Abstract
Objective
The aim of this study was to identify temporal associations of anxiety dimensions with menopausal hot flashes in women progressing through the menopause transition. We hypothesized that associations of both somatic and affective dimensions of anxiety with hot flashes increased in the menopause transition, and that somatic anxiety was an independent risk factor for menopausal hot flashes.
Methods
Hot flashes, anxiety symptoms, hormone levels and other psychosocial variables were assessed annually for 14 years of follow-up. The 233 women were premenopausal at baseline and continued through one year or more after the final menstrual period. Anxiety dimensions were assessed with the Zung Anxiety Scale (ZAS), a validated measure of affective anxiety and somatic anxiety. Summed item scores were divided by the number of items rated, so that ranges of the two dimensions were comparable.
Results
Seventy-two percent of the sample reported moderate/severe hot flashes during the 14-year interval. There was no significant interaction between anxiety dimensions and menopausal stages. However, when adjusted for menopausal stage, the magnitude of association between somatic anxiety and hot flashes dramatically increased (OR 3.03, 95% CI: 2.12, 4.32, P<0.001), while the association between affective anxiety and hot flashes increased to a lesser extent (OR 1.27, 95% CI: 1.03, 1.57, P=0.024). Women with high levels of somatic anxiety (top third of the sample) had the greatest risk of hot flashes (P<0.001). When the anxiety dimensions were considered in combination, the additive effect of high affective anxiety symptoms was minimal, with no significant difference between the group with high affective/low somatic symptoms and the low symptom group in incident hot flashes at each menopausal stage (P=0.54). In multivariable analysis, somatic anxiety increased the risk of hot flashes more than 3 times (OR 3.13, 95% CI: 2.16, 4.53, P<0.001), but affective anxiety was not significantly associated with hot flashes after adjustment for other study variables (OR 1.19, 95% CI: 0.96, 1.48, P=0.117). Time-lagged somatic anxiety scores significantly predicted hot flashes, with a 71% increase in risk (OR 1.71, 95% CI: 1.21, 2.41, P=0.002). Time-lagged affective anxiety scores did not predict hot flashes, (OR 1.06, 95% CI: 0.87, 1.31, P=0.58).
Conclusions
This study showed a strong predictive association of somatic anxiety with the risk of menopausal hot flashes. The temporal associations suggest that somatic anxiety is not simply a redundant measure of hot flashes but predicts the risk of menopausal hot flashes and may be a potential target in clinical management of perimenopausal women.
Keywords: Anxiety, hot flashes, menopause transition, somatic symptoms
INTRODUCTION
Menopausal hot flashes are associated with biological changes of ovarian aging, as indicated by their associations with altered endogenous hormone levels1, 2 and alterations in thermoregulatory control mechanisms3, 4 and many women seek medical treatment to relieve their discomfort.5 However, the pathophysiology of menopausal hot flashes remains poorly understood, and apart from hormone therapy, which significantly relieves hot flashes, scientific information for the management of this common problem remains limited.6
There are many reports of anxiety as a risk factor for hot flashes, but its association is controversial.7–14 Conflicting findings are in part due to the varied manifestations of anxiety syndromes and disorders and to the nature of the somatic symptoms of anxiety, which are similar to somatic complaints of hot flashes, and the consequent difficulty of disentangling the two conditions.11, 14, 15 There is also little evidence to indicate whether anxiety is a precursor or a consequence of hot flashes, and whether anxiety influences perceptions of hot flashes or augments the event of hot flashes.12
While anxiety symptoms are part of everyday life, anxiety disorders are highly prevalent and are the most common mental disorders in the community.16 Moreover, anxiety disorders tend to have a chronic and persistent course, are frequently comorbid with other physical and mental disorders, and result in substantial impairment.16–18 Anxiety syndromes with shorter time periods can be as seriously impairing as syndromes that meet full criteria for generalized anxiety disorder (GAD).19 Anxiety appears to be a particular problem for women inasmuch as the risk of anxiety disorders in women is double that of men (22.6% versus 11.8%), and this risk extends throughout the reproductive years.18
Anxiety manifests in many ways, ranging from symptoms to fully-diagnosed disorders that include generalized anxiety disorder (GAD), panic disorder, phobias and post-traumatic stress disorder. All these conditions consist of anxiety symptoms but differ in the types of situations that induce the anxiety and associated thoughts. For example, a diagnosis of generalized anxiety disorder focuses on persistent and excessive worry, nervousness and inability to relax, while panic disorder involves repeated unexpected panic attacks that include fearfulness and physical symptoms such as shortness of breath, heart palpitations and fear of “going crazy.” Furthermore, anxiety syndromes or high levels of anxiety symptoms that do not meet diagnostic criteria for an anxiety disorder may nonetheless be clinically significant and diminish quality of life.20–22
The aim of this study was to identify temporal associations of anxiety and menopausal hot flashes, using a validated instrument to assess somatic and affective anxiety symptoms in women progressing through the menopause transition. We hypothesized an effect modification of menopausal stage on dimensions of somatic and affective anxiety, with increased associations in the menopause transition. Based on evidence in previous studies,8,11 we also hypothesized that somatic anxiety was an independent risk factor of menopausal hot flashes.
MATERIALS AND METHODS
Study participants
The study sample included 233 women in the Penn Ovarian Aging Study (POAS) cohort. We included only participants who reached at least one year beyond natural menopause during the follow-up period (1996–2012) and reported no troublesome hot flashes at the first assessment, in order to clearly address the temporal association of anxiety with incident hot flashes in the menopause transition. The Institutional Review Board of the University of Pennsylvania approved the study, and all participants provided written informed consent.
