Abstract
Reading has been shown to rely on a dorsal brain circuit involving the temporoparietal cortex (TPC) for grapheme-to-phoneme conversion of novel words (Pugh et al., 2001), and a ventral stream involving left occipitotemporal cortex (OTC) (in particular in the so-called “visual word form area”, VWFA) for visual identification of familiar words. In addition, portions of the inferior frontal cortex (IFC) have been posited to be an output of the dorsal reading pathway involved in phonology. While this dorsal versus ventral dichotomy for phonological and orthographic processing of words is widely accepted, it is not known if these brain areas are actually strictly sensitive to orthographic or phonological information. Using an fMRI rapid adaptation technique we probed the selectivity of the TPC, OTC, and IFC to orthographic and phonological features during single word reading. We found in two independent experiments using different task conditions in adult normal readers, that the TPC is exclusively sensitive to phonology and the VWFA in the OTC is exclusively sensitive to orthography. The dorsal IFC (BA 44), however, showed orthographic but not phonological selectivity. These results support the theory that reading involves a specific phonological-based temporoparietal region and a specific orthographic-based ventral occipitotemporal region. The dorsal IFC, however, was not sensitive to phonological processing, suggesting a more complex role for this region.
Keywords: reading, orthography, phonology, VWFA, neural specificity, homophones
1 Introduction
Much attention and research has been devoted to trying to understand the processes and neural mechanisms involved in reading (Bolger et al., 2005; Houdé et al., 2010; Jobard et al., 2003; Martin et al., 2015; Turkeltaub et al., 2002). A widely held theory is that beginning readers heavily rely on phonological information already acquired through speech and language development and apply this knowledge for the mapping of phonemes to graphemes (Bartl-Pokorny et al., 2013; Goswami, 2008; Ziegler and Goswami, 2005). At the neuroanatomical level a left hemisphere dorsal brain circuit involving the temporoparietal cortex (TPC) and the dorsal aspect of the inferior frontal cortex (IFC), is thought to subserve these aspects of phonological analysis and phonological recoding, respectively, allowing the beginning reader to decode novel words (Pugh et al., 2001; Schlaggar and McCandliss, 2007). As children become skilled readers, they are thought to utilize learned visual experiences to draw on word representations (orthographic units) in the left ventral occipitotemporal cortex (OTC), in particular in the so-called “visual word form area”, VWFA (Dehaene and Cohen, 2011; McCandliss et al., 2003). This allows for direct, rapid identification of frequently encountered written words (those represented in the child’s sight word vocabulary) without the need for phonological decoding. This facilitates reading fluency, which in turn is thought to lead to increased reading comprehension. A recent meta-analysis of reading and reading-related tasks demonstrates that studies of adults show more consistent findings in the OTC than children, supporting the idea of a shift to the OTC in advanced literacy (Martin et al., 2015). The same meta-analysis also showed relatively greater consistency across studies in children in left superior temporal gyrus, indicating the need for beginning readers to use grapheme-to-phoneme mapping.
A key prediction of this theory is that the TPC and dorsal IFC show sensitivity to phonological features whereas the OTC containing the VWFA holds orthographic representations and not phonological representations. However, it has been debated, whether the OTC is part of the grapho-phonological route, processing at the sublexical level (Warrington and Shallice, 1980). It might also encode or be modulated by non-orthographic stimuli, in particular phonological information (Price and Devlin, 2011; Twomey et al., 2011). This brain region has been shown to be modulated by phonological demands, such as pseudoword over real word reading (Dietz et al., 2005; Hagoort et al., 1999) and pseudoword over real word rhyming (Xu et al., 2001). These results may reflect local sensitivity to the phonological aspects of the stimuli, but could also reflect task-dependent top-down modulations from other brain regions more directly involved in phonological processing. The topic of orthographic versus phonological selectivity in other brain regions involved in reading also merits further investigation. Whereas it is largely agreed that the TPC is an important area for decoding and phonological processing (Fiez and Petersen, 1998; Pugh et al., 2001; Schlaggar and McCandliss, 2007; Turkeltaub et al., 2003), less is known about the role of the IFC. Both the ventral and dorsal pathways converge onto the inferior frontal gyrus (IFG), which has been shown to be active during reading in both developing and skilled readers (Martin et al., 2015; Turkeltaub et al., 2003). It is thought that the ventral IFG, which includes the pars orbitalis and the pars triangularis (BA 47 and 45), subserves semantic processing (Poldrack et al., 1999) and is thought to be important for mapping orthographic-lexical stimuli onto semantic representations (Sandak et al., 2004). On the other hand, the dorsal IFG, which includes the pars opercularis (BA 44), is involved in phonological processing, specifically word decoding (Fiez and Petersen, 1998; Jobard et al., 2003), phonological access (Wu et al., 2012), phonological decision making (Poldrack et al., 1999; Rumsey et al., 1997) and phonological output (Taylor et al., 2012).
A problem that has plagued prior studies in determining phonological or orthographic involvement of these areas during word processing is that written word stimuli are associated with both phonological and orthographic information, and as such, make it difficult to identify representations unique to orthography and phonology (Rumsey et al., 1997).
To resolve this ambiguity and better understand the nature of word representations with regards to orthographic and phonological selectivity in the OTC, TPC, and IFC, we conducted two fMRI rapid adaptation (fMRI-RA) experiments (Krekelberg et al., 2006). In fMRI-RA experiments, two stimuli are presented sequentially in each trial. If the two stimuli activate overlapping neuronal populations, neurons that are activated by both stimuli show adaptation on the second presentation (also referred to as repetition-suppression), leading to a smaller blood oxygenation level dependent (BOLD)-contrast response following the second stimulus relative to the first (Dehaene et al., 2001, 2004; Grill-Spector et al., 2006). Specifically, the BOLD-contrast response to the pair is taken to reflect similarity of the neuronal activation patterns corresponding to the two individual stimuli, with the lowest response for two stimuli activating identical neuronal populations, and maximum signal if the two stimuli activate disjoint groups of neurons. Adaptation has also been used to infer if the BOLD signal is coming from a population of neurons processing a specific attribute of the stimulus, as opposed to another population tuned to a different attribute (Grill-Spector et al., 1999). As such fMRI-RA provides a tool for characterizing functional properties of neural populations (Grill-Spector and Malach, 2001) and has been applied to determine the specialization of brain areas involved in face (Jiang et al., 2006) and word processing (Glezer et al., 2009).
We have previously used the fMRI-RA approach to examine neural selectivity to real words over pseudowords in the VWFA (Glezer et al., 2009), as well as changes in this region during the learning of new words (Glezer et al., 2015). These results provide evidence that the VWFA contains neurons highly tuned to familiar words: Two real words (or two pseudowords learned by the participant,) that differ by just one letter cause as little adaptation as two words that share no letters at all, suggesting that a high degree of specificity of tuning exists in the VWFA such that familiar words are represented by disjoint groups of neurons (Glezer et al., 2009, 2015). Here we extended this work to include words that are homophones, as a way to examine neuronal selectivity to orthographic versus phonological properties of words. A pair of homophone words (e.g. rain and reign) has the same phonology but differ in orthography. Therefore those neurons that code phonological information will respond both times, with the presentation of the phonologically identical second stimulus resulting in an adaptive (lower) signal. However, neurons that are tuned to the orthography of words would not show adaptation since the second stimulus differs in orthography, thereby not causing adaptation.
