Abstract
Obesity is associated with chronic low-grade inflammation that drives the development of metabolic diseases, including non-alcoholic fatty liver disease (NAFLD). We recently showed that white adipose tissue (WAT) constitutes an important source of inflammatory factors. Hence, interventions that attenuate WAT inflammation may reduce NAFLD development. Male LDLr−/− mice were fed a high-fat diet (HFD) for 9 weeks followed by 7 weeks of HFD with or without rosiglitazone. Effects on WAT inflammation and NAFLD development were analyzed using biochemical and (immuno)histochemical techniques, combined with gene expression analyses. Nine weeks of HFD feeding induced obesity and WAT inflammation, which progressed gradually until the end of the study. Rosiglitazone fully blocked progression of WAT inflammation and activated PPARγ significantly in WAT. Rosiglitazone intervention did not activate PPARγ in liver, but improved liver histology and counteracted the expression of genes associated with severe NAFLD in humans. Rosiglitazone reduced expression of pro-inflammatory factors in WAT (TNF-α, leptin) and increased expression of adiponectin, which was reflected in plasma. Furthermore, rosiglitazone lowered circulating levels of pro-inflammatory saturated fatty acids. Together, these observations provide a rationale for the observed indirect hepatoprotective effects and suggest that WAT represents a promising therapeutic target for the treatment of obesity-associated NAFLD.
The prevalence of obesity has increased dramatically over the last 30 years and metabolic disorders associated with obesity have become a major health and economic problem worldwide1. Obesity is associated with a state of low-grade chronic inflammation, frequently referred to as systemic inflammation or metabolic inflammation2, which is thought to drive the development of several metabolic diseases including non-alcoholic fatty liver disease (NAFLD)3,4. We recently showed that adipose tissue is a critical source of inflammation in obesity and causally involved in NAFLD progression5. However, it is unclear whether suppression of adipose tissue inflammation would attenuate NAFLD progression.
White adipose tissue (WAT) is the primary site of energy storage. This storage function involves expansion of WAT through adipocyte hyperplasia (increase in cell number) and adipocyte hypertrophy (increase in cell size)6. Adipocyte hypertrophy is closely associated with WAT inflammation: in an in vitro experiment with isolated primary human adipocytes7, only very hypertrophic cells were found to secrete MCP-1, a key mediator of immune cell recruitment into WAT. Consistent with this observation, adipocyte hypertrophy is associated with infiltration of macrophages and formation of crown-like structures (CLS)8, a histological hallmark of inflamed WAT. Notably, a strong increase in CLS is observed at the time point at which a WAT depot has reached its maximal mass as shown very recently in a model of diet-induced obesity5.
It is thought that the inflamed WAT is less insulin sensitive, which enhances lipolysis of stored fat, thereby contributing to ectopic fat deposition and the development of liver steatosis9. In line with this, Kolak and colleagues10 have shown that obese patients with inflamed WAT have more liver fat than equally obese subjects without WAT inflammation. In addition to the increased fat flux, inflamed WAT may produce inflammatory factors that can contribute to systemic inflammation and promote the progression from liver steatosis to non-alcoholic steatohepatitis (NASH)2,11,12. However, experimental support for a causal role of WAT in the development of NASH has long been lacking. Recently we have shown that surgical removal of inflamed abdominal (epididymal) WAT in mice reduced lobular inflammation and attenuated NASH development5, suggesting that WAT constitutes an possible target for the treatment of NASH.
WAT inflammation may be reduced via the nuclear hormone receptor peroxisome proliferator-activated receptor-γ (PPARγ) which is predominantly expressed in adipose tissue, controlling inflammatory and metabolic processes13. Previous studies in humans14 and animals15,16,17, provide indication that pharmacological activators of PPARγ such as rosiglitazone may reduce the inflammatory state of WAT in obesity. We herein investigated whether rosiglitazone intervention can reduce manifest WAT inflammation and would attenuate subsequent NAFLD development. To do so, we first determined the time point at which WAT inflammation develops during high-fat diet treatment in LDLr−/− mice. Subsequently, we studied the therapeutic effect of rosiglitazone on WAT inflammation and associated NAFLD development.
Results
WAT inflammation starts in epididymal WAT during high-fat diet-induced obesity
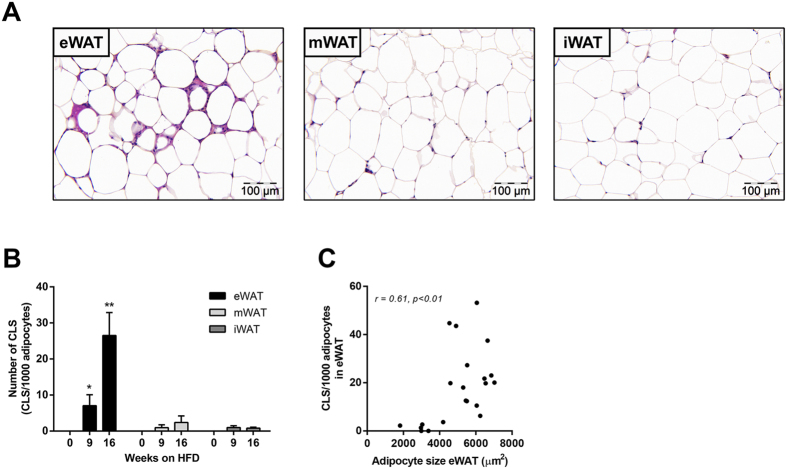
After 16 weeks, CLS formation was most pronounced in epididymal WAT (eWAT) (Fig. 1A), while CLS were hardly observed in mesenteric WAT (mWAT) and inguinal WAT (iWAT). Quantitative analysis showed a marked increase in CLS number in eWAT (p < 0.05; Fig. 1B). CLS number correlated with eWAT mass (r = 0.80, p < 0.001, not shown) and with average adipocyte size, a measure of adipocyte hypertrophy (r = 0.61, p < 0.01; Fig. 1C). The average adipocyte size in eWAT was greater than in mWAT and iWAT (not shown). Hence, eWAT is most susceptible to develop CLS, with substantial inflammation established after 9 weeks of high-fat feeding.
Figure 1. Effect of HFD feeding on development of WAT inflammation.
