maturation of DCs (43, 44). Whether CD103+ DCs in the LP can acquire high levels of αvβ8, such as during inflammation, remains to be determined. Although RA and TGF-β can potentially be derived from various stromal cells in the intestine (27, 45), work from the Rescigno laboratory strongly supports epithelial cells as a likely source of RA and TGF-β for DC conditioning. Their laboratory have previously shown that co-culture of spleen DCs with intestinal epithelial cells or epithelial cell-derived culture supernatant promotes the ability of spleen DCs to generate gut-trophic Tregs (26), and that this is dependent on epithelial cell-derived TGF-β and RA. Our data indicate that up-regulation of expression of αvβ8 on DCs is the likely mechanism for this observation. Furthermore, the close association of CD103+ DCs with the intestinal epithelium (46, 47) suggests this DC subset is ideally placed to receive these signals. Furthermore, there is increasing evidence that DCs associated with the intestinal epithelium interact with intestinal microbes or their products, providing a potential source of TLR signals to promote αvβ8 expression (47–49). It is likely, therefore, that intestinal DCs differ in their exposure to some or all of these stimuli due to their localization, and this may contribute to differential expression of αvβ8 in DC subsets. However, even when cultured under conditions that strongly promote αvβ8 upregulation, expression remained lower in CD103+CD11b+ mucosal DCs than CD103+CD11b− DC. Our observation that CD8α+ DCs from spleen and CD24+ FL-DCs also show high and sustained expression of αvβ8 when cultured in TGF-β, RA and TLR ligands led us to conclude that DC lineage also contributes to αvβ8 expression. Recent studies indicate that these DCs share a common cDC1 lineage with CD103+CD11b− DCs, distinct from CD11b+ DCs found in the intestine and spleen (38, 39, 50). We therefore propose a model in which the cDC1 lineage is programmed to respond to stimulation through TGF-β, RA and TLRs to express high levels of Itgb8, and that this leads to preferential expression of αvβ8 in CD103+CD11b− DCs in vivo. Similar lineage-specific regulation of the Itgb8 promoter has been reported by the Nishimura laboratory in mesenchymal cells, where the pro-inflammatory cytokine IL-1β promotes expression of Itgb8 in lung fibroblasts and astrocytes, but not dermal fibroblasts. In further parallels with our studies, they show that this requires p38 and NF-κB signaling, which are activated downstream of both IL-1β and TLR signaling. IL-1β activates Itgb8 expression by chromatin remodeling and exposure of binding sites for additional transcription factors, and the expression of Itgb8 in different cell types corresponds with the chromatin state of the Itgb8 core promoter (51, 52). p38α is reported to be required for Itgb8 expression by CD103+ MLN DCs, as well as for Treg generation and induction of oral tolerance in mice (53). Hence, we speculate that TLR stimulation likewise promotes expression of Itgb8 through regulating accessibility of the core promoter, possibly governing access of TGF-β and RA-induced transcription factors, and this is further controlled by lineage-specific factors acting at more distal sites. Our model is supported by the presence of an enhancer 74 kb upstream of Itgb8, which is preferentially active in the developmentally related CD24+ BMDCs. It is currently unclear what transcription factors dictate this lineage specificity, and this is an active ongoing area of investigation. However, likely candidates include Batf3 and IRF8, which both bind this enhancer and are required for effective generation and maintenance of CD8α+ and CD103+CD11b− DCs in vivo (Figure 8) (17, 38, 50). Batf3 and IRF8 might thus contribute to licensing the CD8α+/CD103+CD11b− DCs lineage to express β8.
