Abstract
Polydopamine (PDA) prepared in the form of a layer of polymerized dopamine (DA) in a weak alkaline solution has been used as a versatile biomimetic surface modifier as well as a broadly used immobilizing macromolecule. This review mainly discusses the progress of biomaterial surface modification inspired by the participation of PDA in bone tissue engineering. A comparison between PDA-assisted coating techniques and traditional surface modification applied to bone tissue engineering is first presented. Secondly, the chemical composition and the underlying formation mechanism of PDA coating layer as a unique surface modifier are interpreted and discussed. Furthermore, several typical examples are provided to evidence the importance of PDA-assisted coating techniques in the construction of bone biosubstitutes and the improvement of material biocompatibility. Nowadays, the application of PDA as a superior surface modifier in multifunctional biomaterials is drawing tremendous interests in bone tissue scaffolds to promote the osteointegration for bone regeneration.
1. Introduction
Tissue engineering aims to engineer biosubstitutes to repair and regenerate injured tissues to improve the quality of life of individuals. The key to successfully fabricate such biosubstitutes requires the optimal combination of isolated cells, tunable factors, and supportive scaffolds [1–3]. Due to the complexity of tissue regeneration regarding various cases of malformations, occasional accidents in regeneration, chronic infections, and functional failure of end-organs, the biosubstitute prototyping becomes extremely intricate and individualized [4–7]. Musculoskeletal diseases are facing challenges generated by the rapid increase of worldwide aging problem, most of which need the assistance of foreign substitutes to heal during common clinic orthopedic operations such as bone filling and articular replacement [7]. Living tissue donation is considered the best choice for most cases regardless of some implants that may have the nonnegligible drawbacks such as allogeneic diseases, foreign body reactions, and immunogenicity [8–10]. However, due to increasing demand and shortage of living tissue sources, the artificial biosubstitutes become a promising alternative. Nowadays, biosubstitute has benefited from different materials and processing technologies, such as decellularized matrix, biomimetic scaffolds, and multidimensional organ printing [11]. For example, in order to prevent inflammation, the use of cationic Zn with high concentration has been incorporated into biomaterials as an anti-inflammatory molecule to trigger immune cells (polymorphonuclear cells, PMNs) in acute responses [12–14]. A vessel network was used to construct thick 3D bone implants in order to improve vascularization by integrating microfabrication, signaling cell coculturing, and prolonging the release of angiogenesis growth factors during the regeneration of injured bone tissues [15, 16].
The surface modification of bone biosubstitutes is an advanced technology that is capable of awarding biosubstitutes multiple functions to better meet the requirements of bone regeneration, such as cell affinity, material biocompatibility, nontoxicity, and biodegradability. Approaches to traditional surface modification are usually limited by tedious preparation steps and rigorous reaction conditions, which are thereby restrained to limited number of material categories [17]. Comparably, surface modification inspired by polymerized dopamine (polydopamine, PDA) has recently become a simple, safe, effective, and cost-saving alternative way to satisfy the requirements of bone tissue engineering. PDA-assisted surface modification is strongly subjected to the formation of PDA coating on the biomaterial interface caused by the oxidation reaction of dopamine (DA) in weak alkaline solution. PDA coating exhibits extensive chemical properties spanning from the protection against light damage to the role as biosensors transmitting the biological information, among which the adhesive function of PDA coating is one of the most useful features associated with bone tissue regeneration [18]. It can act as a modifier on the interface of different bone biosubstrates and improve their interfacial properties that may provide considerable options for tissue constructs.
2. PDA and the Formation Mechanism of PDA Coating Layer
The adhesive function of polydopamine originates from the study of mussel adhesive proteins (MAPs) secreted by marine mussel (e.g., Mytilus edulis). It was found that MAP is able to form adhesive “feet” to attach to different materials in humid environment and even rigorously trembling environment [19]. MAPs contain abundant 3,4-dihydroxy-L-phenylalanine (DOPA) that enable mussel organism to adhere to almost all kinds of materials via covalent and noncovalent bonding [19, 20]. As a precursor of PDA, DA has functional groups (catechol and phenethylamine) and adhesive mechanism similar to DOPA, which offers DA the potential as a versatile surface modifier in various biomedical applications [21–23]. DA was first applied as an important hormone and neurotransmitter of central nervous system in the treatment of Parkinson's disease in the early 1970s [24]. It plays an essential role in acquisition, emotional regulation, drug addiction, and behavioral control [25–28]. The bioadhesive application of DA has been documented, which highlights PDA for the same adhesive purpose because PDA is derived from DA and it has characteristics similar to DA [29]. In addition, due to the composition similar to some proteins in organism, PDA can also offer some bioprotection functions, such as photoprotection, reactive oxygen species scavenging (ROS), and metal cation sequestering [30, 31].