(A) Representative photomicrographs of three WAT depots after 16 weeks of high-fat feeding. (B) Quantitative analysis of CLS formation over time in the major adipose tissue depots, eWAT, mWAT and iWAT. (C) Positive correlation between CLS number and adipocyte size in eWAT. Data are mean ± SEM (n = 8/group), *p < 0.05 compared with t = 0; **p < 0.05 compared with t = 0 and 9 weeks of high-fat feeding.
Rosiglitazone attenuates WAT inflammation independent of obesity and targets WAT
Mice were treated with high-fat diet for 9 weeks to induce obesity (Table 1). At this time point, intervention with rosiglitazone was started. The caloric intake was comparable between the HFD control group and the HFD + Rosi group (14.6 ± 0.7 and 13.4 ± 0.6 kcal/day, respectively). Continuous high-fat feeding increased fasting plasma glucose, while rosiglitazone had a significant lowering effect (Table 1). Rosiglitazone also significantly lowered fasting plasma insulin and HOMA-IR relative to HFD mice (Table 1). Weight gain and total fat mass were comparable between HFD and HFD + Rosi (Table 1), indicating that the observed metabolic effects were independent of obesity.
Table 1. Metabolic parameters of experimental groups.
| Parameter | Chow | REF | HFD | HFD + Rosi |
|---|---|---|---|---|
| BW gain (g) | 3.2 ± 0.5 | 9.1 ± 1.8a | 17.5 ± 0.9b | 17.1 ± 1.2b |
| Total adiposity (g) | 1.0 ± 0.1 | 2.6 ± 0.6a | 4.4 ± 0.3b | 4.1 ± 0.2b |
| Glucose (mM) | 11.1 ± 0.3 | 12.5 ± 0.7a | 15.0 ± 0.5b | 10.6± 0.2c |
| Insulin (ng/ml) | 0.7 ± 0.2 | 2.9 ± 0.8a | 4.65 ± 0.9b | 1.4 ± 0.2c |
| HOMA-IR | 8.0 ±2.4 | 43.1 ± 12.8a | 78.6 ± 16.1b | 16.3 ± 2.5c |
Abbreviations: Chow, mice fed a chow diet for 16 weeks; REF, reference, mice receiving a HFD for 9 weeks to define condition prior to intervention; HFD, control mice after 16 weeks of HFD; HFD + Rosi, rosiglitazone-treated mice (intervention from 9–16 weeks). a, Significantly different from chow; b, Significantly different from chow and REF; c, Significantly different from HFD (all, p < 0.05).
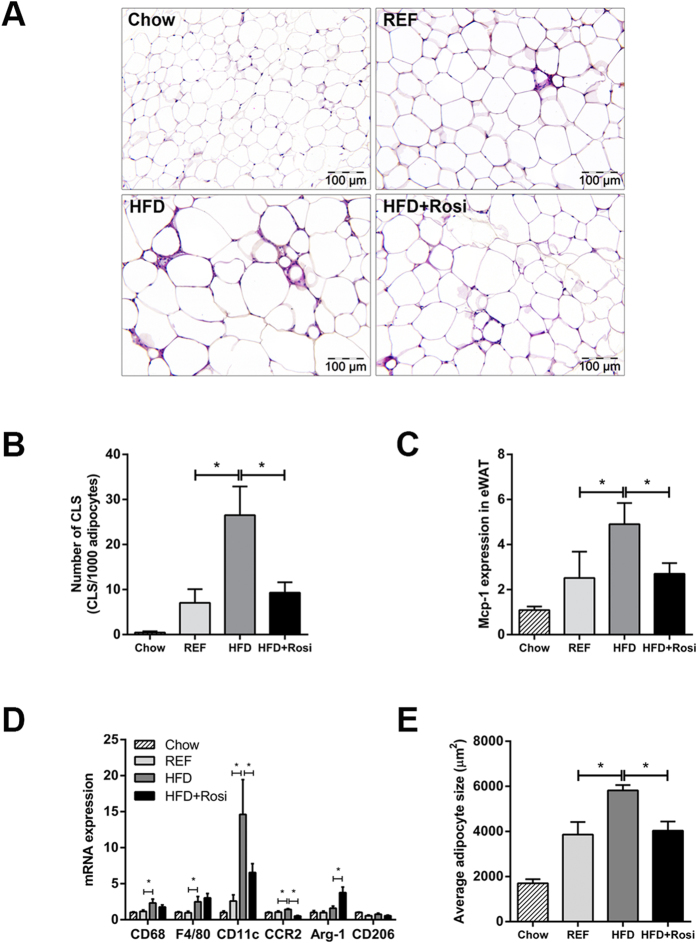
Quantification of CLS in eWAT revealed that CLS numbers were increased in HFD relative to REF, but remained constant in HFD + Rosi (Fig. 2A). Hence, rosiglitazone fully blocked further CLS formation but did not resolve existing inflammation (Fig. 2B). These effects were paralleled by decreased gene expression of MCP-1 in HFD + Rosi (Fig. 2C). Gene expression of macrophage markers revealed that rosiglitazone intervention reduced the pro-inflammatory M1 macrophage markers CD11c and CCR2 (Fig. 2D). In addition, rosiglitazone increased the expression of anti-inflammatory M2 macrophage marker Arginase-1, but did not affect CD206 (Fig. 2D). Consistent with this, we found less immunoreactivity against CCR2 and CD11c in adipose tissue of mice treated with rosiglitazone as determined by immunohistochemical analysis (Supplement 1). Refined analysis of CLS revealed that CLS contain CCR2 + and CD11c + cells and some cells expressed both markers in the HFD group as well as the HFD + Rosi group (Supplement 1). Furthermore, rosiglitazone influenced the expression of genes involved in inflammatory and oxidative stress pathways as shown by microarray analysis (Supplement 2). The observed reduction of eWAT inflammation in HFD + Rosi mice was paralleled by a decreased adipocyte size (Fig. 2E).
Figure 2. Effects of rosiglitazone intervention on eWAT inflammation.
(A) Representative photomicrographs of HPS-stained eWAT cross-sections (magnification x200). (B) High-fat feeding strongly increased CLS formation in eWAT between 9 weeks (REF) and 16 weeks (HFD), while rosiglitazone fully blocked further CLS formation. (C) MCP-1 gene expression was increased in HFD mice, but not in HFD + Rosi. (D) Gene expression of macrophage markers. Rosiglitazone reduced HFD-induced expression of M1 markers (CD11c and CCR2) and increased gene expression of M2 marker Arginase-1 (Arg-1). HFD-induced expression of general macrophage markers, CD68 and F4/80, were not affected by rosiglitazone. (E) Morphometric analysis of average adipocyte size revealed that rosiglitazone attenuated HFD-induced increase in adipocyte size in eWAT. Data are mean ± SEM (n = 7–10/group), *p < 0.05. Mean expression of RT-PCR data was set 1 for chow-fed mice.