DC expression of αvβ8 is essential for generation of intestinal Tregs (8–11), and our data therefore implicate CD103+CD11b− DCs as the major DC subset responsible for Treg generation in the intestine. CD103+ DCs have also been implicated in intestinal Treg generation in previous studies (28, 29, 54), although this function has also been attributed to mucosal CD103+CD11b+ DCs, CD103−CX3CR1intF4/80− DCs or CD103−CX3CR1+F4/80+ macrophages (55, 56). Deletion of all CD103+ DC subsets also leads to a significant loss of intestinal Tregs in mice (57), further supporting the key role for this subset in Treg generation. However, although other in vitro studies support the specific involvement of the CD103+ CD11b− subset in this process (53), deletion of either the CD103+CD11b− population, through knockout of the transcription factors IRF8 or Batf3 (38, 50) or the CD103+CD11b+ population (41) alone does not lead to a detectable loss of intestinal Tregs or intestinal inflammation. Hence it is likely that both populations make some contribution to Treg generation in vivo, or can compensate for each other in this process. Although we have focused on CD103+CD11b− DCs, low levels of Itgb8 transcript can be detected in CD103+CD11b+ DCs at homeostasis, and these and other DC subsets can upregulate expression during infection or inflammation. Further studies will therefore be needed to determine the exact contribution of different DC subsets to Treg generation and regulation of intestinal inflammation.
In this study, we have focused on the factors that regulate αvβ8 expression by DCs during immune homeostasis, where the principal immunological role is likely to be generation of Tregs. In inflammatory settings, where DC αvβ8 is required for Th17 cell generation (16, 58), different mechanisms may be involved. Atarashi and colleagues have reported that microbe-derived ATP triggers Th17 responses in vivo, and that exogenous ATP promoted αvβ8 expression in DCs (25). However, in our experiments, ATP did not induce significant αvβ8 expression in spleen DCs when given alone or in combination with RA and TGF-β (data not shown). This discrepancy most likely reflects responses of different DC subsets, and whereas we focused on CD103+ DCs, during intestinal infection ATP promotes expression of αvβ8 in CD103− CD11b+ CX3CR1+ CD70hi DCs. Hence Treg and Th17 responses appear to differ in both the conditioning signals that induce αvβ8 expression and the subsets of DCs involved. In this context, it is interesting that TGF-β, RA and TLR stimulation (our studies) or ATP stimulation (25) induce only transient expression of αvβ8 in inflammatory DCs, which is likely to favor Th17 over Treg differentiation (59). Conversely, we find that CD103+CD11b− DCs exhibit high and sustained αvβ8 expression, which would be expected to induce regulatory responses. Despite these differences, our data indicate that regulation of αvβ8 expression is a major factor determining the ability of DCs to generate Tregs and Th17 cells.
TGF-β and RA have been previously implicated in promoting Treg generation through direct effects on T cells, inducing expression of Foxp3 and intestinal homing receptors (28, 29, 32, 60–62). Our findings, and those of Rescigno and colleagues, that these same factors promote differentiation of Treg-generating DCs indicate that these environmental factors produce coordinated effects on the immune system, and highlight a ‘feed-forward’ effect, in which TGF-β signaling in DCs promotes expression of αvβ8, which then promotes TGF-β signaling in T cells. Likewise, RA signaling induces the expression of RA-synthesizing enzymes in DCs (30, 34). In this way, the intestinal microenvironment promotes DCs to ‘recapitulate’ the same environment in the MLN during T cell presentation, which we propose allows fine spatio-temporal regulation of TGF-β signaling and thus induction of appropriate mucosal responses and homing in T cells. In addition, it has recently been shown that αvβ8 is expressed by Tregs, and contributes to ongoing immune regulation (63); whether β8 expression is also activated by RA and TGF-β in T cells remains to be determined.
In conclusion, here we show that cell lineage and gut-derived factors together shape DCs into critical gatekeepers of TGF-β dependent intestinal immune responses via regulation of β8 expression. These results also provide a detailed demonstration of how acquisition of a specialized function comes from the unique ability of DC to integrate signals from the periphery and communicate this contextual information forward for induction of appropriate adaptive immunity.
Supplementary Material
Acknowledgments
The authors thank Joseph McCarty (MD Anderson Cancer Center, Houston, TX) for the kind gift of antibodies to β8 integrin, Thibault Andrieu, Sébastien Dussurgey and Céline Couturier for technical assistance for flow cytometry and cell sorting (AniRA – flow cytometry platform, SFR BioSciences Gerland – Lyon Sud, UMS34444/US8, Lyon, France), Pascaline Bogeat for help at the animal facility (PBES, SFR BioSciences, Lyon, France), and Dominique Kaiserlian, Thierry Defrance and members of their laboratories (CIRI, Lyon, France) for useful discussions and help.