The original cohort was randomly identified by telephone digit dialing in Philadelphia County, PA, with sampling stratified to obtain equal numbers of African American and white women as previously described.7 At enrollment, all women were premenopausal with regular menstrual cycles of 22–35 days for the previous three cycles, ages 35–47 years, had an intact uterus and at least one ovary. Exclusion criteria at enrollment included current use of any hormonal or psychotropic medications, alcohol or drug abuse, major psychiatric disorder in the past year, pregnancy or breast feeding, uncontrolled hypertension, and serious health problems known to compromise ovarian function. Attrition since POAS inception has been acceptable at 33% over 14 years (293/436). The attrition has been non-differential with respect to the sample characteristics, as reported in Nelson et al (23), and has not significantly differed by race or education. Attrition through Period 14 was classified as lost to follow-up (n=51), no reason given (n=40), withdrew consent (n=22), personal constraints or problems (n=16), and deceased (N=14).
Study design
Following cohort enrollment, full follow-up assessments were conducted for 14 years at intervals of approximately 9 months in the first five years and then annually, with a two-year gap between assessments 10 and 11. Study data were collected at two in-home visits timed to the menstrual cycle (days 2–6) in two consecutive menstrual cycles, or approximately one month apart in non-cycling women for 14 assessment periods. The study was described to participants as a general women’s health study. Trained research interviewers obtained menstrual dates, structured interview data on overall health, blood samples for hormone assays, and anthropometric measures. Participants completed a set of validated self-report measures to assess health and other behavioral measures of the study at each assessment period.
Study variables
The primary outcome variable was moderate or severe hot flashes as reported by participants at each follow-up assessment, using a menopausal symptom list that was validated for the cohort and embedded in the structured interview questionnaire.24 Participants were asked whether hot flashes or night sweats occurred in the past month, whether they occurred in the past year, and rated the severity for each time period as 0 (none), 1 (mild), 2 (moderate), 3 (severe).
Anxiety was rated at each assessment using the Zung Anxiety Scale (ZAS), a validated measure that was constructed to measure anxiety symptoms as a clinical entity and included 20 common symptoms of anxiety.25 The scale is based on a strong theoretical model and discriminates well between patients diagnosed with and without anxiety disorders.26 Participants rated each item for its frequency in the past week, from none or a little (1), to most or all of the time (4). The rating scores were summed for each participant. Zung identified summed score ranges as indicating normal anxiety (20 to 35), minimal to moderate anxiety (36 to 47), marked to severe anxiety (48–59), and most severe anxiety (60–80). The two subscales measure affective anxiety (items 1–5) and somatic anxiety (items 6–20). We calculated the mean scores for each subscale (the summed item scores divided by the number of items rated), in order to provide comparable score ranges for interpretation of the affective and somatic anxiety dimensions. In each subscale, the top third was defined as the high symptom group; the lower two-thirds were defined as the low symptom group.
Menopausal stages were adapted from the Staging System for Reproductive Aging in Women27 as follows: premenopausal: regular menstrual cycles in the 22- to 35-day range; early transition: changes >=7 days in either direction in the participants’ own cycle length through 2 months (60 days) amenorrhea; late transition: 3 to 11 months amenorrhea; early postmenopause: final menstrual period (FMP) to <6 years post-FMP; late postmenopause: >=6 years post-FMP.
Covariates were selected for their associations with hot flashes in previous studies and the goals of this study. Time-varying covariates included body mass index (BMI >=30, <30), perceived stress,28 alcohol use >=1/week, current smoking, estradiol, follicle stimulating hormone (FSH), current age, employment (yes, no) and use of psychotropic medications in the follow-up interval (yes, no); fixed variables were history of depression (yes, no) as identified at cohort enrollment by medical history interview, race (African American or white) and education (>HS, <=HS).
Estradiol and follicle stimulating hormone (FSH) were measured by radioimmunoassay in the Translational Research Center of the University of Pennsylvania using Coat-A-Count commercial kits (Siemens). Non-fasting blood samples were collected at each study visit between days 2 and 6 of the menstrual cycle, or one month apart in non-cycling women, through assessment period 14, providing a possible maximum of 28 samples per woman. The blood samples were centrifuged and frozen in aliquots at −80C. Assays were conducted in batches that included four visits per participant to reduce the within-women variability resulting from assay conditions. All assays were performed in duplicate and repeated if values differed by greater than 15%. Inter-assay and intra-assay coefficients of variation were less than 5%.
Statistical analysis
The primary outcome variable was a dichotomous assessment of hot flash severity (moderate or severe versus mild or none), which was rated by the participants at each assessment period. The 233 participants provided 3,112 hot flash observations. All participants in this study were premenopausal at baseline and had observations for at least one year after the FMP.
Generalized linear mixed effects regression models for repeated measures were used to estimate unadjusted and adjusted associations of the study variables with moderate/severe hot flashes. The anxiety scores and hot flash severity were measured at each assessment period throughout the study. All available data were included in the analyses. Models were adjusted for menopausal stage, and all potentially time-varying covariates were treated as such in modeling. Variance estimates for the statistical tests on the regression coefficients were adjusted for repeated observations from each participant using generalized estimating equations.29 In all models, observations during hormone use, pregnancy and breast feeding were censored at the time of their occurrence. Women who had a hysterectomy prior to natural menopause were excluded in this report. To test the predictive values of the anxiety symptom dimensions, the models were rerun, with the somatic and affective anxiety scores lagged by one assessment period relative to each time of the hot flash reports. To test whether hot flash-type symptoms in the somatic anxiety dimension (facial flushing, heart pounding) accounted for the association between anxiety and hot flashes, the models were rerun, omitting these possible symptoms of hot flashes in the somatic subscale scores.