To validate that rosiglitazone affected PPARγ-regulated genes in eWAT under the experimental conditions employed an upstream transcriptional regulator analysis was performed. This analysis demonstrated a highly significantly increased transcriptional activity of PPARγ (Z-score: 4.1, p = 5.92e-24). More specifically, rosiglitazone significantly affected the expression of 1049 genes (FDR < 0.05), of which 71 are established PPARγ-regulated genes (including fatty acid transporter protein 1, fatty acid binding proteins, perilipin, uncoupling protein-1, acyl-CoA synthetase) (for detailed list, see Supplement 2). By contrast, microarray analysis of corresponding livers under the same statistical cut-off (FDR < 0.05) revealed that only 36 genes (among which 4 PPARγ-regulated genes) were differentially expressed by rosiglitazone (Supplement 3), and upstream transcriptional regulator analysis showed no activation of PPARγ. There were also no indications for off-target activation of PPARα or PPARδ from this microarray analysis (Supplement 3). Altogether, these data demonstrated that rosiglitazone significantly activated PPARγ in WAT and attenuated high-fat diet-induced WAT inflammation.
Rosiglitazone prevents progression of NAFLD
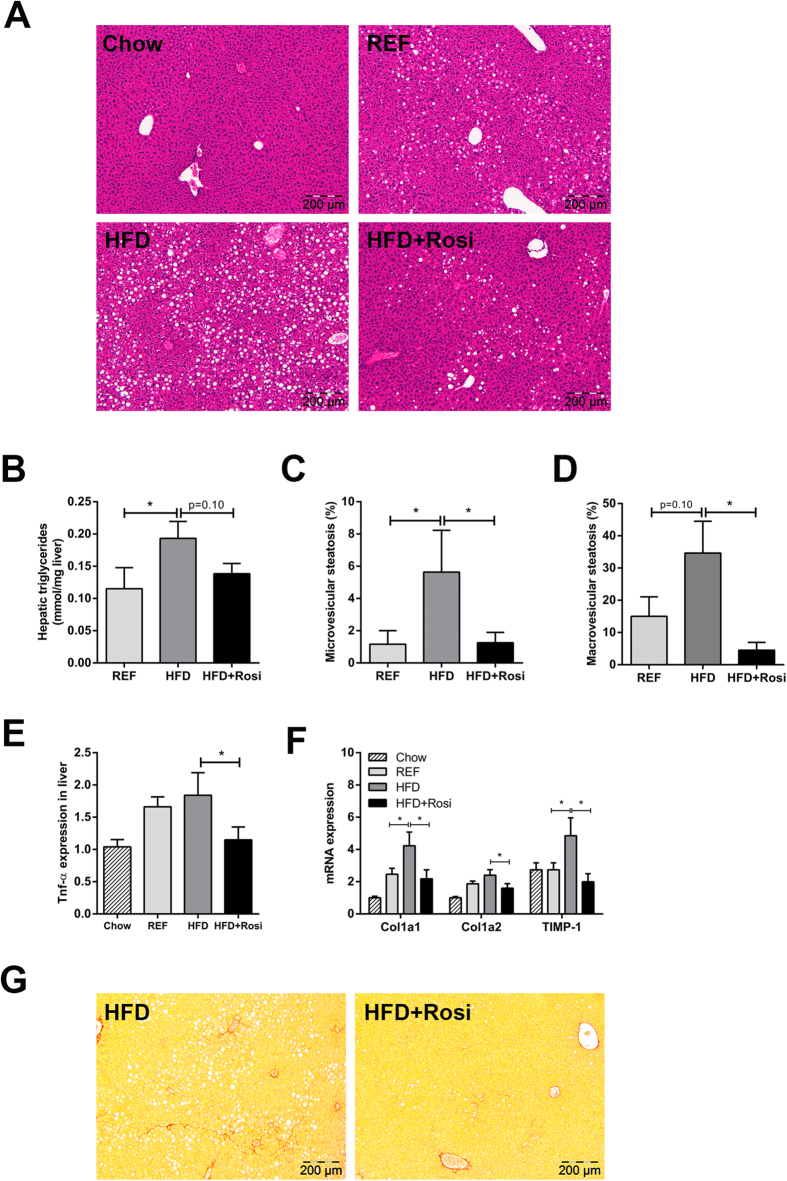
Next, we investigated the effects of rosiglitazone intervention on the liver. High-fat feeding resulted in mild/moderate hepatic steatosis after 9 weeks (REF), which was markedly aggravated after 16 weeks (HFD) (Fig. 3A). Rosiglitazone blunted the progression of NAFLD and livers resembled those of REF. Biochemical intrahepatic triglyceride analysis showed a significant increase in HFD relative to REF and liver triglyceride concentrations tended to be lower in HFD + Rosi (Fig. 3B). Histological analysis revealed a strong increase in microvesicular steatosis in HFD compared with REF and rosiglitazone fully prevented this increase (Fig. 3C). Macrovesicular steatosis, a hallmark of NASH in humans18, was also elevated in HFD and reduced by rosiglitazone (Fig. 3D). High-fat treatment activated several pro-inflammatory and pro-fibrotic pathways in liver including those induced by TNFα (Z-score: 2.79; p = 3.8e-03), IL-6 (Z-score: 2.03; p = 2.9e-07) and TGFβ1 (Z-score: 1.3; p = 1.38e-05) as demonstrated by pathway analysis (FDR < 0.05). Moreover, high-fat treatment induced several genes which were recently identified in human NASH/fibrosis patients19 (Table 2). Rosiglitazone treatment attenuated this effect and counteracted the expression of genes including Col14a1, TaxIBP3, EFEMP2, EGFBP7, THBS2, BICC1 and DKK3. Furthermore, RT-PCR analysis of TNFα, which plays an essential role in NASH, showed increased TNFα gene expression in HFD mice and that rosiglitazone treatment quenched this induction (Fig. 3E). Similarly, HFD-induced expression of pro-fibrotic genes Col1a1, Col1a2 and TIMP-1 were suppressed by rosiglitazone intervention (Fig. 3F). High-fat feeding also resulted in infiltration of neutrophils (MPO-positive inflammatory cells) and formation of inflammatory cell aggregates characteristic for NASH20 between 9 and 16 weeks which was attenuated by rosiglitazone (Supplement 4). Analysis of Sirius-red stained liver cross-sections of the HFD group revealed onset of perisinusoidal fibrosis, which was not observed in HFD + Rosi (Fig. 3G). Altogether, intervention with rosiglitazone attenuated the progression from steatosis to NASH.