The design of the current study focused on three regions of interest (ROI) that were defined under separate experimental conditions on a single-subject basis, using functional localizers of phonological (TPC and IFC) or orthographic (OTC) processing. These ROI were examined for responses under three types of word pairs. First, consider the sequential presentation of identical words, such as “bike”-”bike”, which have the exact same orthography and phonology. Whether a particular ROI contained neurons responsive to orthographic processing only, phonological processing only, or both, these two stimuli would repeatedly activate the same neural populations in all three cases, resulting in the maximum amount of adaptation possible (Glezer et al., 2009). Secondly, consider the other extreme, that is, the sequential presentation of two completely different words (with no letters or phonemes in common, e.g., bike-dog). In that case, there should be no adaptation in the BOLD response to the second word in the pair, irrespective of whether the ROI contained neurons tuned to phonology, orthography or both, as both stimuli are different in phonology and orthography relative to the first (no repetition suppression). The interesting case for our study is a third condition, in which the sequentially presented words are homophone pairs (e.g. rain-reign). Here, the two words share the same phonology, but have different orthography. For neurons tuned to phonological information, an adaptation response would ensue at the same level as for identical word pairs, since the same phonological information is presented twice. In contrast, in an area showing selectivity exclusively for orthographic information, word pairs that share the same phonology but have different orthography would be expected to activate different populations of neurons, leading to a release from adaptation and higher BOLD signal.
We conducted two separate experiments, with each experiment involving different participants. Both experiments used fMRI-RA with the different kinds of word pairs described above and both examined three, functionally-defined brain areas known to be critical in reading (TPC, IFC and OTC) for their specific role in phonological or orthographic processing of words. Importantly, both experiments used oddball detection tasks for which the inter-item similarity in an RA stimulus pair was irrelevant (as participants had to analyze each word equally to determine whether it was an oddball word), and top-down factors therefore were not expected to vary across different RA conditions. Indeed, as we have shown previously (Glezer et al., 2009), this produces highly consistent profiles for release from adaptation for real words even if total response amplitude varies, e.g., due to variation of attention or task difficulty. In particular, we have previously shown robust patterns of release from adaptation in the VWFA (compatible with highly selective tuning for real words) irrespective of whether participants performed a semantic or an orthographic task. In the current study, the two experiments employed different oddball tasks, with one experiment using an orthographic, and the other using a phonological task, allowing us to investigate the robustness of selectivity for orthographic and phonologic stimulus attributes across the reading system across these different tasks.
2 Material and Methods
We conducted two fMRI-RA experiments to probe the nature of the representation in the TPC, the dorsal IFG in the IFC, and the VWFA. Experiment 1 was conducted in one group of adults and used a phonological oddball task and Experiment 2 involved another group of adults and used an orthographic oddball task. Importantly, both experiments presented pairs of words that had either (i) same orthography and phonology (same word), (ii) different phonology and different orthography (different words) or (iii) same phonology and different orthography (homophone).
2.1 Participants
Eleven participants were run in Experiment 1 and 16 in Experiment 2. All participants were recruited through fliers posted on the university campus. The Georgetown University Institutional Review Board approved all experimental procedures, and written informed consent was obtained from each individual prior to testing. All participants were monolingual English speakers with good reading skills and in good physical health. Participants were excluded from further analysis if they had excessive head motion (see Methods) or if they were not able to stay awake for the experimental scanning sessions. The fMRI data from 1 participant for Experiment 1 and 3 participants for Experiment 2 were excluded for these reasons. Therefore the results from 10 participants for Experiment 1 and 13 for Experiment 2 are reported here.
2.2 MRI Acquisition
MRI data were acquired at The Center for Functional and Molecular Imaging (CFMI), Georgetown University Medical Center on a 3T Siemens Trio scanner with a 12-channel head coil. For both the functional localizers and the fMRI-RA, 35 interleaved axial slices (4mm thick, no gap; 3.2×3.2mm2 in-plane resolution) were acquired for fMRI data using an echo-planar sequence (flip angle=90°, TR=2.04s, TE=29ms, FOV=205°, 64×64 matrix). For the event-related fMRI-RA scans, trial order was randomized and counterbalanced using M-sequences (Buračas and Boynton, 2002) and the number of presentations was equalized for all stimuli in each experiment. Stimuli were presented using E-Prime (www.pstnet.com), back-projected on a screen located at the rear of the scanner, and viewed through a headcoil-mounted mirror.
2.3 Functional Localizers design, stimuli and individual ROI Selection
For Experiment 1 and 2 functional localizers were used to identify brain regions in the left hemisphere involved in phonological or orthographic processing on a single subject basis to create ROI by which to focus the fMRI-RA data analysis. Intersubject variability in the location and size of such ROI, particularly the VWFA, has been shown to hamper analysis approaches that do not take individual variability into account (Glezer and Riesenhuber, 2013). Localizer ROI were acquired with a traditional block design fMRI. We were interested in separately identifying ROI associated specifically with orthographic processing (VWFA) and also identifying ROI specifically associated with phonological processing (IFC and TPC). To do this, we needed to include one localizer to identify orthographic regions, which would comprise stimuli, tasks, and contrasts that would allow us to identify ROI associated with orthography (e.g. all written words > fixation) and also separately include another localizer to identify phonological regions which would include a different set of stimuli, tasks and contrasts to identify ROI associated with phonological processing (e.g. rhyming > fixation). Generally speaking, the approaches were similar across both experiments, with the goal of identifying ROIs in the left hemisphere that were functionally responsive during phonological (TPC and IFC) and orthographic (VWFA in OTC) processing. There were minor differences between the localizers employed in the two experiments, described in detail below. To briefly summarize here, Experiment 1 included fixation, scrambled images of words, and three conditions with written words in which the participant needed to perform three different tasks depending on the set of words (matching, rhyming, meaning). All of these conditions were included in different blocks within each run of the localizer (two runs were presented in total). In Experiment 2 we conducted separate orthographic and phonological localizers in different runs. Because of these different designs between the experiments we needed to define the ROI slightly differently across the experiments (described below). Yet, results were consistent across both experiments.
2.3.1 Experiment 1 Design and Stimuli
Functional localizers (for phonological and orthographic regions) were derived from data acquired in two runs. Following an initial 14.28s fixation period, ten pairs of words or ten pairs of visually scrambled words (pixel-scrambled words, tile size: 2×2 pixels) (Glezer et al., 2009) were presented to participants in blocks of 30.6s, separated by a 14.28s fixation block. Within each block, each pair of images were displayed for 1500ms (one above and one below a fixation cross that was located at the center of screen, followed by a 1560ms blank screen). Each block began with a 2040ms cue, which instructed participants to perform one of the following tasks in the block: Word Matching – participants needed to decide whether the two simultaneously presented words were the same or different; Word Rhyming – participants needed to decide whether the two words in a pair rhymed with each other; Word Meaning – participants needed to decide whether the two words in a pair belonged to the same or different semantic category; Scrambled Words – participants needed to decide whether the two visual patterns (pixel-scrambled words) in a pair had the same or different contrast. This last condition served as a visual control.