All covariates were defined a priori and evaluated for their unadjusted association with hot flashes. Covariates that were associated with hot flashes at P<=0.20 in unadjusted analysis were included in multivariable models to determine the independent contribution of the covariates to the outcome of hot flashes. Inclusion in the final multivariable models was guided by whether each variable remained statistically significant at P<0.05 or modified other significant associations by >=15%. Hypothesized interactions between the two anxiety subscales and menopausal stage were examined. Hormone covariates were modeled using natural log transformations to reduce the influence of skewed distributions. The subject mean of the two hormone measures obtained at each assessment period was used in analysis. Estradiol and FSH were evaluated in separate multivariable models due to their high inter-correlation. Odds ratios for the hormones are presented per unit (1 standard deviation) change.
Statistical power calculations were computed using the sampclus program in STATA version 14 (College Station, TX) to compare whether the women defined by high somatic (or affective) anxiety symptoms (33% of the sample) versus the remainder of the sample had an increased prevalence of moderate/severe hot flashes. Each woman contributed numerous visits prior to and following menopause. Based on assumptions of 2-sided tests with type 1, alpha, error of 5%, a mean of 8 repeated measures per participant, and a correlation among the repeated measures of 0.45, the study has 80% power to detect an increase in the likelihood (odds ratio) of moderate to severe hot flashes of 1.43 or greater.
Data analyses were conducted using the SAS 9.3 statistical package (SAS, Inc., Cary, NC). Statistical tests were two-sided, with P<=0.05 considered significant.
RESULTS
Sample description
Participants were fully evaluated for 14 years and contributed a mean of 13.4 observation points per participant. One-hundred-sixty-eight women in the study sample (72%) reported moderate or severe hot flashes during the 14-year interval. The average Zung anxiety score for the total sample at baseline was 34 (SD 7) (high normal range). Table 1 shows the sample characteristics at baseline for the affective and somatic dimensions of anxiety with each dimension divided into high and low symptom-level groups as defined above.
Table 1.
Characteristics of the Sample by Somatic/Affective Anxiety Groups1 at Baseline, N=233
| Variable Mean (SD) |
HighS/HighA N=42 |
HighS/LowA N=28 |
LowS/HighA N=36 |
LowS/LowA N=127 |
P Value |
|---|---|---|---|---|---|
| Age, y | 41.75 (3.66) | 41.94 (3.36) | 41.85 (3.31) | 42.41 (3.32) | 0.63 |
| Anxiety (Zung total)2 | 44.33 (5.81) | 37.29 (2.99) | 35.61 (3.66) | 29.38 (4.06) | <0.001 |
| Perceived stress (PSS)3 | 25.74 (6.15) | 20.81 (9.36) | 22.78 (7.27) | 18.20 (6.71) | <0.001 |
| Depression (CES-D)4 | 23.62 (10.85) | 14.39 (9.34) | 19.60 (10.35) | 9.13 (7.03) | <0.001 |
| Estradiol, pg/mL | 47.05 (23.80) | 44.89 (21.71) | 38.62 (22.05) | 45.03 (36.49) | 0.67 |
| FSH, mIU/mL | 8.29 (4.01) | 6.97 (2.96) | 9.23 (5.25) | 7.74 (3.04) | 0.07 |
| Variable, N (%)5 | |||||
| History of depression | 34 (81.0) | 14 (50.0) | 23 (63.9) | 27 (21.3) | <0.001 |
| Body mass index (BMI), <=30 | 13 (31.7) | 14 (50.0) | 9 (27.3) | 45 (36.0) | 0.28 |
| Alcohol >=1/wk | 3 (7.1) | 2 (7.1) | 3 (8.3) | 15 (11.8) | 0.75 |
| Current smoker | 20 (47.6) | 13 (46.4) | 11 (31.4) | 46 (36.2) | 0.36 |
| Employed | 33 (78.6) | 21 (75.0) | 31 (86.1) | 113 (89.0) | 0.16 |
| Education >HS | 19 (45.2) | 15 (53.6) | 20 (55.6) | 86 (67.7) | 0.05 |
| Race African American White |
23 (54.8) 19 (45.2) |
14 (50.0) 14 (50.0) |
13 (36.1) 23 (63.9) |
54 (42.5) 73 (57.5) |
0.34 |
. Somatic dimension = S; affective dimension = A. In each dimension, the top third of scores defined the high symptom group; the lower two-thirds defined the low symptom group.
. Scores indicating anxiety status as defined by Zung are normal (20–35), moderate 36–47, severe (48–59) very severe (60–80).
. Mean score for community-based adult females is 25.6 (SD 8.2).
. The standard cut point for high depressive symptoms is >=16.
. Any slight differences in percents are due to missing data.