Figure 3. Effects of rosiglitazone intervention on NAFLD development.
(A) Representative photomicrographs of HE-stained liver sections of REF, HFD and HFD + Rosi. (B) Biochemical analysis of hepatic triglyceride content. Histological quantification of (C) microvesicular steatosis and (D) macrovesicular steatosis show that steatosis was ameliorated by rosiglitazone compared with HFD (n = 7–10/group). (E) TNFα gene expression in liver was diminished in rosiglitazone-treated mice (n = 7–8/group). (F) Gene expression of fibrotic genes determined by RT-PCR. Rosiglitazone reduced HFD-induced expression of Col1a1, Col1a2 and TIMP-1. (G) Onset of fibrosis in Sirius Red-stained liver cross-sections in HFD mice, but not in HFD + Rosi. Pictures are shown in magnification x100. Data are mean ± SEM, *p < 0.05. Mean expression of RT-PCR data was set 1 for chow-fed mice.
Table 2. Microarray analysis of hepatic gene expression profile based on genes identified in human NAFLD.
| Probe ID | Gene symbol | Gene name | HFD vs. Chow | HFD + Rosi vs. HFD | ||||
|---|---|---|---|---|---|---|---|---|
| Fold-Change | p-value | Fold-Change | p-value | |||||
| ILMN_2635229 | Thbs2 | thrombospondin 2 | 1,740 | ↑ | 9,47E-06 | 0,650 | ↓ | 4,62E-04 |
| ILMN_2764588 | Igfbp7 | insulin-like growth factor binding protein 7 | 1,465 | ↑ | 1,79E-05 | 0,834 | ↓ | 3,52E-02 |
| ILMN_1217309 | Tax1bp3 | Tax1 (human T-cell leukemia virus type I) binding protein 3 | 1,360 | ↑ | 7,45E-04 | 0,826 | ↓ | 3,28E-02 |
| ILMN_2866901 | Efemp2 | epidermal growth factor-containing fibulin-like extracellular matrix protein 2 | 1,556 | ↑ | 7,62E-04 | 0,751 | ↓ | 2,70E-02 |
| ILMN_2636424 | Itgbl1 | integrin, beta-like 1 | 1,528 | ↑ | 1,11E-03 | 0,905 | 4,33E-01 | |
| ILMN_2746556 | Dkk3 | dickkopf homolog 3 (Xenopus laevis) | 1,402 | ↑ | 1,16E-03 | 0,744 | ↓ | 4,27E-03 |
| ILMN_1258629 | Col3a1 | collagen, type III, alpha 1 | 1,887 | ↑ | 1,61E-03 | 0,746 | 1,39E-01 | |
| ILMN_2939138 | Bicc1 | bicaudal C homolog 1 (Drosophila) | 1,460 | ↑ | 2,69E-03 | 0,666 | ↓ | 1,28E-03 |
| ILMN_2746086 | Tax1bp3 | Tax1 (human T-cell leukemia virus type I) binding protein 3 | 1,334 | ↑ | 2,94E-03 | 0,787 | ↓ | 1,27E-02 |
| ILMN_2980663 | Aqp1 | aquaporin 1 | 0,812 | ↓ | 7,68E-03 | 1,158 | ↑ | 5,74E-02 |
| ILMN_2606210 | Dpt | dermatopontin | 1,459 | ↑ | 1,08E-02 | 0,714 | ↓ | 2,26E-02 |
| ILMN_3007428 | Sox9 | SRY-box containing gene 9 | 0,694 | ↓ | 1,11E-02 | 1,245 | 1,23E-01 | |
| ILMN_2831656 | Epha3 | Eph receptor A3 | 1,334 | ↑ | 1,76E-02 | 0,901 | 3,84E-01 | |
| ILMN_2687872 | Col1a1 | collagen, type I, alpha 1 | 1,471 | ↑ | 3,99E-02 | 0,921 | 6,58E-01 | |
| ILMN_2747959 | Dcn | decorin | 1,151 | ↑ | 4,22E-02 | 0,874 | ↓ | 5,21E-02 |
| ILMN_2591027 | Col14a1 | collagen, type XIV, alpha 1 | 1,176 | ↑ | 4,74E-02 | 0,820 | ↓ | 1,61E-02 |
| ILMN_1223552 | Fbn1 | fibrillin 1 | 1,181 | 6,25E-02 | 0,885 | 1,69E-01 | ||
| ILMN_1233545 | Lbh | limb-bud and heart | 0,782 | 6,40E-02 | 1,089 | 5,19E-01 | ||
| ILMN_2669189 | Lima1 | LIM domain and actin binding 1 | 1,226 | 8,23E-02 | 0,956 | 6,98E-01 | ||
| ILMN_1253806 | Col1a2 | collagen, type I, alpha 2 | 1,278 | 8,24E-02 | 0,837 | 2,08E-01 | ||
| ILMN_2852957 | Dkk3 | dickkopf homolog 3 (Xenopus laevis) | 1,184 | 8,82E-02 | 0,941 | 5,38E-01 | ||