For the Word Matching task, sixty 3–6 letter high and low frequency words were used and there were two conditions – 1) same (two words were the same, e.g., desk-desk), and 2) different (two words differing by one letter, e.g., desk-disk); For the Word Rhyming task, eighty 3–6 letter, low and high frequency words were used, and there were four conditions – 1) words with the same orthography that rhymed (e.g. cave-save), 2) words with different orthography that rhymed (e.g. bows-toes), 3) words with the same orthography that did not rhyme (hose-lose), and 4) words with different orthography that do not rhyme (plow-fear). For the Word Meaning task, forty high frequency animate and forty high frequency inanimate words were used, and there were two conditions – both words in a pair belonged to 1) the same category (Animate vs Inanimate) (e.g., lion-fish, or shoe-book), or 2) different category (e.g., lion-book). Finally, for the Visual Pattern task, pixel-wise scrambled images from sixty words were used, and there were two conditions – the two scrambled images were 1) the same image with the same contrast, or 2) different images with additional 30% difference in contrast. Each condition was repeated twice in a pseudorandomized order in each run with new stimuli not seen before during the scanning session, which lasted for 389.64s, and participants were asked to perform the tasks according to the cues while keeping their fixation at the center of the screen.
2.3.2 Experiment 1 ROI Selection and Identification
the left hemisphere TPC and IFC ROI were identified for each participant individually using the contrast of Word Rhyming > Fixation (p<0.00001, uncorrected) masked by Word Rhyming > Word Matching (p<0.05, uncorrected). This contrast typically yielded 1–2 ROI in the TPC and 2–3 in the IFC. ROI in these regions were selected by identifying in each participant the cluster that was significant at the corrected cluster-level of at least p<0.05 in the TPC and IFC in a location closest to those regions identified in previous literature as being involved in phonological processing during reading (Paulesu et al., 2014; Richlan et al., 2011) (these ROI also show reduced activation in dyslexia). In order to select ROI that were of equivalent size (Goh et al., 2010; Park et al., 2004) the thresholds were further adjusted in order to obtain a cluster size that was between 10 and 100 voxels. The VWFA region was identified for each individual participant independently with the data from the localizer scans, using the contrast of Word Processing (Word Matching, Word Rhyming and Word Meaning combined) versus Fixation (p<0.00001, uncorrected) masked by the contrast of Word Processing (data acquired during Word Matching, Word Rhyming and Word Meaning combined) versus Visual Pattern (scrambled words task) (p<0.05, uncorrected). This contrast typically resulted in only 1–2 foci in the left ventral occipitotemporal cortex (p<0.05, corrected). ROI were selected by identifying in each participant the most anterior cluster that was significant at the corrected cluster-level of at least p<0.05 in the ventral occipitotemporal cortex (specifically, the occipitotemporal sulcus/fusiform gyrus region) in a location closest to the published location of the VWFA, approximate Talairach coordinates −43 −54 −12 ±5 (Cohen and Dehaene, 2004). Thresholds were adjusted in order to obtain a cluster that was between 10–50 voxels.
2.3.3 Experiment 2 Design and Stimuli
Two functional localizers (one phonological and one orthographic) were obtained. Each localizer included two separate pseudorandomized runs (therefore 4 runs in total were run for localizers). The first localizer was a phonological localizer used to identify the IFC and TPC. During each of the two runs for this localizer, following an initial 14.28s fixation period, ten pairs of words or ten pairs of line drawings were presented to participants in blocks of 20.4s, separated by a 14.28s fixation block. There were ten trials within each block, and a pair of images (each containing either a word or a set of lines, see below) was presented sequentially within each trial. Each trial began with a 100ms fixation, then the first stimulus was presented for 300ms, followed by a 200ms blank screen, and then the second stimulus for another 300ms, which was followed by a 1140ms blank screen before the onset of next trial. Each block began with a 2040ms cue, which instructed the participants to perform one of following tasks in the block: Word Rhyming – participants needed to decide whether the two words in a pair rhymed; Semantic – participants needed to decide whether the two words in a pair belonged to the same or different semantic categories (animate vs. inanimate); Line Judgment – participants needed to decide whether the two sets of line drawings were same or different. TPC and IFC regions of interest were defined by contrasting Word Rhyming > Line Judgment masked by Word Rhyming > Fixation. The Semantic stimuli were not used for analysis for this study.
The second localizer was an orthographic localizer used to identify the VWFA identical to prior reports (Glezer and Riesenhuber, 2013; Glezer et al., 2009, 2015). Briefly, participants passively viewed blocks of images of Written Words (high frequency nouns, >50 per million, different from those used in the event-related scans), Scrambled Words, Faces, and Objects. Each block lasted 20.4s (stimuli were displayed for 500ms and were separated by a 100ms blank interval), and stimulus blocks were separated by a 10.2s Fixation block. There were two pseudorandomized runs for this localizer and each run consisted of 2 blocks of each stimulus type (words, scrambled words, faces, objects) and 8 fixation blocks. Each stimulus block contained a new set of stimuli not seen before in the scanning session. ROI were identified for each individual participant independently by contrasting Written Words versus Fixation masked by the contrast of Written Words versus Scrambled Words. The faces and objects stimulus categories were not used for analysis for this study and details on ROI identification are provided below.
2.3.4 Experiment 2 ROI Selection and Identification
The ROI in the left hemisphere were again identified for each individual participant independently. This time, due to different rhyming words than in Experiment 1 (consisting of less difficult rhymes) and baseline stimuli (lines), we use a slightly different contrast than in Experiment 1 to identify the TPC and IFC, namely the contrast of Word Rhyming > Line Judgment (p<0.00001, uncorrected) masked by Word Rhyming > Fixation (p<0.05, corrected). To identify the VWFA, we used a similar procedure as for Experiment 1, based on the contrast of Written Words versus Fixation (p<0.00001, uncorrected) masked by the contrast of Written words versus Scrambled Words (p<0.05, uncorrected), with VWFA ROI selection as described above in Experiment 1.
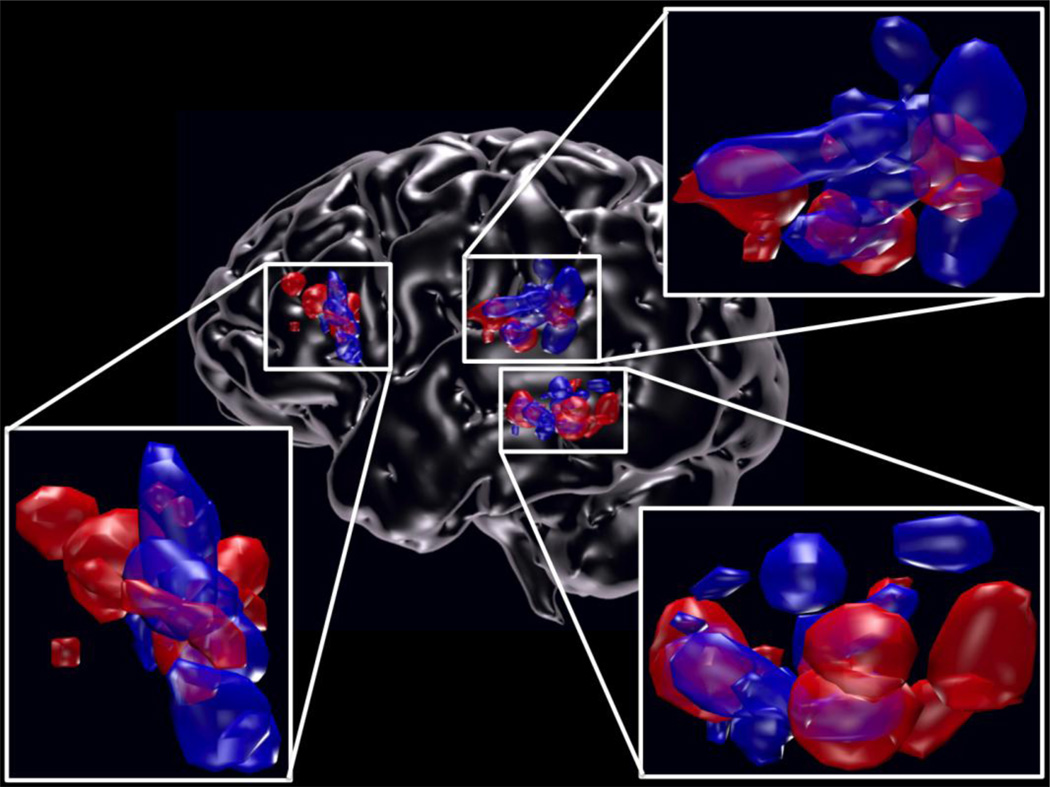
Using these criteria for ROI selection, we were able to obtain VWFA ROI in all participants for both experiments, and we were able to obtain 9/10 and 11/13 TPC ROI and 10/10 and 11/13 IFC ROI for Experiment 1 and 2, respectively. For visualization in Figure 1, ROI and glass brain were rendered using Blender (http://www.blender.org).