Anxiety symptoms in relation to menopausal hot flashes in unadjusted analysis
The total summed ZAS score was significantly associated with moderate/severe hot flashes over the 14-year follow-up, with the likelihood of association between anxiety and hot flashes increasing 2% with each point increase in the ZAS scores (OR 1.02, 95% CI: 1.01 – 1.04, P=0.005). We then evaluated the two dimensions of the ZAS for their associations with hot flashes. The baseline mean score (the summed item scores divided by the number of items) was 1.68 (SD 0.35) for the somatic dimension and 1.74 (SD 0.54) for the affective dimension. The dimension of somatic anxiety was strongly associated with hot flashes over the follow-up interval, with the likelihood of hot flashes increasing 4% with each 1-point increase in mean scores (OR 1.04, 95% CI: 1.02, 1.07, P<0.001). The dimension of affective anxiety was not significantly associated with hot flashes in unadjusted analysis (OR 1.01, 95% CI: 0.96, 1.05, P=0.78).
Anxiety in relation to hot flashes adjusted for menopausal stage
There was no significant interaction between somatic anxiety or affective anxiety and menopausal stage, indicating that the patterns of association with hot flashes in the menopause transition were similar (interaction for somatic anxiety: P=0.83; interaction for affective anxiety: P=0.18). In models adjusted only for menopausal stage, the magnitude of the association between somatic anxiety and hot flashes dramatically increased in the follow-up interval (OR 3.03, 95% CI: 2.12, 4.32, P<0.001), while the association between affective anxiety and hot flashes increased to a lesser extent (OR 1.27, 95% CI: 1.03, 1.57, P=0.024). To test whether hot flash-type symptoms in the somatic anxiety dimension (facial flushing, heart pounding) accounted for the association between anxiety and hot flashes, we recalculated the somatic anxiety subscale, subtracting these 2 items. When the models were rerun, omitting these dual symptoms both singly and together, the associations between somatic anxiety and hot flashes remained significant (OR 2 20, 95% CI: 1.59, 3.06, P<0.001 with both symptoms removed).
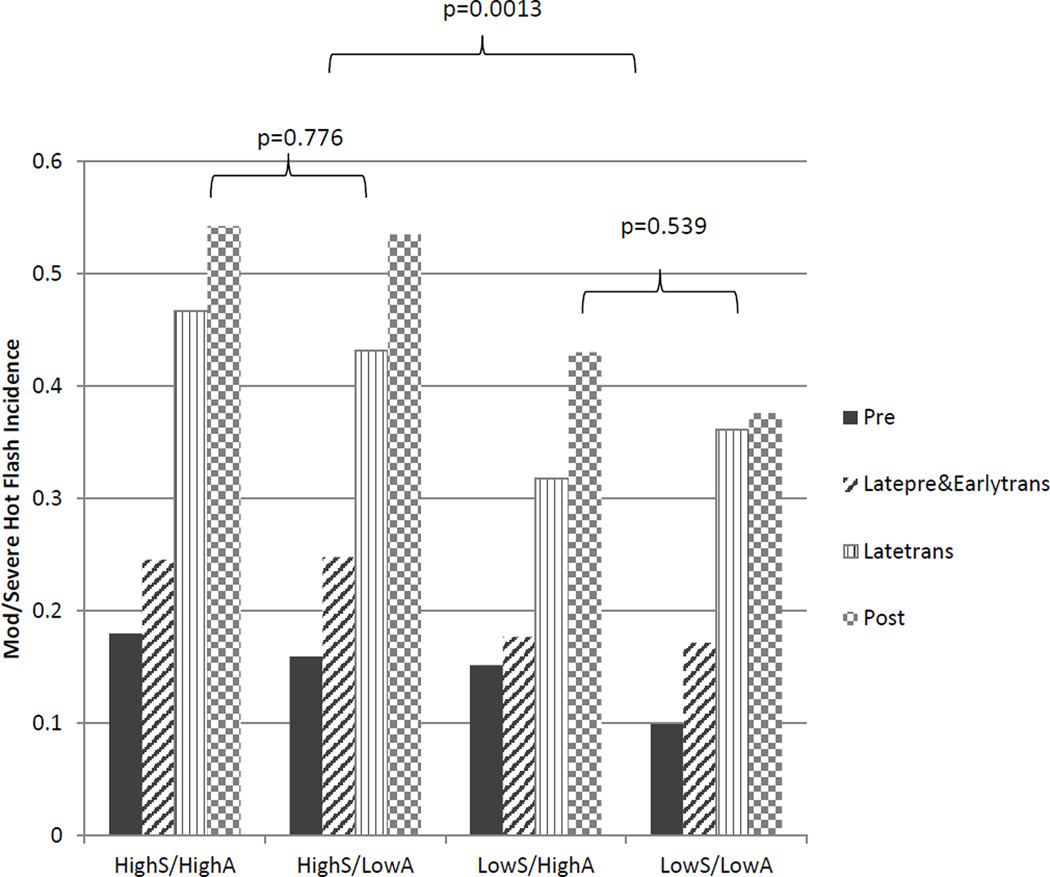
When each dimension of anxiety was divided into high and low symptom groups and considered in combination, the two groups with high somatic anxiety had the greatest risk of hot flashes compared to women with low anxiety symptoms and also had nearly identical reports of incident hot flashes at each stage in the menopause transition (Figure 1). Figure 1 further shows that the additive effect of affective anxiety symptoms was minimal, inasmuch as the high affective anxiety group did not differ from the low affective group in reports of incident hot flashes. Furthermore, the group with high affective/low somatic symptoms did not differ from the group with low symptoms in the reports of incident hot flashes at each menopausal stage (P=0.54), again suggesting that the association of affective symptoms in relation to hot flashes was minimal.