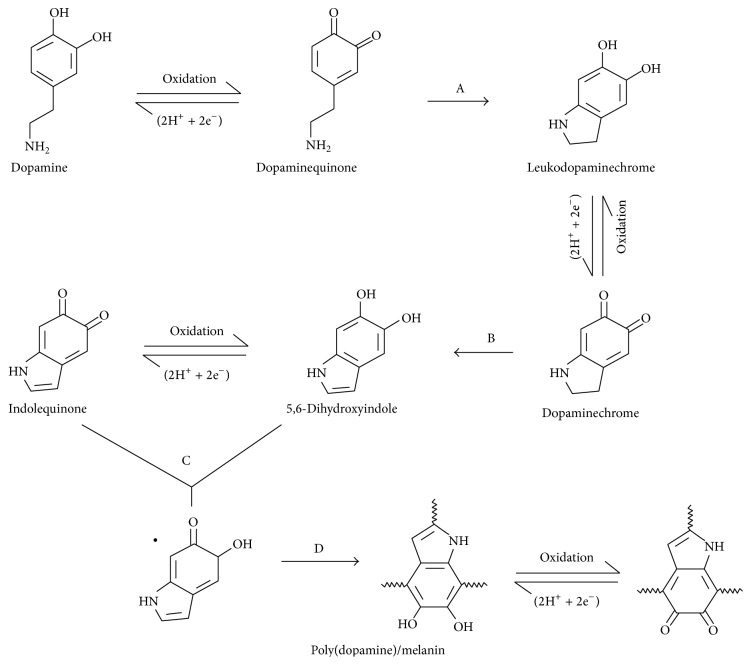
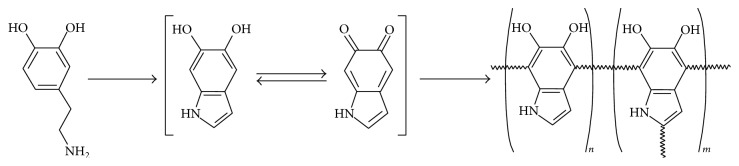
To date, the adhesive function of PDA enables it to become a useful biomimetic surface modifier and biomolecule immobilizer. However, the adhesive mechanism of PDA on the interface of various substrates still remains debating due to the complexities of structural analysis of PDA and its analogous molecules such as eumelanin [32–35]. The analogy of PDA to eumelanin is a useful approach to understand the chemical formation of PDA layer [36]. Eumelanin is naturally formed with the L-3,4-dihydroxyphenylalanine (L-DOAP) or tyrosine as precursors. These DA based monomers and other important conditions of PDA polymerization become decisive factors to impact the formation of PDA layer [31, 37]. Several mechanisms of the PDA coating layer have been developed and they all consist essentially of two steps as shown in Figure 1, including the autooxidation and intramolecular rearrangement of DA monomers and polymerization of DA monomers [29, 31, 38]. The first step involves the occurrence of autooxidation of DA monomers where dopaminequinone is synthesized in the presence of dissolved oxygen, alkaline buffer, and adequate supply of initial dopamine monomers. Dopaminequinone is subjected to intramolecular cyclization (A substep), reversible oxidation to form dopaminechrome, and intramolecular rearrangement to lead to 5,6-dihydroxyindole (B substep). The reverse dismutation reaction occurs between 5,6-dihydroxyindole and its further oxidized intermediate (indolequinone) (C substep) finally leads to PDA (D sub-step) [31]. The homomonomer and heteromonomer of indoles in various oxidation states are shown in Figure 2 [39]. The second step of PDA formation still remains elusive. Several popular models have clearly addressed the polymerization of DA monomers. The first model exhibits a “covalent bonding” polymerization fashion where DA is polymerized via 5,6-dihydroxyindole monomers following further oxidization and subsequent intermolecular cross-linking of PDA [29, 40–44]. The second model is the “noncovalent bonding (quinhydrones)” polymerization fashion where DA monomers are linked together by noncovalent interaction including hydrogen bonding, charge transfer, ionic interaction, and π-stacking to aggregate into a structural macroassembly [35, 45–47]. As PDA polymer units, the polymeric synthesis of quinhydrones has been well documented by the oxidative polymerization using biomacromolecules or supramolecules as a template [48, 49]. The “noncovalent bonding” fashion is a controversial assumption that has not been broadly acknowledged while the “covalent bonding” assumption has been partly proved via C-C bonding among the benzene rings of the dihydroxyindoline and indolinedione and open-chain monomer units [38, 50].