| ILMN_1214954 | Cldn10 | claudin 10 | 0,836 | 1,39E-01 | 1,143 | 2,68E-01 | ||
| ILMN_1228374 | Lima1 | LIM domain and actin binding 1 | 1,190 | 1,48E-01 | 0,916 | 4,66E-01 | ||
| ILMN_2980661 | Aqp1 | aquaporin 1 | 0,895 | 1,89E-01 | 1,124 | 1,65E-01 | ||
| ILMN_1226183 | Antxr1 | anthrax toxin receptor 1 | 1,211 | 1,91E-01 | 0,815 | 1,63E-01 | ||
| ILMN_2848305 | Pnma1 | paraneoplastic antigen MA1 | 1,144 | 1,96E-01 | 0,993 | 9,43E-01 | ||
| ILMN_2666018 | Mgp | matrix Gla protein | 1,162 | 1,99E-01 | 0,914 | 4,39E-01 | ||
| ILMN_2816180 | Lbh | limb-bud and heart | 1,137 | 2,24E-01 | 0,902 | 3,29E-01 | ||
| ILMN_1257077 | Jag1 | jagged 1 | 1,160 | 2,33E-01 | 0,941 | 6,22E-01 | ||
| ILMN_2734683 | Fstl1 | follistatin-like 1 | 1,119 | 2,71E-01 | 0,949 | 6,11E-01 | ||
| ILMN_2596346 | Dcn | decorin | 1,102 | 3,26E-01 | 0,834 | 6,82E-02 | ||
| ILMN_2597515 | Ehf | ets homologous factor | 1,147 | 3,35E-01 | 1,055 | 7,05E-01 | ||
| ILMN_3001540 | Lum | lumican | 1,101 | 4,53E-01 | 0,817 | 1,17E-01 | ||
| ILMN_1227817 | Ank3 | ankyrin 3, epithelial | 1,109 | 4,57E-01 | 0,940 | 6,58E-01 | ||
| ILMN_2769479 | Lama2 | laminin, alpha 2 | 1,116 | 4,87E-01 | 0,968 | 8,38E-01 | ||
| ILMN_2893417 | Sox4 | SRY-box containing gene 4 | 0,929 | 5,57E-01 | 0,965 | 7,75E-01 | ||
| ILMN_1223963 | Ank3 | ankyrin 3, epithelial | 1,081 | 5,62E-01 | 0,835 | 1,83E-01 | ||
| ILMN_2836637 | Glt8d2 | glycosyltransferase 8 domain containing 2 | 1,078 | 5,91E-01 | 1,229 | 1,45E-01 | ||
| ILMN_1249021 | Bcl2 | B-cell leukemia/lymphoma 2 | 1,058 | 5,99E-01 | 0,937 | 5,50E-01 | ||
| ILMN_1229643 | Antxr1 | anthrax toxin receptor 1 | 1,066 | 6,02E-01 | 0,779 | ↓ | 4,37E-02 | |
| ILMN_2620563 | Nexn | nexilin | 1,081 | 6,07E-01 | 0,771 | 8,88E-02 | ||
| ILMN_1238000 | Srpx | sushi-repeat-containing protein | 1,056 | 6,75E-01 | 1,095 | 4,83E-01 | ||
| ILMN_2621643 | Col4a1 | collagen, type IV, alpha 1 | 1,044 | 7,12E-01 | 1,125 | 3,12E-01 | ||
| ILMN_2629486 | Srpx | sushi-repeat-containing protein | 0,958 | 7,70E-01 | 0,832 | 2,12E-01 | ||
| ILMN_2686036 | Tax1bp3 | Tax1 (human T-cell leukemia virus type I) binding protein 3 | 1,030 | 8,04E-01 | 1,004 | 9,72E-01 | ||
| ILMN_2701712 | Plcxd3 | phosphatidylinositol-specific phospholipase C, X domain containing 3 | 0,982 | 8,80E-01 | 0,943 | 6,22E-01 | ||
| ILMN_2629804 | Epha3 | Eph receptor A3 | 0,987 | 9,04E-01 | 1,100 | 3,67E-01 | ||
The table lists the genes that were recently reported to be associated with NAFLD severity in humans19. HFD feeding of LDLr−/− mice resulted in a significant effect on 16 genes compared to chow (arrows indicate significant up- (↑) or downregulation (↓)). Rosiglitazone counteracted the effect of a HFD as shown by the comparison of HFD + Rosi vs. HFD.
Rationale for the hepatoprotective effects of rosiglitazone
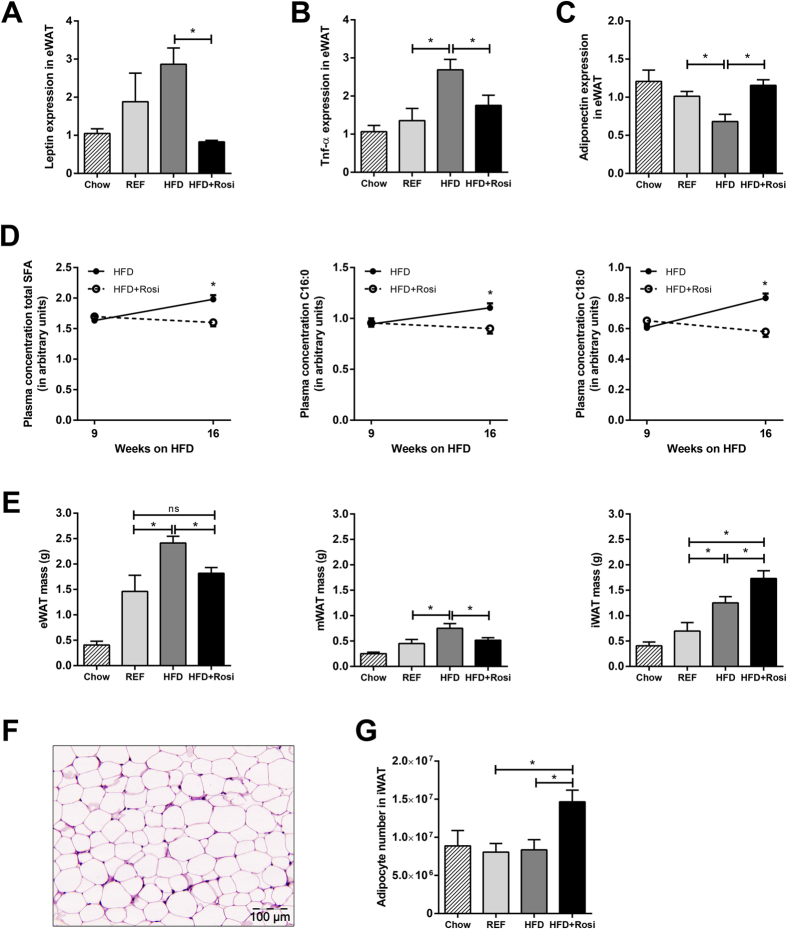
In eWAT, rosiglitazone blocked the HFD-induced gene expression of leptin and TNFα (Fig. 4A,B). These effects were paralleled in plasma; HFD + Rosi reduced concentrations of leptin and TNFα (Supplement 5). By contrast, rosiglitazone fully restored the HFD-induced decrease in adiponectin gene expression in eWAT (Fig. 4C) which was also reflected in plasma (Supplement 5).