Figure 1.
Incidence of moderate/severe hot flashes by anxiety symptom groups and menopausal stages. Main effect menopausal stage, P<0.001; anxiety symptom groups, P<0.001.
Associations with covariates and hormone levels
Table 2 shows associations of the hypothesized covariates with hot flashes. Covariates with a significant unadjusted association with hot flashes included somatic anxiety, menopausal stage, current age, history of depression, body mass index, current psychotropic medications, education, log FSH and log estradiol.
Table 2.
Risk factors for moderate to severe hot flashes during 14 years of follow–up (N=233)
| Variable | Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model with Somatic Anxiety | Model with Affective Anxiety | ||||||||
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| Anxiety (Zung) | |||||||||
| Total (mean) | 1.02 | 1.01–1.04 | 0.005 | ||||||
| Somatic dimension (mean) | 1.91 | 1.36–2.67 | <0.001 | 3.13 | 2.16–4.53 | <0.001 | |||
| Affective dimension (mean) | 1.03 | 0.82–1.30 | 0.780 | 1.19 | 0.96–1.48 | 0.117 | |||
| Menopausal stage | <0.001 | <0.001 | <0.001 | ||||||
| Premenopausal | Reference | ––– | –– | Reference | ––– | ––– | Reference | ___ | ___ |
| Early transition | 2.54 | 1.82–3.54 | <0.001 | 1.89 | 1.32–2.73 | < 0.001 | 1.75 | 1.22–2.50 | 0.002 |
| Late transition | 6.40 | 4.45–9.20 | <0.001 | 2.77 | 1.75–4.40 | <0.001 | 3.31 | 2.12–5.17 | <0.001 |
| Early postmeno | 7.22 | 4.94–10.55 | <0.001 | 3.89 | 2.24–6.75 | <0.001 | 3.42 | 1.98–5.91 | <0.001 |
| Late postmeno | 5.23 | 3.24–8.44 | <0.001 | 2.21 | 1.04–4.70 | 0.040 | 1.95 | 0.92–4.10 | 0.080 |
| Age, current (y) | 1.11 | 1.09–1.14 | <0.001 | 1.10 | 1.05–1.15 | <0.001 | 1.09 | 1.04–1.14 | <0.001 |
| History of depression3 | 1.62 | 1.14–2.29 | 0.007 | 1.36 | 0.90–2.05 | 0.145 | 1.60 | 1.06–2.42 | 0.023 |
| log Estradiol, pg/mL1,2,3 | 0.84 | 0.80–0.90 | <0.001 | 0.96 | 0.90–1.03 | 0.236 | 0.96 | 0.90–1.02 | 0.162 |
| log FSH, mIU/mL1,2,3 | 1.21 | 1.16–1.26 | <0.001 | 1.06 | 1.00–1.13 | 0.059 | 1.05 | 0.99–1.12 | 0.126 |
| Education3 | |||||||||
| >HS | 0.65 | 0.46–0.92 | 0.015 | 0.68 | 0.46–1.01 | 0.056 | 0.64 | 0.43–0.96 | 0.029 |
| ≤HS | Reference | ––– | ––– | Reference | ––– | ––– | Reference | ––– | ––– |
| Current psychotropic medications | 2.82 | 1.97–4.04 | <0.001 | 0.41 | 1.29–2.95 | 0.002 | 2.02 | 1.34–3.07 | <0.001 |
| BMI ≥ 30 | 1.51 | 1.15–1.98 | 0.003 | ||||||
| Race | |||||||||
| African American | 1.38 | 0.98–1.94 | 0.069 | ||||||
| White | Reference | ––– | –– | ||||||
| Alcohol < 1/wk | 0.76 | 0.56–1.03 | 0.076 | ||||||
| Perceived stress (PSS) | 1.01 | 0.99–1.02 | 0.236 | ||||||
| Employed vs not employed | 0.76 | 0.47–1.23 | 0.267 | ||||||
| Current smoker | 0.97 | 0.69–1.38 | 0.885 | ||||||
. OR is for mean log hormone per 1 SD.
. Hormones were added separately to the models.
. Variables at P>0.05 were retained in the models for consistency between the 2 models.
In multivariable analysis, the association of somatic anxiety with hot flashes remained highly significant, with the risk of hot flashes increasing over 3 times with each point increase in the mean somatic anxiety score (OR 3.13, 95% CI: 2.16, 4.53, P<0.001). Menopausal stage (P<0.001) and current age (P<0.001) were independent contributors to hot flashes after adjusting for all other variables in the model. Current psychotropic medications were protective in the multivariable model, with a 59% lower risk of hot flashes, but importantly, did not confound the association between somatic anxiety and hot flashes (i.e., the association of somatic anxiety with hot flashes was nearly identical in models with and without medications). Although significantly associated with hot flashes in unadjusted analysis, the associations of estradiol and FSH were not significant in multivariable analysis (P=0.24 and P=0.06, respectively), due to the impact of menopausal stage, which had less variability and explained the same association with hot flashes.