Figure 1.
Individual ROI locations of the VWFA, IFC, TPC. Lateral view of left hemisphere showing the locations of all of the participants’ individual ROI for the VWFA (n=23), IFC (n=21), and TPC (n=20) in Experiment 1 (blue) and Experiment 2 (red).
2.4 fMRI-RA Experiment 1
MRI images from four event-related (ER) scans were collected for each participant. Each run lasted 579.36s and had two 30.6s fixation periods, one at the beginning and the other at the end. Between the two fixation periods, a total of 127 trials were presented to participants at a rate of one every 4.08s. During each trial, following a 1000ms fixation, two words were displayed sequentially (400ms each with a 200ms blank screen in-between), and followed by a 2080ms blank screen. For each run, there were 25–26 trials each condition. There were three conditions of interest (making a “triplet”) of varying intra-pair stimulus similarity: 1) the same target repeated, such as pole-pole (S), 2) a different word from the target, such as poke-pole (D), and 3) a homophone of the target, such as poll-pole (H). The different word (D) stimulus always contained the same number of overlapping and repeated letters (i.e. matched for identity and position) as the H stimulus. All the words in the triplet were one syllable and had the same orthographic and phonological length. All lists were matched for part-of-speech (POS) which was based on the English Lexicon Project (Balota et al., 2007), and frequency and orthographic neighborhood were based on MCWord (Medler, D.A., & Binder, 2005). For the POS matching, all words occurred as nouns, verbs or adjectives or a combination. Word lists were matched based on the most frequently occurring POS for each word. Trial order was randomized and counterbalanced using M-sequences. To engage participants’ attention yet avoid potential task-related confounds of the BOLD-contrast response to the conditions of interest (Grady et al., 1996; Sunaert et al., 2000), participants were asked to read all words silently and perform an “oddball” detection task (Glezer et al., 2009; Jiang et al., 2000; Xue et al., 2006) in the scanner. In this experiment, a phonological oddball task was used and participants were asked to press a button with their right hand every time they saw a two-syllable word (all experimental stimuli were one-syllable words, yet there were no differences in word length between target and non-target words). The two-syllable oddball stimulus appeared as either the first or the second one of the pair of words.
2.5 fMRI-RA Experiment 2
MRI images from four ER scans were collected for each participant. Each run lasted 558.96s and had two 20.4s fixation periods, one at the beginning and the other at the end. Between the two fixation periods, a total of 127 trials were presented to participants at a rate of one every 4.08s. During each trial, two words were displayed sequentially (300ms each with a 400ms blank screen in-between), and followed by a 3080ms blank screen. For each run, there were 25–26 trials for each condition. There were three conditions of interest of varying intra-pair stimulus similarity, just as in Experiment 1. All lists were matched and trial order was randomized as in Experiment 1. The same oddball task was used as in Experiment 1, however this time an orthographic oddball task was used. Participants were asked to press a button every time they saw a word in which three letters were replaced with the letter strings, “abc” or “xyz”.
All stimuli for localizers and fMRI-RA for both experiments were rendered in Courier font (36 point size, 100 ppi), average letter size ¼ × ¼ inch (25 × 25 pixels), for an approximate size of 0.5 degrees of visual angle per letter in the scanner.
2.6 General MRI Data Preprocessing and Analysis: MRI data analysis
For both the functional localizers and the fMRI-RA scans, all preprocessing and most statistical analyses were done using the SPM2 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm2/). After discarding the first five acquisitions of each run, the EPI images were temporally corrected to the middle slice (for event-related scans only), spatially realigned, resliced to 2 × 2 × 2 mm3 and normalized to a standard MNI reference brain in Talairach space. Images were then smoothed with an isotropic 6mm Gaussian kernel. Participants who had > 1 mm scan-to-scan motion for more than 25% of the scan were excluded from further analysis. After removing low frequency temporal noise from the EPI runs with a high pass filter (1/128Hz), fMRI responses were modeled with a design matrix comprising the onset of trial types and movement parameters as regressors using a standard canonical hemodynamic response function (HRF). For the fMRI-RA ER scans a proportional scaling was applied to remove the effects of global variations (Aguirre et al., 1998) as we expected differences between the conditions of interest to be small and limited to local VWFA regions based on previous findings (Glezer et al., 2009). We then extracted the mean percent signal change of the VWFA, TPC and IFC ROI for each participant with the MarsBar toolbox (Brett et al., 2002), see below, and conducted statistical analyses within-subject repeated measures ANOVA with Greenhouse-Geisser correction, followed by planned t-tests, a=0.05, two-tailed) on the percent signal change.
3 Results
Locations of the three individually defined ROI of interest (TPC, IFC and VWFA in the OTC) for all the participants from both experiments are shown in Figure 1. The average MNI location with standard deviation for the 3 ROI for Experiments 1 and 2, respectively, were: angular /superior temporal gyri in the TPC (BA 39/22) −57±6 −49±6 12±6 and −55±6 −44±9 9±5; IFC (BA 44) −53±4 8±3 10 ±6 and −51±5 13±8 15±8, both located in the pars opercularis; VWFA in the OTC (BA 37) −47±4 −55±7 −18±4 and −44±5 −61±9 −16±5. There was no significant difference between coordinate location in the x, y, or z planes for any of the three ROI between the two experiments (p>0.05, one-way ANOVA).