Grant support
This work was supported by grants from the Agence Nationale de la recherche (ANR-13-PDOC-0019) and the People Programme (Marie Sklodowska-Curie Actions) of the European Union’s Seventh Framework Programme FP7 (REA grant agreement n° PIIF-GA-2012-330432) and funds from the Mitchell Trust (UK) to H.P., by grants from the National Institutes of Health (R01DK093695) and the Crohn’s and Colitis Foundation of America to A.L-H, and by a grant from the UK Medical Research Council (G0802069) to J.S.
Abbreviations
- αvβ8
alpha-v beta8 integrin
- BMDC
bone marrow-derived dendritic cell
- ChIP-seq
chromatin immunoprecipitation sequencing
- CpG ODN
CpG oligodeoxynucleotides
- DC
dendritic cell
- Flt3-L
FMS-like tyrosine kinase 3 ligand
- FL-DCs
BMDCs generated in the presence of Flt3-L
- Itgb8
integrin beta8 gene
- l-TGF-β
latent TGF-β
- LP
lamina propria
- MLN
mesenteric lymph node
- RA
retinoic acid
- SI
small intestine
- Treg
regulatory T cell
- TGFBR2
TGF-β receptor 2
- TRIF
Toll/IL-1R-domain-containing adapter-inducing IFN-β
- VAD
vitamin A deficient
- VA+
vitamin A sufficient
- WT
wild-type
Footnotes
DISCLOSURE
The authors declare they have no conflicting interests.
References
- 1.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nature Immunology. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 4.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 5.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, Umemoto E, Ebisuno Y, Yang BG, Seoh JY, Lipp M, Kiyono H, Miyasaka M. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 7.Bekiaris V, Persson EK, Agace WW. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol Rev. 2014;260:86–101. doi: 10.1111/imr.12194. [DOI] [PubMed] [Google Scholar]
- 8.Païdassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, Stuart LM, Savill J, Lacy-Hulbert A. Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology. 2011;141:1813–1820. doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worthington JJ, Czajkowska BI, Melton AC, Travis MA. Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8. Gastroenterology. 2011;141:1802–1812. doi: 10.1053/j.gastro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Chytil A, Magnuson MA, Wright CVE, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 14.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 15.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 16.Acharya M, Mukhopadhyay S, Païdassi H, Jamil T, Chow C, Kissler S, Stuart LM, Hynes RO, Lacy-Hulbert A. αv Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grajales-Reyes GE, Iwata A, Albring J, Wu X, Tussiwand R, WKC, Kretzer NM, Briseño CG, Durai V, Bagadia P, Haldar M, Schönheit J, Rosenbauer F, Murphy TL, Murphy KM. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α (+) conventional DC clonogenic progenitor. Nature Immunology. 2015;16:708–717. doi: 10.1038/ni.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. Journal of Experimental Medicine. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hägerbrand K, Westlund J, Yrlid U, Agace W, Johansson-Lindbom B. MyD88 Signaling Regulates Steady-State Migration of Intestinal CD103+ Dendritic Cells Independently of TNF-α and the Gut Microbiota. The Journal of Immunology. 2015;195:2888–2899. doi: 10.4049/jimmunol.1500210. [DOI] [PubMed] [Google Scholar]
- 23.McNamee EN, Masterson JC, Veny M, Collins CB, Jedlicka P, Byrne FR, Ng GY, Rivera-Nieves J. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn’s-like murine ileitis. J Leukoc Biol. 2015;97:1011–1022. doi: 10.1189/jlb.3HI0614-303R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 26.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 27.Rescigno M. Dendritic cell-epithelial cell crosstalk in the gut. Immunol Rev. 2014;260:118–128. doi: 10.1111/imr.12181. [DOI] [PubMed] [Google Scholar]
- 28.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villablanca EJ, Wang S, de Calisto J, Gomes DCO, Kane MA, Napoli JL, Blaner WS, Kagechika H, Blomhoff R, Rosemblatt M, Bono MR, von Andrian UH, Mora JR. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141:176–185. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramalingam R, Larmonier CB, Thurston RD, Midura-Kiela MT, Zheng SG, Ghishan FK, Kiela PR. Dendritic cell-specific disruption of TGF-β receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. The Journal of Immunology. 2012;189:3878–3893. doi: 10.4049/jimmunol.1201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 34.Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O’Toole T, Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. The Journal of Immunology. 