Affective anxiety was not significantly associated with hot flashes in multivariable analysis (OR 1.19, 95% CI: 0.96, 1.48, P=0.117) (Table 2). The covariates of menopausal stage, current age, history of depression, and education were independent contributors to hot flashes in this model. A history of depression had an independent contribution to hot flashes when modeled with affective anxiety (P=0.023) but did not reach significance in the model with somatic anxiety (P=0.145). Higher education levels were protective, with a 36% lower risk of hot flashes. Current psychotropic medication had a significant association with hot flashes, but did not confound the association between somatic anxiety and hot flashes (i.e., the association of somatic anxiety with hot flashes was nearly identical in models with and without medications). Neither FSH nor estradiol were significantly associated with hot flashes when included in the model with affective anxiety (P=0.13 and P=0.16 respectively), due to the impact of menopausal stage.
The covariate of BMI was significantly associated with hot flashes in unadjusted analysis but was not an independent contributor to hot flashes in the final multivariable models. Other hypothesized covariates of perceived stress, current smoking, alcohol use, employment and race were not significantly associated with hot flashes in either adjusted or unadjusted analysis in this study (data shown in Table 2).
Anxiety as a predictor of hot flashes
To address whether anxiety preceded hot flashes and could therefore be considered a predictor of the risk of menopausal hot flashes, the anxiety symptom scores adjusted for menopausal stage were lagged by one assessment period relative to each time of the hot flash reports to identify whether anxiety occurred before incident hot flashes. Lagged somatic anxiety scores significantly predicted risk of hot flashes, with a 69% increase in risk for each point increase in mean somatic anxiety scores (OR 1.69, 95% CI: 1.23, 2.32, P=0.001). When the somatic anxiety model was re-run with the possible dual symptoms (facial flushing and heart pounding items) removed from the somatic anxiety scores, the associations of somatic anxiety with hot flashes remained significant in time-lagged models (OR 1.50, 95% CI: 1.10, 2.05, P=0.011 with 2 items removed). In contrast, lagged affective anxiety scores did not predict hot flashes, a further indication of the weak association of affective anxiety with hot flashes (OR 1.06, 95% CI: 0.87, 1.31, P=0.58).
DISCUSSION
Anxiety, specifically the somatic symptom dimension, was strongly associated with hot flashes in the menopause transition in a 14-year follow-up interval. The association remained strong after adjusting for important factors known to be associated with hot flashes including age, menopausal stage, reproductive hormone levels, obesity, history of depression, education, race, perceived stress, alcohol use and smoking. Importantly, similar results persisted when we examined the association of somatic anxiety preceding reports of hot flashes, which suggested that somatic anxiety was not simply a redundant measure of hot flashes but significantly predicted the risk of moderate/severe hot flashes. Importantly, when possible dual symptoms were removed from the somatic anxiety scores, the time-lagged association between somatic anxiety and hot flashes remained.
In contrast, affective anxiety, which was characterized by anxiousness, apprehension and fearfulness and by definition was not sensitive to somatic symptoms, did not predict the risk of menopausal hot flashes. Although each dimension had similar average scores at baseline, and their patterns with hot flashes in the menopause transition were similar, only somatic anxiety strongly predicted the risk of hot flashes. While the association between affective anxiety and hot flashes increased modestly in the menopause transition, affective anxiety had no predictive association with incident hot flashes when the temporality of the association was considered.
These findings add information to several previous studies of anxiety in relation to menopausal hot flashes. In an earlier study of this cohort,7 women with moderate anxiety were nearly three times more likely to report hot flashes and women with high anxiety were nearly five times more likely to report hot flashes compared to women with anxiety scores in the normal range. Notably, anxiety preceded hot flashes, although interpretation was limited by the predominance of observations in the premenopause and early transition stages. In another study that assessed anxiety with the ZAS, somatic anxiety but not affective anxiety was significantly associated with hot flashes, as was found in the present study.11 However, the researchers interpreted the somatic anxiety symptoms as manifestations of hot flashes and were unable to evaluate whether anxiety symptoms preceded hot flashes, due to the cross-sectional design. In a study that evaluated anxiety as somatosensory amplification (the experience of sensing bodily sensations as intense, agitating, and unpleasant), the findings indicated that anxiety was significantly associated with hot flash interference and perceived control over hot flashes.30
Our predictive findings of association between somatic anxiety and moderate/severe hot flashes could also be interpreted as early subthreshold hot flashes/vasomotor symptoms that registered on the anxiety measure and were not anxiety symptoms. While it is possible that the women experienced sub-threshold vasomotor symptoms that they did not report as hot flashes, it is also possible that these dual symptoms were also symptoms of somatic anxiety, which amplified experiences of physical sensations such as hot flashes. When we tested the contribution of hot-flash type symptoms to the somatic anxiety score by omitting these symptoms from the scores, the associations between anxiety and hot flashes remained significant. These findings are consistent with concepts that somatic anxiety and other similar anxiety constructs such as anxiety sensitivity31 and somatosensory amplification30 describe a trait-like cognitive condition where perceptions are linked with anxiety-related sensations or aversive physical sensations. We suggest that somatic anxiety is derived from a heightened apprehension of arousal symptoms in thermoregulation12 and links with the perceived severity and/or troublesomeness of hot flashes.
The selected covariates in this study were previously identified as risk factors for menopausal hot flashes in numerous studies. In this study, the significant independent risk factors of hot flashes in addition to menopausal stage and somatic anxiety included current age, a history of depression and education.. These diverse factors encompass social, behavioral and biological influences and add further support to considering hot flashes as not simply as a result of ovarian aging but as a multifactorial condition with important behavioral influences.