Figure 1.
The schematic formation of PDA coating layer [31].
Figure 2.
Different monomer states during the polymerization of DA to form PDA layer [39].
3. Immobilization of Functionalized Biomolecules
Upon the occurrence of oxidative polymerization of DA, PDA coating layer can be perfectly formed on various interfaces of substrates including noble metals, oxides, ceramics, polymers, and semiconductors [29]. Such a PDA coating layer with tunable thickness of 10–100 nm as functions of incubation time and various material interfaces [51, 52] can offer good surface properties such as high hydrophilicity, long-term corrosion resistance, and moderate physicochemical properties [31, 53, 54]. In order to optimize versatile functions of PDA coating in real environments, some additional biomolecules are considered to anchor on the PDA coating layer. Nucleophilic agents containing functional groups such as amino, thiol, imidazole, and mercapto can readily attack the catechol or diketone groups exposed on the PDA layer via Shiff-base and Michael addition reactions so that to provide improved surface properties to the biosubstitutes [29, 55, 56]. In addition, biomacromolecules, such as proteins, peptides, and DNA, can anchor on the surface of biosubstitutes via the PDA coating layer [50, 57–60]. Such coordination bonding and strong chelating bonding with transition metal compound occur between catechol or diketone groups on the PDA coating layer and the interface of inorganic biosubstitutes along with the protein cross-linking [61–66]. Meanwhile, on certain specific surfaces of inorganic biosubstitutes (e.g., negatively charged oxidized silicone), the electrostatic interaction from protonated amino group can also enhance the adhesion of biomolecules to the surface of biosubstitutes [62, 67]. For the surface of organic material, the oxidation of catechol group exposed on the PDA coating layer results in the formation of quinone/semiquinone to form irreversible covalent bonding [19] between biomolecules and the surface of organic material via similar Michael addition and Schiff formation reactions [29] and thereby further trigger the intermolecular cross-linking reactions among PDA polymers. It should be noted that the DA and DOPA exhibit similar surface-modification function due to the same functional groups and similar structures. However, such a function of surface modification inspired by PDA coating layer varies according to different biosubstitutes and different immobilized biomolecules [68].
4. Advantages for Applications of PDA in Bone Biosubstitute
Although bone graft is commonly used in clinical treatment (such as autograft, allograft, and xenograft) it is still not ideal for treatment of bone defects. The ideal scaffold of biosubstitute materials for bone defects aims to enable cell attachment and proliferation, possess osteodifferentiation ability, and implement mineralization performance. PDA-assisted surface modification can offer these properties described in the following section.
PDA has been reported to be one of the mineral inducers to promote the formation of mineralized surface. It is known that nanohydroxyapatite (nano-HAp) and collagen are two major components of natural bone. The capability of bone biosubstitute inducing the mineralization containing the nucleation of calcium and the crystallization of nano-Hap in a real wound environment is an important index to evaluate the effectiveness of bone repair [69]. The bone biosubstitutes can be fabricated from many different material categories from metals, inorganics to organics, such as noble metals, metal oxides, ceramics, and polymers. The formation of PDA coating layer on the silicon-based biosubstitutes has been found to assist in the mineralization [23]. In addition, under the assistance of PDA coating layer, the HA coated biosubstitute surface (e.g., titanium alloy, Ti6Al4V) can immobilize some active biomolecules such as BMP-2, and these immobilized biomolecules can be uniformly distributed on the surface and exhibit a sustainable release profile once in a real bone repair environment [70].