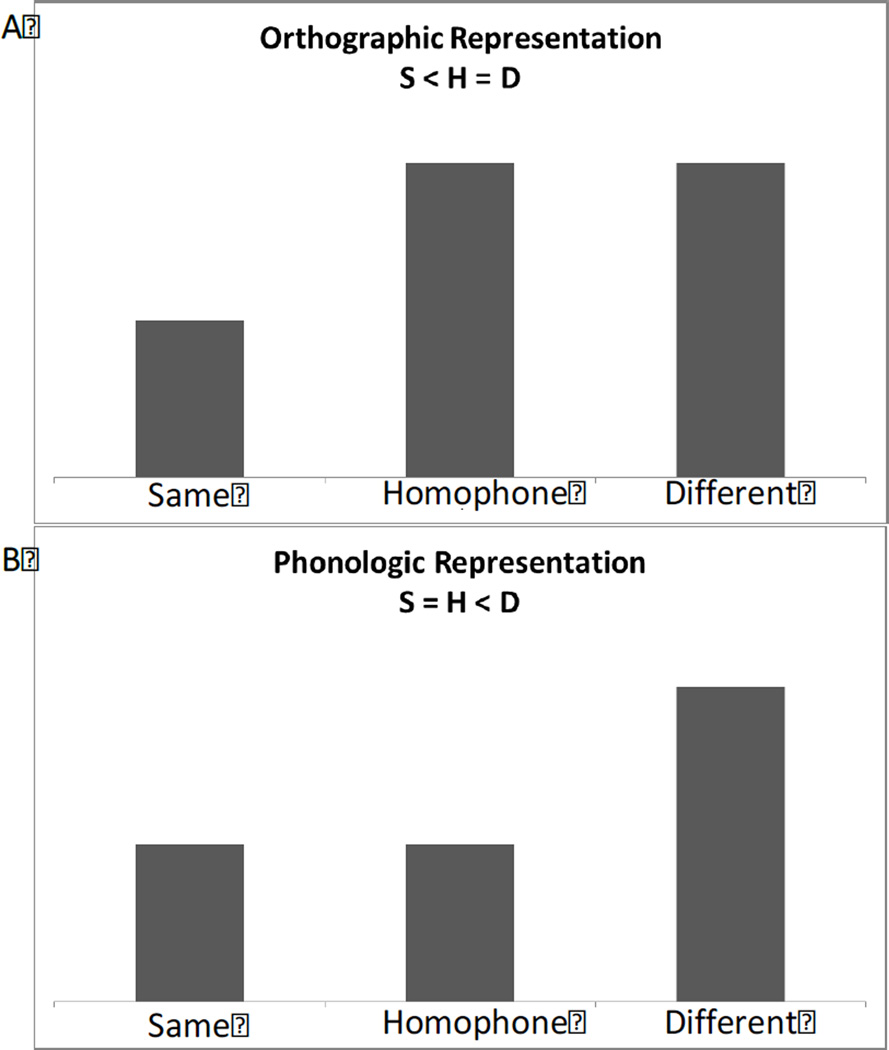
In all ROI for both experiments, we predicted that signal would be lowest in the same (S) condition, as the two word stimuli were identical and would therefore repeatedly activate the same neural populations, causing maximum adaptation (Glezer et al., 2009). On the other hand, we predicted the least amount of adaptation for the different (D) condition because the two word stimuli differ in orthography and phonology. The second stimulus would therefore be activating a new subpopulation of neurons. The crucial prediction that differentiates an orthographic representation from a phonological representation is the response of the homophone (H) condition: For regions tasked with phonological processing, the averaged response would be equivalent to that observed for processing of the same words (S) given the identical phonology of word pairs in the H condition. That is, even though the two words in an H pair differ in orthography, an ROI tuned to phonology should treat them identically, leading to maximum adaptation, as in the S condition. In contrast, in brain areas showing orthographic whole-word selectivity, like the VWFA (Glezer et al., 2009), responses to the H condition should be identical to the D condition, given the orthographic differences of H word pairs. Finally, areas that have sensitivity to phonology and orthography should exhibit a pattern where S < H < D (see Figure 2).
Figure 2.
Predictions for fMRI-RA response to the different conditions for orthographic (A) and phonological (B) representations.
3.1 TPC: Phonological but not orthographic selectivity
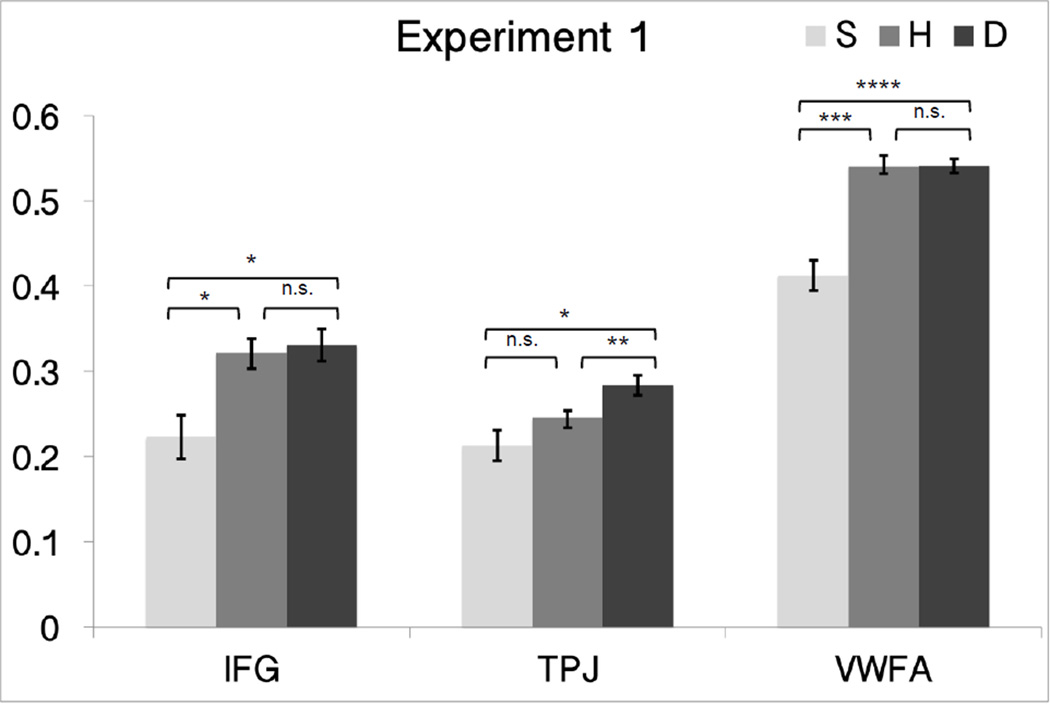
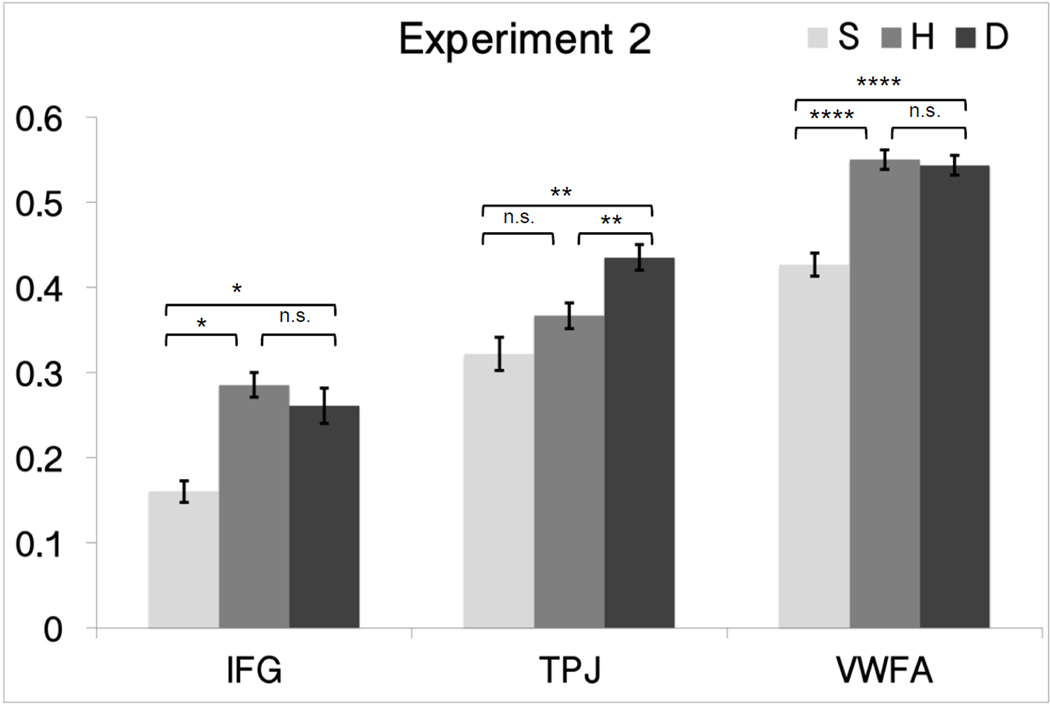
As expected, we observed responses modulated by phonology in TPC, with full adaptation for the H condition in both Experiments 1 and 2. That is, adaptation to H pairs was as strong as in the S condition – the difference in orthography in H did not lead to a release from adaptation; instead, only phonological similarity mattered for TPC responses. H was similar in response to S (the two were not significantly different from each other in both Experiments 1 and 2; p> 0.153) and both H and S were significantly lower than D in both Experiment 1 and 2 (Experiment 1, H: p=0.005 and S: p= 0.041, Experiment 2, H: p=0.008 and S: p= 0.003).