Figure 4. Effects of rosiglitazone on adipokine expression in eWAT, pro-inflammatory fatty acids in plasma and WAT morphology.
High-fat feeding increased gene expression in eWAT of pro-inflammatory adipokines (A) leptin, (B) TNFα and decreased expression of (C) anti-inflammatory adipokine adiponectin, while rosiglitazone counteracted these effects. (D) Plasma levels of total saturated fatty acids (SFA) and specific SFAs, palmitic acid (C16:0) and stearic acid (C18:0), were increased between week 9 and 16 of high-fat feeding. This increase was blunted by rosiglitazone (all p < 0.01; paired t-test; n = 9–12/group). (E) The mass of WAT depots was increased in HFD, while rosiglitazone specifically increased iWAT mass. (F) Representative photomicrograph of iWAT in HFD + Rosi, showing absence of CLS. (G) Expansion of iWAT mass in HFD + Rosi was mainly attributable to an increase in adipocyte number. Data are mean ± SEM, *p < 0.05. Mean expression of RT-PCR data was set 1 for chow-fed mice (n = 7–8/group). Fatty acid plasma concentration was expressed as arbitrary units relative to internal standard.
In addition, rosiglitazone prevented the high-fat diet-induced increase in total saturated fatty acids in plasma (Fig. 4D). In line with this, total NEFA were significantly increased (by 26%, p < 0.05) in the HFD group, whereas no significant increase was observed in the HFD + Rosi group (11%, n.s.). More specifically, plasma concentrations of palmitic acid (C16:0) and stearic acid (C18:0) were not increased in HFD + Rosi (Fig. 4D).
Since WAT inflammation correlated with WAT mass and adipocyte hypertrophy we analyzed effects of rosiglitazone on eWAT, iWAT and mWAT in more detail (Fig. 4E). During intervention with rosiglitazone, eWAT mass did not further increase while iWAT mass almost doubled, indicating a shift of fat mass from eWAT towards iWAT. Despite the increase in iWAT mass, this depot did not become inflamed (Fig. 4F). Quantification of adipocyte size showed that the expansion in iWAT was mainly attributable to an increase in adipocyte number rather than adipocyte size (Fig. 4G). This suggests that increased capability of iWAT to store fat may prevent the development of hypertrophy and associated inflammation in eWAT, and may thereby contribute to beneficial effects of rosiglitazone on NAFLD development.
Discussion
Recent findings indicate that inflamed (abdominal) WAT plays a causal role in the development of NASH in the context of obesity5. WAT may thus constitute a new target for intervention. Compounds that specifically target and quench WAT inflammation have not been developed yet. We therefore used rosiglitazone, an activator of PPARγ with reported anti-inflammatory properties14,15,16, as a model compound to intervene in manifest WAT inflammation. Here, we show that rosiglitazone attenuates WAT inflammation and reduces NASH development.
Under the experimental conditions employed herein, rosiglitazone activated PPARγ in WAT, but not in liver, based on a comprehensive analysis of PPARγ-regulated genes. The significant activation of PPARγ in WAT may be important for the observed hepatoprotective effects, because PPARγ activation in liver could cause detrimental effects: Recent knock-out studies have shown that targeted PPARγ deletion in hepatocytes or macrophages protected mice against high-fat induced steatosis21, while deletion of PPARγ in adipose tissues increased liver steatosis upon high-fat feeding22. Furthermore, rosiglitazone treatment remained effective in mice lacking PPARγ specifically in the liver23, supporting the view that adipose tissue is an important site of thiazolidinedione action.
Consistent with our findings, beneficial effects of rosiglitazone in NAFLD were also observed in aged (12 months old) LDLr−/− mice that develop a more severe disease phenotype than young mice (3 months old) as used herein24. However, this study did not examine the effects of rosiglitazone in a therapeutic (intervention) setting and its effects in adipose tissue were not analyzed. In the study by Gupte and colleagues24, the diet was supplemented with cholesterol which may explain some of the differences observed on liver gene expression and inflammation. Dietary cholesterol has been shown to be a strong inducer of inflammatory gene expression in the liver25,26. For instance, treatment with a HFD supplemented with small amounts (0.2% w/w) of cholesterol triggered Kupffer cell activation and inflammatory gene expression after already 2 weeks in LDLr−/− mice, whereas the same diet without cholesterol hardly had an effect on liver inflammation26. High-fat diets without cholesterol supplementation induce liver inflammation typically at a slower pace and, importantly, this liver inflammation is at least partly mediated by the inflamed white adipose tissue (WAT)5. However, it is unclear to which extent WAT may contribute to liver inflammation when cholesterol is added to a high-fat diet.
We found that eWAT is more susceptible to develop chronic inflammation than mWAT or iWAT. This observation may be related to the fact that adipocytes in eWAT are more prone to hypertrophy than those in other adipose depots27. In the present study, CLS numbers in eWAT correlated with adipocyte size supporting the importance of adipocyte hypertrophy in the development of WAT inflammation6,7,8. Consistent with this, metabolically healthy obese subjects were found to have significantly smaller adipocytes compared with metabolically unhealthy obese patients who had more ectopic liver fat at a comparable body mass index28. This suggests that the ability to expand WAT through mechanisms of adipocyte hyperplasia may prevent: a) WAT inflammation and b) ectopic fat accumulation, thereby contributing to a healthy metabolic state.
We observed that rosiglitazone stimulated hyperplasia specifically in subcutaneous WAT thereby preventing adipocyte hypertrophy, which is also observed in patients treated with thiazolidinediones29,30. Consequently, this depot did not become inflamed even though its mass was much greater than in control animals, as is seen in humans treated with rosiglitazone31. The observed stimulation of hyperplasia specifically in iWAT by rosiglitazone may be explained by depot-specific regulation of perilipin, which is essential for enlargement of lipid droplets. Kim and co-workers showed that perilipin protein expression increased after rosiglitazone treatment in subcutaneous adipose tissue, but did not change in visceral adipose tissue32.