PDA is noncytotoxic and the formed PDA coating layer can control cellular behaviors. It has been reported that PDA enhances the proliferation and calcium deposition of osteoblast cells and the effect can be further strengthened in the combination of growth factors [71, 72]. The immobilization of rabbit chondrocytes has been improved with the formation of PDA coating layer on biosubstitutes as compared to unmodified surface. Additionally, the PDA coated surface can enhance the attachment of NT3T3 fibroblast cell [73, 74]. The amine-(thiol-) terminated methoxy-poly(ethylene glycol) (mPEG-SH/mPEG-NH2) with PDA coating layer can improve the cell adhesion behavior although this biosubstitute material exhibits an adverse effect of inhibiting the growth of NIH 3T3 cells [29]. Meanwhile, PDA can be used as controllable surface modifier to reinforce the selective antifouling of biomaterial surface or the cell affinity of scaffold. Tsai et al. [75] gave an example of performing one-step codeposition of poly(ethyleneimine)-graft-poly(ethylene glycol) (PEI-g-PEG) and PEI-g-biotin via PDA coating layer on various substrates. This enables cells to selectively attach on such PDA modified surfaces. Investigation in the cell adhesion on the material surface via codeposition of PDA and PEI-g-galactose obtained a similar outcome where cells were attached in the form of specific micropatterning profile [76]. Furthermore, mediating various parameters, such as using different cell lines, preprocessing PDA, and culturing cells under different conditions, the formed PDA coating layer may offer tunable regulating properties. For example, modulating the incubation temperature of DA (PDA precursor) could result in the improvement of the ratio of quinone versus phenolic hydroxyl groups to better regulate the proliferation of smooth muscle cells attached on the biosubstitutes [77].
For some sensitive biomolecules that are easily deactivated because of changes of environmental conditions, the formed PDA coating layer can immobilize them on the biosubstitutes and meanwhile exert no or tiny impact on their activities. Growth factors including most sensitive biomolecules, such as bone morphogenic protein (BMP), Arg-Gly-Asp peptide (RGD), and alkaline phosphatase (ALP), are able to adhere to the surface of biosubstitutes via the formed PDA coating layer. Studies have shown that the activity of these biomolecules was not affected by the PDA coating layer itself except for the forming condition of PDA coating layer and the inherent properties of biomolecules (incubation time, concentration, molecule weight, and isoelectric point) [78]. Compared to traditional immobilization approaches, PDA-assisted immobilization exhibits a merit to significantly improve the anchoring performance of biomolecules [79].
In an in vivo environment, it has been found that the PDA coating layer enhances the biocompatibility of bone biosubstitutes. For a PDA-coated surface of poly-L-lactic acid (PLLA) biosubstitute, the PDA coating layer reduced the blood toxicity of CdSe quantum dots while it enhanced the immunogenicity and the biocompatibility of biosubstitute material [80]. PDA-coated substrate of biosubstitute made of nylon/cellulose/polyethersulfone composite, grafted with bovine serum albumin (BSA), can reduce the attachment of blood platelets and selectively enable BSA to anchor on the surface, which leads to the decrease of unwanted blood clotting [81]. Additionally, recent studies have shown a positive outcome of restoration and regeneration of bone tissue by means of the use of PDA-coated technology to the bone implanted materials. One reported on a collagen membrane coated with PDA on which calcitriol was absorbed [82]. The osteogenic competency of this membrane composite was examined when it was implanted into rat mandibular bone defects, suggesting that calcitriol bound to PDA-coated membrane showed strong potential in prohibition of osteoclastogenesis while it could promote the osteogenic differentiation leading to a rapid regeneration of new bone and reunion of the bone marrow cavity. Ge and coworkers [83] developed a small intestinal submucosa (SIS) modified polypropylene (PP) scaffold via the PDA-coated technology for pelvic reconstruction. The in vivo rat model study showed that SIS attached to PP via PDA indicated a low expression of proinflammatory but a high expression of promacrophages which significantly contributed to pelvic tissue reconstruction. Study conducted by Madhurakkat Perikamana and coworkers [84] used a poly(L-lactic acid) (PLLA) nanofibril scaffold with an aligned morphology to explore the in vivo collagen assembly of regenerated bone via a mouse calvarial defect model. They utilized PDA-coated technology to immobilize bone morphogenic protein-2 (BMP-2) on the aligned PLLA nanofibers. The surface immobilized BMP-2 via PDA coating successfully controlled collagen fiber assembly in the in vitro environmental, which may be promising to be used to engineer structurally relevant bone. This scaffold also played an active role in the new bone formation where the in vivo bone regeneration was significantly better than control groups without PDA binding BMP-2. Li and coworkers [85] also exploited PDA-coated technology to immobilize bone forming peptide-1 (BFP-1) onto the poly(lactic-co-glycolic acid) (PLGA) surface. BFP-1 modified PLGA scaffold achieved a higher osteogenic activity than BMP-2 and the in vivo study further demonstrated that it significantly promoted bone formation in a nude murine model. Gao and coworkers [86] used PDA-coated technology to bind nanohydroxyapatite (nano-HA) rather than bone growth factors onto the polycaprolactone (PCL) surface. The results showed that nano-HA modified PCL via PDA coating enhanced the cell adhesion and proliferation, as well as promoting the bone regeneration even in the absence of osteogenesis soluble inducing factors in in vivo tests. In all the above examples, the use of PDA-coated technology is a relatively simple and rapid process requiring only a material-immersing preparation. Various active molecules can be immobilized on various surfaces via such a fascinating PDA-coating chemistry, which thereby greatly offers considerable merits to bone regeneration.