Further, the mixed-design ANOVA analyses showed similar response profiles between the two experiments: Condition (F(2,36)=13.281, p<0.001), Experiment (F(1,18)=2.189, p=0.156), and Condition × Experiment (F(2,36)=0.763, p=0.446). These results indicate that the TPC is sensitive to phonological features during reading, irrespective of task.
3.2 IFC: Orthographic, but not phonological sensitivity
Unexpectedly, the IFC ROI, even though defined by a phonological localizer, showed no phonological tuning in both experiments. In fact, its response profile was what one might expect in a region involved in orthographic processing: S was significantly lower than D and H (p < 0.022); and D and H were not significantly different (p > 0.44 for both experiments).
Results were consistent for both Experiment 1 and 2, as again the mixed-design ANOVA analyses revealed no difference between the two experiments: Condition (F(2,38)=18.587, p<0.001), Experiment (F(1,19)=0.283, p=0.601), and Condition × Experiment (F(2,38)=0.375, p=0.674). These results suggest that, counter to expectations, this dorsal portion of the IFC, in the pars opercularis, while responsive to words in phonological tasks (as used in the localizer), is not tuned to phonology, but instead demonstrated sensitivity to orthography, irrespective of task.
3.3 VWFA: Orthographic, but not phonological sensitivity
As expected, the response in the VWFA ROI showed whole word orthographic tuning in Experiment 1 (Figure 3) and Experiment 2 (Figure 4), compatible with prior studies (Glezer et al., 2009, 2015). That is, there was no adaptation for the H condition (D vs. H was not significant, paired t-test p > 0.678 for both experiments).
Figure 3.
Plots of mean percent signal change in relation to orthographic and phonological similarity in the fMRI-RA scans for Experiment 1 (phonological task) in the VWFA, IFC, TPC. Error bars represent within-subject SEM.
* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Figure 4.
Plots of mean percent signal change in relation to orthographic and phonological similarity in the fMRI-RA scans for Experiment 2 (orthographic task) in the VWFA, IFC, TPC. Error bars represent within-subject SEM.
* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
We conducted a mixed-design ANOVA of VWFA responses, with two factors, one within-subject factor, Condition (S vs. H vs. D), and one between-subject factor, Experiment (Experiment 1 vs. 2). These additional analyses revealed similar response profiles between the two experiments: Condition (F(2,42)=55.248, p<0.001), Experiment (F(1,21 )=0.015, p=0.904), and Condition × Experiment (F(2,42)=0.092, p=0.880). Together, these results provide evidence that the VWFA is not modulated by phonology and is highly tuned to orthographic information, independent of whether participants engage in an orthographic or phonological task.
3.4 Overall Between-Experiment Comparisons
Finally, to bring all these findings for the ROIs across the two experiments together, we conducted repeated-measures ANOVAs to compare the neural tuning in the three ROI for both experiments, with two within-subject factors, conditions (S vs. H vs. D), and ROI (VWFA vs. TPC vs. IFC), and one between-subject factor, Experiments (Experiment 1 vs. 2). This analysis revealed a significant effect of conditions (F(2,34) = 42.701, p<0.001) and ROI (F(2,34) = 11.359, p=0.001), and a significant interaction between ROI and conditions (F(4,68) = 3.611, p=0.018), but no significant interactions between conditions and Experiments (F(2,34) = 0.162, p>0.8), nor between ROI and experiments (F(2,34) = 1.580, p>0.2), nor between the three factors (F(4,68) = 0.988, p>0.4). We ran post-hoc tests to confirm which regions and for which contrast they differed by conducting three 2×2 ANOVAs (region × condition: VWFA vs. IFC × S vs. H; VWFA vs. TPC × S vs. H; IFC vs. TPC × S vs. H). The results show that there were significant interactions for VWFA and TPC (F(1,19)= 8.82, p=0.008) and IFC and TPC (F(1,18) = 10.07 p=0.005), but not IFC and VWFA (F91,20 = 0.14 p= 0.713). These post-hoc tests confirm that the adaptation profiles in the VWFA and IFC differ from that in the TPJ while the VWFA and IFC do not differ from each other. These results confirm that ROI differed in their selectivity profiles, and that these differences were consistent across the two experiments.
4 Discussion
This study employed fMRI-RA to address the sensitivity to phonological and orthographic information in regions known to be involved in reading. As expected, the TPC was found to be tuned to phonological factors of the written word. We found the VWFA in the OTC to be highly sensitive to orthography, consistent with our previous studies (Glezer and Riesenhuber, 2013; Glezer et al., 2009, 2015) and compatible with its location in high-level visual cortex (Dehaene and Cohen, 2011; Vinckier et al., 2007). This extends previous findings to show that the VWFA is not modulated by phonology, demonstrating that it is truly a visual word form area. Surprisingly we found that the IFC did not exhibit a phonological profile, instead it showed a profile that suggests orthographic selectivity. The results from all three ROI were consistent across Experiment 1 and 2, demonstrating that they are not dependent on the task performed during the experiment (phonological or orthographic).