Clinical evaluation of women seeking treatment for hot flashes should include assessment of anxiety symptoms and consider various treatments for reducing anxiety as potentially beneficial in reducing hot flashes. Given the strong association between hot flashes and somatic anxiety, which reflects a trait-like cognitive condition, cognitive-behavioral therapy (CBT), which reduced anxiety sensitivity compared to control conditions in a meta-analysis of CBT, may be an important treatment to consider, although more studies are needed to confirm current information.41 The strong association between anxiety and hot flashes may also partly explain why selective serotonin reuptake inhibitors, which are indicated for treatment of anxiety, have shown modest efficacy for hot flashes32–36 and are considered a possible alternative to hormone therapy.37 Gabapentin is another medication that has been shown to reduce both hot flashes and anxiety symptoms and may provide therapeutic benefit,38–40 although confirmatory studies are needed for these preliminary findings. Non-pharmacological treatments that specifically target anxiety sensitivity might reduce the severity of hot flashes without the side effects of hormone and pharmacologic treatments. Exercise training resulted in clinically significant changes in anxiety sensitivity and improved panic disorder and GAD, but well-designed trials to confirm efficacy for hot flashes are needed.42, 43
Several limitations of the study are noted. Anxiety symptoms were assessed using a validated and widely-published self-report questionnaire that measured affective and somatic dimensions of anxiety. It is underscored that other measures of anxiety may yield different results, due to the heterogeneity of anxiety disorders and scale-specific metrics.25 Anxiety as measured in this study indicated that most participants had normal to moderate levels of anxiety, and only 5% had severe anxiety levels; studies of women with diagnosed anxiety disorders might provide further clarification of the role of anxiety in the subjective experience of hot flashes. Women who had surgical menopause and current users of hormone therapy were not included in the study, and further studies that evaluate these conditions are needed. Although we examined several important biological, behavioral and demographic variables associated with hot flashes, many factors beyond the scope of this study, such as physical activity, food consumption and negative life events, may be important to evaluate in other studies. While the sample had multiple assessments throughout the menopause transition with an acceptable attrition rate that was non-differential with respect to the sample characteristics, undetected bias due to loss to follow-up is possible. Finally, our findings are based on a population-based cohort of African-American and white urban women who were in general good health with no current hormone use and may not be generalizable to all perimenopausal women.
The primary strengths of this report are the 14-year follow-up, with annual evaluations using validated measures. The women were followed from premenopausal status through one or more years after the final menstrual period, which provided identification of menopause and the menopause transition with minimal recall bias. All study participants reported no troublesome hot flashes at baseline, which enabled clear identification of incident hot flashes in the menopause transition. The population-based sample was randomly identified and stratified to have similar numbers of African-American and white women for analysis of racial associations. Hormone measures were obtained concurrently with the study questionnaires and were collected in the early follicular phase in menstruating women to provide consistent timing of the assessments in relation to the menstrual cycle.
CONCLUSIONS
This study showed a strong predictive association of somatic anxiety with the risk of menopausal hot flashes. Although anxiety and hot flashes have dual symptoms that apply to both conditions, the temporal associations suggest that somatic anxiety is not simply a redundant measure of hot flashes but significantly predicts the risk of hot flashes and may be a potential target for treatment. Additional studies are needed to determine whether treatments that target somatic anxiety effectively reduce menopausal hot flashes.
Acknowledgments
Supported by the National Institute of Aging, grant RO1 AG12745 (EWF, principal investigator) and UL1TR000003 (to the Clinical and Translational Research Center, Perelman School of Medicine, University of Pennsylvania.
Footnotes
Conflict of interest and financial disclosures. The authors declare no conflicts of interest and have no financial disclosures.
REFERENCES
- 1.Overlie I, Moen MH, Holte A, Finset A. Androgens and estrogens in relation to hot flashes during the menopause transition. Maturitas. 2000;41:69–77. doi: 10.1016/s0378-5122(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 2.Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–4030. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 3.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13:453–464. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopause hot flashes. Fertil Steril. 1998;70:332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 5.Williams RE, Kalilani L, DiBenedetti DB, Zhoe X, Fehnel SE, Clark RV. Healthcare seeking and treament for menopause symptoms in the United States. Maturitas. 2007;58:348–358. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Richardson MK. Alternatives to hormone therapy for hot flashes: many choices but science is lacking. Menopause. 2013;20:980–982. doi: 10.1097/GME.0b013e3182982436. [DOI] [PubMed] [Google Scholar]
- 7.Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12:258–266. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Again Study cohort. Menopause. 2014;21:924–932. doi: 10.1097/GME.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberger JT, Kravitz HM, Chang Y, et al. Does risk for anxiety increase during the menopausal transition? Menopause. 2013;20:488–495. doi: 10.1097/GME.0b013e3182730599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods NF, Mitchell ES, Landis C. Anxiety, hormonal changes and vasomotor symptoms during the menopause transition. Menopause. 2005;12:242–245. doi: 10.1097/01.gme.0000161054.45892.01. [DOI] [PubMed] [Google Scholar]