5. Material Categories of Bone Biosubstitutes Adapted to PDA-Assisted Modification
Most applications of PDA-assisted surface modification are used in bone tissue engineering. As a qualified bone biosubstitute, it should offer good biocompatibility, multiple functions, and other superior physical properties. Compared to traditional surface modification approaches including physical deposition/absorption, chemical modification/grafting, and plasma technology, the PDA-assisted surface modification may remedy some drawbacks from traditional approaches [71]. Different material categories of bone biosubstitutes must be adapted to PDA-assisted modification and the processing of PDA coating layer may also vary with different material categories to achieve the best outcome. Two kinds of typical materials that are used to prepare bone biosubstitutes can achieve a significant improvement of surface properties via PDA-assisted surface modification, which would be conducive to the cell growth and tissue regeneration.
Metallic materials and their alloys are one important category of materials used to fabricate bone biosubstitutes for many orthopedic operations, such as bone fracture and articular replacement. For orthopedic and dental implants, Ti6Al4V is a broadly used metallic composite material consisting of inorganic materials, metallic titanium, and its alloy. Once it is coated by the PDA layer, a special strong charge transfer interaction would be formed between the formed PDA coating layer and Ti, which can offer the biosubstitute a strong corrosive resistance, an improved mechanical properties, and a high superficial energy [87]. In addition, the PDA-assisted surface modification to Ti and Ti alloys can also offer the osteoinductivity and osteoconductivity to bioinert metallic bone biosubstitutes and enhance their bioactivities regarding the viability, proliferation, and differentiation of cell and material mineralization [23, 71, 72, 88]. Furthermore, the PDA coating layer can effectively immobilize vascular endothelial growth factor (VEGF) on the Ti-based biosubstitute without the adverse effect on the activity of VEGF [89]. Such a layer-by-layered composite bone biosubstitute combining VEGF, PDA layer, and Ti material significantly facilitates the attachment and proliferation of endothelial cells, as well as benefiting the differentiation of human mesenchymal stem cells (hMSC) to endothelial cells. In light of various functions obtained by immobilizing different biomolecules, endeavors related to the PDA-assisted surface modification on diverse metallic surfaces are being or have been performed to introduce new concepts and new processing strategies in order to enhance the revascularization ability, haemocompatibility, and antibacterial ability during the period of bone regeneration [89].
Synthetic and bioderived polymers are another important category of materials used to fabricate bone biosubstitutes, such as poly(l-lactide-co-glycolide) (PLGA), polycaprolactone (PCL), PLLA, collagen, and cellulose. Although most polymeric biomaterials are biodegradable, biocompatible, and erosion-resistant and have appropriate mechanical properties, they lack satisfactory osteointegration [90]. Through the PDA-assisted surface modification, the surface properties of these polymers can be greatly improved. Upon immobilization of BMP, the property of osteoblast cells responsive to polymeric materials can be remarkably improved, and the material mineralization can be expedited in the presence of immobilized growth factors [91–95]. Bone filling materials are broadly used in bone tissue engineering, such as plastic surgery and bone defect. The PDA coating layer on poly(dimethylsiloxane) (PDMS) can enhance the cytoaffinity of PDMS based bone cement where NIH3T3 fibroblast cells can attach more readily to the material substrate which further improves the cell proliferation [96].