Clinical trials have shown that treatment with thiazolidinediones can improve liver histology in patients with NASH33,34. However, the underlying mechanisms mediating the beneficial effects of thiazolidinediones in NASH development are unclear. Data from the present study support the view that rosiglitazone may attenuate the development of NAFLD via an effect on WAT. Several studies showed that infiltration of macrophages into WAT is strongly associated with NAFLD development10,35,36. More specifically, an increase in CD11c + CD206 + and CCR2 + macrophages in WAT is associated with enhanced production of pro-inflammatory adipokines and cytokines in WAT, and NASH severity36. Herein we show that rosiglitazone intervention reduced the expression of pro-inflammatory M1 markers, CD11c and CCR2 and increased the expression of anti-inflammatory M2 marker, Arginase-1. An increase in Arginase-1 expression has also been observed in HFD-fed Sv129 mice after treatment with rosiglitazone17, but rosiglitazone did not alter the expression of CD11c which may be related to the relatively short intervention period. Long-term rosiglitazone treatment in ob/ob mice resulted in lower CD11c expression level in WAT37, consisted with our findings. Analysis of CLS in the present study shows that long-term rosiglitazone intervention attenuates WAT inflammation by reducing CLS numbers (Fig. 2B), rather than altering the activation state of immune cells within a CLS (as determined CD11c and CCR2 immunoreactivity).
Our study indicates that the hepatoprotective effects on NASH by rosiglitazone may at least partly be mediated by adipokines, since plasma leptin and TNFα levels were reduced and plasma adiponectin levels were increased. It is known that leptin can exert pro-inflammatory effects and can activate hepatic stellate cells thereby promoting fibrosis38. TNFα plays a crucial role in human and animal NAFLD and neutralization of TNFα activity attenuated the disease39. For instance, adiponectin is a potent TNFα-neutralizing cytokine that counteracts inflammation that is relevant for NASH progression38,39. It has been demonstrated that also saturated fatty acids can activate inflammatory cascades leading to activation of TNFα40. We found that the saturated fatty acids; palmitic acid and stearic acid, were markedly increased by high-fat feeding and reduced with rosiglitazone. Notably, these fatty acids are also increased in patients with diagnosed NASH41. Furthermore, surgical excision of inflamed WAT in mice lowered palmitic acid in plasma and reduced progression towards NASH5. In vitro experiments have shown that conditioned medium from palmitic acid-treated hepatocytes induces the expression of pro-fibrotic genes in hepatic stellate cells42, providing mechanistic support for a crucial role of inflammatory lipid mediators in NASH.
We also observed that rosiglitazone attenuated the HFD-induced hepatic expression of the genes encoding for Col1a1, Col1a2 and TIMP-1. This hepatoprotective effect of rosiglitazone was further substantiated by an effect on genes that are associated with severity of human NAFLD as shown by Moylan et al.19. These findings support the view that the experimental conditions established herein (HFD-induced obesity, hyperinsulinemia, WAT inflammation concurrent with histologic NASH) may facilitate preclinical research that aims at translation to the human setting.
In all, intervention with rosiglitazone reduces WAT inflammation, lowers circulating inflammatory mediators and attenuates NAFLD progression. These effects were independent of total adiposity and body weight, indicating that adipose tissue quality (i.e. inflammatory state) rather than absolute mass is critical for NAFLD development. Our results suggest that intervention in WAT may present a new therapeutic option for the treatment of NAFLD.
Methods
Animal experiments
All animal experiments were approved by the institutional Animal Care and Use Committee of the Netherlands Organization of Applied Scientific Research (Zeist, The Netherlands; approval number DEC2935) and were conducted in accordance with the Dutch Law on Animal Experiments, following international guidelines on animal experimentation. Mice (aged 12–14 weeks at the start of the experiment) had ad libitum access to food and water.
Time-course study
Male LDLr−/− mice were fed a high-fat diet (HFD: 45 kcal% lard fat, D12451, Research Diets, New Brunswick, NJ, USA) and were sacrificed after 0, 9 and 16 weeks to collect epididymal WAT (eWAT), mesenteric WAT (mWAT) and inguinal WAT (iWAT). Tissues were prepared essentially as reported5.
Intervention study
Tissues and plasma were obtained from a large cohort study in which rosiglitazone and other interventions (e.g. fenofibrate) were analyzed43. Briefly, one group (n = 9) was sacrificed after 9 weeks of HFD to define the condition prior to intervention (reference, REF). The remaining mice continued on HFD (HFD, n = 13) or HFD supplemented with 0.01% w/w rosiglitazone (HFD + Rosi, n = 9, Avandia, GSK, Zeist, The Netherlands). A separate control group was kept on chow as a baseline control for microarray and RT-PCR gene expression analysis. In week 16, all animals were sacrificed and WAT depots and liver were collected. Mice (n = 2) that did not become obese after 9 weeks of high-fat feeding (i.e. body weight gain 50% less than group mean), were excluded from the analyses.
Histological, biochemical, metabolomics and gene expression analyses
Briefly, WAT characteristics and NAFLD development were quantified histologically as described5,44. Immunohistochemistry was performed on frozen, acetone-fixed WAT sections using primary antibodies specific for CCR2 (PA5-23044, Thermo Fisher Scientific, Rockford, IL, USA) and CD11c (BD553800, BD Biosciences, San Diego, CA, USA). After incubation, biotinylated antibodies were detected by incubation with streptavidin-HRP using Nova Red as a substrate (both, Vector Laboratories, Burlingame, CA, USA). All sections were counterstained with hematoxylin. Immunopositive cells were quantified in four different cross-sections per mouse using ImageJ. Intrahepatic triglyceride concentrations were analyzed by high performance thin-layer chromatography (HPTLC)43. Plasma parameters were determined with commercially available assays as previously specified43. Plasma fatty acids were determined by gas chromatography/mass spectrometry (GC/MS)43. The plasma concentration of total free non-esterified fatty acids (NEFAs) was determined with NEFA-HR kit (Instruchemie, Delfzijl, The Netherlands). Illumina microarray gene expression and subsequent pathway analysis of eWAT and liver was performed following established protocols. To analyze potential off-target effects of rosiglitazone in the liver, an upstream transcriptional activator analysis was performed45. Microarray data were validated and confirmed by RT-PCR and changes in expression were calculated using the comparative Ct (ΔΔCt) method, expressed as fold-change relative to chow.