- 11.Lermer MA, Morra A, Moineddin R, Manson J, Blake J, Tierney MC. Somatic and affective anxiety symptoms and menopausal hot flashes. Menopause. 2011;18:129–132. doi: 10.1097/gme.0b013e3181ec58f8. [DOI] [PubMed] [Google Scholar]
- 12.Hanish LJ, Hantsoo L, Freeman EW, Sullivan GM, Coyne JC. Hot flashes and panic attacks: a comparison of symptomatology, neurobiology, treatment, and a role for cognition. Psychol Bull. 2008;134:247–269. doi: 10.1037/0033-2909.134.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Maki PM. Menopause and anxiety: immediate and long-term effects. Menopause. 2008;15:1033–1035. doi: 10.1097/gme.0b013e318186d823. [DOI] [PubMed] [Google Scholar]
- 14.Bryant C, Judd FK, Hickey M. Anxiety during the menopause transition: a systematic review. J Affect Disord. 2012;139:141–148. doi: 10.1016/j.jad.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Hickey M, Bryant C, Judd E. Evaluation and management of depressive and anxiety symptoms in midlife. Climacteric. 2012;15:3–9. doi: 10.3109/13697137.2011.620188. [DOI] [PubMed] [Google Scholar]
- 16.Martin P. The epidemiology of anxeity disorders: a review. Dialogues Clin Neurosci. 2003;5:281–298. doi: 10.31887/DCNS.2003.5.3/pmartin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittchen H-U, Kessler RC, Beesdo K, Krause P, Hofler M, Hoyer J. Generalized anxiety and depression in primary care: prevalence, recognition and management. J Clin Psychiatry. 2002;63(Suppl 8):24–34. [PubMed] [Google Scholar]
- 18.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-II-R psychiatric disorders in the United States: results from the national comorbidity survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Wittchen HU. Patterns and correlates of generalized anxiety disorder in community samples. J Clin Psychiatry. 2002;63(Suppl 8):4–10. [PubMed] [Google Scholar]
- 20.Greenblum CA, Rowe MA, Neff DF, Greenblum JS. Midlife women: symptoms associated with menopausal transition and early postmenopause and quality of life. Menopause. 2013;20:22–27. doi: 10.1097/gme.0b013e31825a2a91. [DOI] [PubMed] [Google Scholar]
- 21.Whiteley J, DiBonaventura MD, Wagner J-S, Alvir J, Shah S. The impact of menopausal symptoms on quality of life, productivity and economic outcomes. J Women’s Health. 2013;22:983–990. doi: 10.1089/jwh.2012.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uguz F, Sahingoz M, Gezginc K, Ayhan MG. Quality of life in postmenopausal women: the impact of depressive and anxiety disorders. Int J Psychiatry Med. 2011;41:281–292. doi: 10.2190/PM.41.3.g. [DOI] [PubMed] [Google Scholar]
- 23.Nelson DB, Sammel MD, Freeman EW, Liu L, Langan E, Gracia CR. Predicting participation in prospective studies of ovarian again. Menopause. 2004;11(5):543–548. doi: 10.1097/01.gme.0000139770.14675.40. [DOI] [PubMed] [Google Scholar]
- 24.Freeman EW, Sammel MD, Liu L, Martine P. Psychometric properties of a menopausal symptom list. Menopause. 2003;10:258–265. doi: 10.1097/00042192-200310030-00014. [DOI] [PubMed] [Google Scholar]
- 25.Zung WWK. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 26.Rose M, Devine J. Assessment of patient-reported symptoms of anxiety. Dialogues Clin Neurosci. 2014;16:197–211. doi: 10.31887/DCNS.2014.16.2/mrose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen S, Kararck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 29.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 30.Carpenter JS, Igega CM, Otte JL, Burns DS, Yu M, Wu J. Somatosensory amplification and menopausal symptoms in breast cancer survivors and midlife women. Maturitas. 2014;78:51–55. doi: 10.1016/j.maturitas.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 32.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopause women: a randomized controlled trial. JAMA. 2011;305:267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 34.Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014;174:1058–1066. doi: 10.1001/jamainternmed.2014.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guthrie KA, LaCrois AZ, Ensrud KE, et al. Pooled analysis of six pharmacologic and nonpharmacologic interventions for vasomotor symptoms. Obstet Gynecol. 2015;126:413–422. doi: 10.1097/AOG.0000000000000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orleans RJ, Li L, Kim M-J, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med. 2014;370:1777–1779. doi: 10.1056/NEJMp1402080. [DOI] [PubMed] [Google Scholar]
- 37.ACOG Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202–216. doi: 10.1097/01.AOG.0000441353.20693.78. [DOI] [PubMed] [Google Scholar]
- 38.Pinkerton JV, Kagan R, Portman D, Sathyanarayana R, Sweeney M Breeze 3 investigators. Phase 3 randomized controlled study of gastroretentive gabapentin for the treatment of moderate-to-severe hot flashes in menopause. Menopause. 2014;21:567–573. doi: 10.1097/GME.0b013e3182a7c073. [DOI] [PubMed] [Google Scholar]
- 39.Hayes LP, Carroll DG, Kelley KW. Use of gabapentin for the management of natural or surgical menopausal hot flashes. Ann Pharmacother. 2011;45:388–394. doi: 10.1345/aph.1P366. [DOI] [PubMed] [Google Scholar]
- 40.Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479–486. doi: 10.1007/s10549-012-2251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smits JA, Berry AC, Tart CD, Powers MB. The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: a meta-analytic review. Behav Res Ther. 2008;46:1047–1054. doi: 10.1016/j.brat.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Herring MP, Lindheimer JB, O’Connor PJ. The effects of exercise training on anxiety. Am J Lifestyle Med. 2013;8:388–403. [Google Scholar]
- 43.Smits JA, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW. Reducing anxiety sensitivity with exercise. Depress anxiety. 2008;25:689–699. doi: 10.1002/da.20411. [DOI] [PubMed] [Google Scholar]