4.1 Temporoparietal Cortex
It is well known that the TPC is involved in phonological processing (Vigneau et al., 2006) and phonological decoding during reading (Jobard et al., 2003; Taylor et al., 2012). Our results of selectivity to phonological information in this region are therefore not surprising; however, our approach avoids any concern that orthographic processing may inadvertently be involved. Specifically, the phonological functional localizer included left superior temporal and angular gyri (AG), a location that is consistent with previous reports of activity during phonological processing and reading (Pugh et al., 2001; Sandak et al., 2004). It is widely believed that these regions supports grapheme-to-phoneme mapping, allowing the reader to pronounce regular, novel words (Herbster et al., 1997) via the “indirect route” (Coltheart and Rastle, 1994). The AG has been featured in the reading literature for many years, with Dejerine (Dejerine, 1892) hypothesizing that this region stores visual letter shapes and is critical in linking letters to sounds. While brain imaging studies have not characterized the AG as a site for storage of visual information about letter or word shapes, they have found the AG to be involved in phonological analysis and grapheme-phoneme mapping (Booth et al., 2003). Our results clearly demonstrate specificity to phonology over orthography in the AG and STG. The STG is thought to be especially important for beginning reading in children (Bitan et al., 2007; Martin et al., 2015; Turkeltaub et al., 2003) and for readers of alphabetic relative to logographic languages (Bolger et al., 2005), especially those alphabetic languages that are transparent (shallow orthography) thereby relying on regular sound-spelling mapping (Paulesu et al., 2000). Due to its proximity to auditory cortex, STG is thought to rely on sensory information and areas involved in speech perception (Hickok and Poeppel, 2007). Together the TPC likely hosts a range of phonological-based skills and, while our results do not speak to each specifically, they do address the specificity of this region to the sound structure associated with visual word stimuli.
4.2 VWFA
We have shown previously (Glezer et al., 2009, 2015) that the VWFA is highly sensitive to orthographic information. Specifically, we have shown that rather than processing prelexical information, such as commonly occurring letter combinations (Binder et al., 2006; Cohen and Dehaene, 2004; Dehaene and Cohen, 2011) the VWFA is finely tuned to whole real words (Glezer et al., 2009) and that experience with words shapes the representation in this region (Glezer et al., 2015). Similarly, studies conducted by Kronbichler and colleagues (Kronbichler et al., 2007, 2009) showed an orthographic familiarity effect in the VWFA (using real words and pseudohomophones) indicating that the VWFA is sensitive to whole words. Such findings were also reported by Bruno and colleagues who showed an orthographic familiarity effect in adults using real words, pseudowords, and pseudohomophones (Bruno et al., 2008) and van der Mark and colleagues who showed an orthographic familiarity and skill-level effect in children (Bruno et al., 2008; van der Mark et al., 2009). At the same time, some have raised the question of whether the VWFA is involved in phonological aspects of word reading, noting that it is more active for pseudowords than words (Binder et al., 2005; Herbster et al., 1997). Our current study allows us to conclude that the role of the VWFA is purely orthographic in nature and is not modulated by phonological information or task demands.
4.3 IFC
Interestingly, we found similar degrees of orthographic selectivity in the IFC as in the VWFA, even though the IFC ROI was identified using a phonological localizer. Our expectation was that this dorsal aspect of the IFG would show selectivity to phonological aspects of the stimuli, based on prior studies implicating it in phonological processing (Bokde et al., 2001; Fiez, 1997; Fiez et al., 1999; Katzev et al., 2013; McDermott et al., 2003; Newman and Joanisse, 2011; Poldrack et al., 1999). While previous studies have attributed the pars opercularis in the inferior frontal cortex to phonological processing during reading including grapheme-to-phoneme conversion (Dietz et al., 2005; Fiebach et al., 2002; Heim et al., 2005; Joubert, 2004) our study suggests a functional specialization for orthography over phonology within this region.
On the other hand, it has to be considered that the selectivity in the IFC could also be due to the semantic attributes of the word stimuli (Binder and Desai, 2011; Binder et al., 2009). Specifically, homophones differ in orthography, but also in meaning. As such we cannot be certain in terms of attribution. While the region was identified using the phonological localizer, and located dorsal and posterior to those areas known to be associated with purely semantic processing (Binder et al., 2009; Fiez, 1997), it clearly showed specificity to the orthography of the word stimuli. Poldrack and colleagues demonstrated this region to be involved in both phonological and semantic processing (Poldrack et al., 1999).
The role of the IFC in reading is still unclear. Indeed, two recent meta-analyses (Cattinelli et al., 2013; Taylor et al., 2012) come to different conclusions as to the role of this region. While both support the idea that this region is involved in phonology each has a different interpretation of its role. Cattinelli and colleagues (Cattinelli et al., 2013) suggest this region is modulated by difficulty and sensitive to computational load. Taylor and colleagues (Taylor et al., 2012) on the other hand suggest this region is involved in phonological output resolution. One aspect about the current study that might help provide insight into differing results is that the region we have identified in the individual participants is relatively small compared to studies to date. Additionally, most other studies have examined this region at the group level. Here we have identified this region at the individual subject level and therefore do not have the effect of intersubject variability and group averaging that might affect the ability to isolate functional specialization.
Interestingly, the meta-analysis by Jobard and colleagues (Jobard et al., 2003) noted that their “the opercular cluster contained as many “indirect route” as “direct route” contrasts” reflecting a mix of phonological and orthographic studies contributing to this aspect of the IFC. Vinckier and colleagues (Vinckier et al., 2007) demonstrated that the kind of posterior-to-anterior word-tuning gradients noted in adults’ VWFA, were also present in the IFC, this time in the medial-to-lateral direction. The same was observed by Olulade and colleagues in children (Olulade et al., 2015). This particular study also showed a positive correlation in brain activity between these two areas (the IFC and VWFA). Indeed, there is evidence for anatomical and functional connectivity between the VWFA and the IFG (Bitan et al., 2006; Koyama et al., 2010, 2011; Mechelli et al., 2005; Schurz et al., 2013), suggesting that tuning in these areas may be due to the neuronal connection between these two areas.
It has previously been suggested that connections between the IFC and the ventral temporal cortex are required for successful reading (Paulesu et al., 1996). Indeed, recent work by Vandermosten and colleagues (Vandermosten et al., 2012) has suggested a crucial role of the left inferior longitudinal fronto-occipital fasciculus (IFOF, thought to connect the VWFA with the IFC) in successful reading. Their study revealed a correlation between orthographic processing and fractional anisotropy of the IFOF. Additionally, another study (van der Mark et al., 2011) systematically demonstrated functional connectivity between the VWFA and the left hemisphere IFC (in a similar location to our individually defined IFC) in Swiss-German speaking, typically reading children that was shown to be significantly weaker in their dyslexic group. Connections between the VWFA region and TPC have also been shown to be important as the development of the inferior longitudinal fasciculus (ILF) is strongly related to children’s reading development (Yeatman et al., 2012, 2013).
Together, our results support the theory that reading involves a phonologically-based, dorsally-located region (TPC, BA 39/22) and a visual-orthographic ventral pathway (VWFA, BA 37) (Pugh et al., 2001). Our results suggest however that the role of dorsal IFC (BA 44) may be more complex.
Highlights.
The temporoparietal cortex is exclusively sensitive to phonology.
The visual word form area (VWFA) is exclusively sensitive to orthography.
The inferior frontal cortex shows orthographic not phonological tuning.
Supports theory of a dorsal phonological region and a ventral orthographic region.