Statistical analysis
All data are presented as mean ± SEM. Data were analyzed using one-way ANOVA and least significant difference (LSD) post-hoc test. Non-normally distributed data were analyzed by Kruskal-Wallis followed by Mann-Whitney U post-hoc test. Correlations were determined by Spearman’s rank correlation. Statistically significant differences in plasma fatty acids over time within HFD and HFD + Rosi were analyzed using Student’s paired t-test. Statistical tests were performed using Graphpad Prism software (version 6, Graphpad Software Inc., La Jolla, USA). P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Mulder, P. et al. Reduction of obesity-associated white adipose tissue inflammation by rosiglitazone is associated with reduced non-alcoholic fatty liver disease in LDLr-deficient mice. Sci. Rep. 6, 31542; doi: 10.1038/srep31542 (2016).
Supplementary Material
Acknowledgments
The authors would like to thank Erik Offerman, Wim van Duyvenvoorde, Karin Toet. Elvira Fluitsma and Anne Schwerk for their excellent technical assistance. This study was supported by the TNO research programs ‘Enabling Technology Systems Biology’ and ‘Predictive Health Technologies’.
Footnotes
Author Contributions P.Y.W., T.K. and R.K. designed the study. P.M., M.C.M., W.L. and L.V. performed the experiments. P.M., P.Y.W., J.H.v.B., T.K. and R.K. interpreted the data. P.M. and R.K. wrote the manuscript. All authors were involved in critical revision of the drafted manuscript and approved the final version.
References
- Ng M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006). [DOI] [PubMed] [Google Scholar]
- Lumeng C. N. & Saltiel A. R. Inflammatory links between obesity and metabolic disease. J Clin Invest 121, 2111–2117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H. & Moschen A. R. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab 19, 371–379 (2008). [DOI] [PubMed] [Google Scholar]
- Mulder P. et al. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int J Obes (Lond) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays H. E. et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 6, 343–368 (2008). [DOI] [PubMed] [Google Scholar]
- Skurk T., Alberti-Huber C., Herder C. & Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92, 1023–1033 (2007). [DOI] [PubMed] [Google Scholar]
- Cinti S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46, 2347–2355 (2005). [DOI] [PubMed] [Google Scholar]
- Fabbrini E., Sullivan S. & Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolak M. et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56, 1960–1968 (2007). [DOI] [PubMed] [Google Scholar]
- Mirza M. S. Obesity, Visceral Fat, and NAFLD: Querying the Role of Adipokines in the Progression of Nonalcoholic Fatty Liver Disease. ISRN Gastroenterol 2011, 592404 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H. & Moschen A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6, 772–783 (2006). [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. & Glass C. K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72, 219–246 (2010). [DOI] [PubMed] [Google Scholar]
- Kolak M. et al. Effects of chronic rosiglitazone therapy on gene expression in human adipose tissue in vivo in patients with type 2 diabetes. J Clin Endocrinol Metab 92, 720–724 (2007). [DOI] [PubMed] [Google Scholar]
- Xu H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112, 1821–1830 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. T. et al. Regulation of chemokine and chemokine receptor expression by PPARgamma in adipocytes and macrophages. PLoS One 7, e34976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R. et al. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem 283, 22620–22627 (2008). [DOI] [PubMed] [Google Scholar]
- Tiniakos D. G., Vos M. B. & Brunt E. M. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 5, 145–171 (2010). [DOI] [PubMed] [Google Scholar]
- Moylan C. A. et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 59, 471–482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W. et al. Metabolically induced liver inflammation leads to NASH and differs from LPS- or IL-1beta-induced chronic inflammation. Lab Invest 94, 491–502 (2014). [DOI] [PubMed] [Google Scholar]
- Moran-Salvador E. et al. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J 25, 2538–2550 (2011). [DOI] [PubMed] [Google Scholar]
- He W. et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100, 15712–15717 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O. et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278, 34268–34276 (2003). [DOI] [PubMed] [Google Scholar]
- Gupte A. A. et al. Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology 52, 2001–2011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters K. et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 48, 474–486 (2008). [DOI] [PubMed] [Google Scholar]
- Funke A. et al. Cholesterol-induced hepatic inflammation does not contribute to the development of insulin resistance in male LDL receptor knockout mice. Atherosclerosis 232, 390–396 (2014). [DOI] [PubMed] [Google Scholar]
- Caesar R. et al. A combined transcriptomics and lipidomics analysis of subcutaneous, epididymal and mesenteric adipose tissue reveals marked functional differences. PLoS One 5, e11525 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell J. et al. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One 5, e9997 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G., Cheung P., Mozzoli M. & Fried S. K. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism 52, 753–759 (2003). [DOI] [PubMed] [Google Scholar]
- McLaughlin T. M. et al. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring) 18, 926–931 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U. & Hammarstedt A. Antagonistic effects of thiazolidinediones and cytokines in lipotoxicity. Biochim Biophys Acta 1801, 377–380 (2010). [DOI] [PubMed] [Google Scholar]
- Kim H. J. et al. Depot-specific regulation of perilipin by rosiglitazone in a diabetic animal model. Metabolism 56, 676–685 (2007). [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B. A., Brunt E. M., Wehmeier K. R., Oliver D. & Bacon B. R. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 38, 1008–1017 (2003). [DOI] [PubMed] [Google Scholar]
- Belfort R. et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355, 2297–2307 (2006). [DOI] [PubMed] [Google Scholar]
- Cancello R. et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 55, 1554–1561 (2006). [DOI] [PubMed] [Google Scholar]
- du Plessis J. et al. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology 149, 635–648 e614 (2015). [DOI] [PubMed] [Google Scholar]
- Prieur X. et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 60, 797–809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra F. & Lotersztajn S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr Pharm Des 19, 5250–5269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis 28, 179–185 (2010). [DOI] [PubMed] [Google Scholar]
- Shi H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116, 3015–3025 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida I. T., Cortez-Pinto H., Fidalgo G., Rodrigues D. & Camilo M. E. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr 21, 219–223 (2002). [DOI] [PubMed] [Google Scholar]
- Wobser H. et al. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res 19, 996–1005 (2009). [DOI] [PubMed] [Google Scholar]
- Radonjic M. et al. Differential effects of drug interventions and dietary lifestyle in developing type 2 diabetes and complications: a systems biology analysis in LDLr−/− mice. PLoS One 8, e56122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W. et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One 9, e115922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W. et al. Coordinated and interactive expression of genes of lipid metabolism and inflammation in adipose tissue and liver during metabolic overload. PLoS One 8, e75290 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.