2011;186:1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- 35.Geuking MB, Cahenzli J, Lawson MAE, Ng DCK, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Campeau JL, Salim SY, Albert EJ, Hotte N, Madsen KL. Intestinal epithelial cells modulate antigen-presenting cell responses to bacterial DNA. Infect Immun. 2012;80:2632–2644. doi: 10.1128/IAI.00288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ, Zeng R, Dent A, Ansel KM, Diamond B, Hadeiba H, Butcher EC. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nature Immunology. 2014;15:98–108. doi: 10.1038/ni.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelson BT, WKC, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung S-SJ, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. Journal of Experimental Medicine. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tailor P, Tamura T, Morse HC, Ozato K. The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood. 2008;111:1942–1945. doi: 10.1182/blood-2007-07-100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc Natl Acad Sci USA. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J, Gudjonsson S, Håkansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Vander Lugt B, Khan AA, Hackney JA, Agrawal S, Lesch J, Zhou M, Lee WP, Park S, Xu M, DeVoss J, Spooner CJ, Chalouni C, Delamarre L, Mellman I, Singh H. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nature Immunology. 2014;15:161–167. doi: 10.1038/ni.2795. [DOI] [PubMed] [Google Scholar]
- 43.Shreedhar V, Moodycliffe AM, Ullrich SE, Bucana C, Kripke ML, Flores-Romo L. Dendritic cells require T cells for functional maturation in vivo. Immunity. 1999;11:625–636. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- 44.Flores-Romo L. In vivo maturation and migration of dendritic cells. Immunology. 2001;102:255–262. doi: 10.1046/j.1365-2567.2001.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vicente-Suarez I, Larange A, Reardon C, Matho M, Feau S, Chodaczek G, Park Y, Obata Y, Gold R, Wang-Zhu Y, Lena C, Zajonc DM, Schoenberger SP, Kronenberg M, Cheroutre H. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol. 2015;8:141–151. doi: 10.1038/mi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farache J, Koren I, Milo I, Gurevich I, Kim K-W, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 49.Chieppa M, Rescigno M, Huang AYC, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. Journal of Experimental Medicine. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markovics JA, Araya J, Cambier S, Jablons D, Hill A, Wolters PJ, Nishimura SL. Transcription of the transforming growth factor beta activating integrin beta8 subunit is regulated by SP3, AP-1, and the p38 pathway. J Biol Chem. 2010;285:24695–24706. doi: 10.1074/jbc.M110.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markovics JA, Araya J, Cambier S, Somanath S, Gline S, Jablons D, Hill A, Wolters PJ, Nishimura SL. Interleukin-1beta induces increased transcriptional activation of the transforming growth factor-beta-activating integrin subunit beta8 through altering chromatin architecture. J Biol Chem. 2011;286:36864–36874. doi: 10.1074/jbc.M111.276790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang G, Wang Y, Chi H. Control of T cell fates and immune tolerance by p38α signaling in mucosal CD103+ dendritic cells. The Journal of Immunology. 2013;191:650–659. doi: 10.4049/jimmunol.1300398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. Journal of Experimental Medicine. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature Immunology. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 56.Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, Karp CL, Pulendran B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. The Journal of Immunology. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyártó BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. Journal of Experimental Medicine. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 61.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cassani B, Villablanca EJ, de Calisto J, Wang S, Mora JR. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol Aspects Med. 2012;33:63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Worthington JJ, Kelly A, Smedley C, Bauché D, Campbell S, Marie JC, Travis MA. Integrin αvβ8-Mediated TGF-β Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity. 2015;42:903–915. doi: 10.1016/j.immuni.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.