Acknowledgments
This work was supported by the National Institutes of Health (NICHD HD067884 and HD056107) and the National Science Foundation (1026934). We thank the staff at the Center for Functional and Molecular Imaging (P30HD040677) and our participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D’Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage. 1998;8:302–306. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. English Lexicon Project. Behav. Res. Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Bartl-Pokorny KD, Marschik PB, Sachse S, Green VA, Zhang D, Van Der Meer L, Wolin T, Einspieler C. Tracking development from early speech-language acquisition to reading skills at age 13. Dev. Neurorehabil. 2013;16:188–195. doi: 10.3109/17518423.2013.773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn. Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam M-M, Booth JR. Weaker top-down modulation from the left inferior frontal gyrus in children. Neuroimage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou T-L, Lu D, Cone NE, Cao F, Bigio JD, Booth JR. The interaction between orthographic and phonological information in children: An fMRI study. Hum. Brain Mapp. 2007;28:880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde aL, Tagamets Ma, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and wordlike stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Hum. Brain Mapp. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Relation between brain activation and lexical performance. Hum. Brain Mapp. 2003;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox; Present. 8th Int. Conf. Funct. Mapp. Hum. Brain; 2002. [Google Scholar]
- Bruno JL, Zumberge A, Manis FR, Lu Z-L, Goldman JG. Sensitivity to orthographic familiarity in the occipito-temporal region. Neuroimage. 2008;39:1988–2001. doi: 10.1016/j.neuroimage.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buračas GT, Boynton GM. Efficient Design of Event-Related fMRI Experiments Using M-Sequences. Neuroimage. 2002;16:801–813. doi: 10.1006/nimg.2002.1116. [DOI] [PubMed] [Google Scholar]
- Cattinelli I, Borghese NA, Gallucci M, Paulesu E. Reading the reading brain: A new meta-analysis of functional imaging data on reading. J. Neurolinguistics. 2013;26:214–238. [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K. Serial processing in reading aloud: Evidence for dual-route models of reading. J. Exp. Psychol. Hum. Percept. Perform. 1994;20:1197–1211. [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Rivière D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline J-B, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychol. Sci. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Contribution à l’étude anatomo-pathologique et clinique des différentes variétés de cécité verbale. Mémoires La Société Biol. 1892;4:61–90. [Google Scholar]
- Dietz NAE, Jones KM, Gareau L, Zeffiro TA, Eden GF. Phonological decoding involves left posterior fusiform gyrus. Hum. Brain Mapp. 2005;26:81–93. doi: 10.1002/hbm.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Müller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez Ja. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum. Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc. Natl. Acad. Sci. U. S. A. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Riesenhuber M. Individual variability in location impacts orthographic selectivity in the “visual word form area”. J. Neurosci. 2013;33:11221–11226. doi: 10.1523/JNEUROSCI.5002-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area”. Neuron. 2009;62:199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer LS, Kim J, Rule J, Jiang X, Riesenhuber M. Adding Words to the Brain’s Visual Dictionary: Novel Word Learning Selectively Sharpens Orthographic Representations in the VWFA. J. Neurosci. 2015;35:4965–4972. doi: 10.1523/JNEUROSCI.4031-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. The development of reading across languages. Ann. N. Y. Acad. Sci. 2008;1145:1–12. doi: 10.1196/annals.1416.018. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ. The neural circuitry involved in the reading of German words and pseudowords: A PET study. J. Cogn. Neurosci. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Heim S, Alter K, Ischebeck AK, Amunts K, Eickhoff SB, Mohlberg H, Zilles K, von Cramon DY, Friederici AD. The role of the left Brodmann’s areas 44 and 45 in reading words and pseudowords. Brain Res. Cogn. Brain Res. 2005;25:982–993. doi: 10.1016/j.cogbrainres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Hum. Brain Mapp. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Houdé O, Rossi S, Lubin A, Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev. Sci. 2010;13:876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rosen E, Zeffiro T, Vanmeter J, Blanz V, Riesenhuber M. Evaluation of a shape-based model of human face discrimination using FMRI and behavioral techniques. Neuron. 2006;50:159–172. doi: 10.1016/j.neuron.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joubert S. Neural correlates of lexical and sublexical processes in reading. BRAIN Lang. 2004;89:9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Katzev M, Tüscher O, Hennig J, Weiller C, Kaller CP. Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: the crucial role of task demands and individual ability. J. Neurosci. 2013;33:7837–7845. doi: 10.1523/JNEUROSCI.3147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. Reading networks at rest. Cereb. Cortex. 2010;20:2549–2559. doi: 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo X-N, Kelly C, Mennes M, Jutagir DR, Castellanos FX, Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. J. Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel RJA. Adaptation: from single cells to BOLD signals. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. taksi: on orthographic word recognition in the left ventral occipitotemporal cortex. J. Cogn. Neurosci. 2007;19:1584–1594. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Klackl J, Richlan F, Schurz M, Staffen W, Ladurner G, Wimmer H. On the functional neuroanatomy of visual word processing: effects of case and letter deviance. J. Cogn. Neurosci. 2009;21:222–229. doi: 10.1162/jocn.2009.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015 doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41:293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph Ma, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of neuronal interactions. J. Cogn. Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. An On-Line Orthographic Database of the English Language. 2005 http://www.neuro.mcw.edu/mcword/
- Newman RL, Joanisse MF. Modulation of brain regions involved in word recognition by homophonous stimuli: An fMRI study. Brain Res. 2011;1367:250–264. doi: 10.1016/j.brainres.2010.09.089. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Flowers DL, Napoliello EM, Eden GF. Dyslexic children lack word selectivity gradients in occipito-temporal and inferior frontal cortex. NeuroImage. Clin. 2015;7:742–754. doi: 10.1016/j.nicl.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119(Pt. 1):143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, et al. A cultural effect on brain function. Nat. Neurosci. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Danelli L, Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front. Hum. Neurosci. 2014;8:830. doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The Interactive Account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner aR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz Ba. Neurobiological studies of reading and reading disability. J. Commun. Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain. 1997;120(Pt. 5):739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Sci. Stud. Read. 2004;8:273–292. [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of Neural Systems for Reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schurz M, Kronbichler M, Crone J, Richlan F, Klackl J, Wimmer H. Top-down and bottom-up influences on the left ventral occipito-temporal cortex during visual word recognition: An analysis of effective connectivity. Hum. Brain Mapp. 2013 doi: 10.1002/hbm.22281. 000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, Davis MH. Can Cognitive Models Explain Brain Activation During Word and Pseudoword Reading? A Meta-Analysis of 36 Neuroimaging Studies. Psychol. Bull. 2012 doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro Ta, Eden GF. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Twomey T, Duncan KJK, Price CJ, Devlin JT. Top-down modulation of ventral occipito-temporal responses during visual word recognition. Neuroimage. 2011;55:1242–1251. doi: 10.1016/j.neuroimage.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, Kronbichler M, Loenneker T, Klaver P, Martin E, et al. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D. The left occipitotemporal system in reading: Disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage. 2011;54:2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Word-form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang B, Yan T, Li X, Bao X, Guo Q. Different roles of the posterior inferior frontal gyrus in Chinese character form judgment differences between literate and illiterate individuals. Brain Res. 2012;1431:69–76. doi: 10.1016/j.brainres.2011.10.052. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, Reeves-Tyer P, DiCamillo P, Theodore W. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb. Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Xue G, Jiang T, Chen C, Dong Q. Language experience shapes early electrophysiological responses to visual stimuli: the effects of writing system, stimulus length, and presentation duration. Neuroimage. 2006;39:2025–2037. doi: 10.1016/j.neuroimage.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 2013;125:146–155. doi: 10.1016/j.bandl.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol. Bull. 2005;131:3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]