6. Conclusions and Prospectives
In order to construct a multifunctional bone biosubstitute, the surface of the biosubstitute is important to combine stable functional factors and provide satisfactory cell behavior containing cell attachment, proliferation, migration/spreading, and differentiation to facilitate the bone tissue regeneration. PDA-assisted surface modification uses PDA as a biocompatible surface modifier to interact with organic and inorganic components on the interface of bone bio-substitutes. Such targeted modification makes the biphasic interface of material more stable and versatile for diverse requirements from bone tissue repair [97]. Two key advantages of PDA-assisted surface modification of bone biosubstitutes are as follows: (1) the PDA coating layer can be formed under a mild alkaline condition only with the contact of oxygen to obtain relatively satisfactory properties and other methods containing enzymatic oxidation and electropolymerization may form PDA with higher quality in a benign environment or in shorter reaction time [98]; (2) the autopolymerization of PDA precursor (DA) can occur virtually on various surfaces of materials, such as noble metals, oxide, polymers, and ceramics. As compared to traditional approaches with regard to surface modification or biomolecule immobilization, the PDA-assisted surface modification exhibits some exceptional characters containing facile processing, stable performance, and versatile application. Although the mechanism behind the formation of PDA coating layer on the material substitute still remains under debate, the PDA-assisted surface modification is expanding its applications to not only bone biosubstitutes but also cardiovascular devices, biosensors, neuron pseudosubstitutes, and sustainable drug delivery [18, 99–101].
Acknowledgments
This work was supported by the Natural Science Foundation of China (31570967, 31370978, and 81472078), the Shenzhen Science and Technology Program (JCYJ20140610152828698), and the Shenzhen Key Laboratory of Marine Biomedical Materials (ZDSY20130401165820356).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Langer R., Vacanti J. P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Williams D. Benefit and risk in tissue engineering. Materials Today. 2004;7(5):24–29. doi: 10.1016/S1369-7021(04)00232-9. [DOI] [Google Scholar]
- 3.Williams D. F. The Williams Dictionary of Biomaterials. Liverpool, UK: Liverpool University Press; 1999. [Google Scholar]
- 4.Burchill L. J., Ross H. J. Heart transplantation in adults with end-stage congenital heart disease. Future Cardiology. 2012;8(2):329–342. doi: 10.2217/fca.12.11. [DOI] [PubMed] [Google Scholar]
- 5.Molzahn A. E., Starzomski R., McCormick J. The supply of organs for transplantation: issues and challenges. Nephrology Nursing Journal. 2003;30(1):17–28. [PubMed] [Google Scholar]
- 6.Macchiarini P., Jungebluth P., Go T., et al. Clinical transplantation of a tissue-engineered airway. The Lancet. 2008;372(9655):2023–2030. doi: 10.1016/s0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 7.Ott H. C., Matthiesen T. S., Goh S.-K., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nature Medicine. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 8.Rose F. R. A. J., Oreffo R. O. C. Bone tissue engineering: hope vs hype. Biochemical and Biophysical Research Communications. 2002;292(1):1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 9.Hench L. L., Polak J. M. Third-generation biomedical materials. Science. 2002;295(5557):1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 10.Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24(13):2133–2151. doi: 10.1016/s0142-9612(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 11.Jaganathan S. K., Supriyanto E., Murugesan S., Balaji A., Asokan M. K. Biomaterials in cardiovascular research: applications and clinical implications. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/459465.459465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velard F., Laurent-Maquin D., Braux J., et al. The effect of zinc on hydroxyapatite-mediated activation of human polymorphonuclear neutrophils and bone implant-associated acute inflammation. Biomaterials. 2010;31(8):2001–2009. doi: 10.1016/j.biomaterials.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Anderson J. M., Miller K. M. Biomaterial biocompatibility and the macrophage. Biomaterials. 1984;5(1):5–10. doi: 10.1016/0142-9612(84)90060-7. [DOI] [PubMed] [Google Scholar]
- 14.Grandjean-Laquerriere A., Laquerriere P., Jallot E., et al. Influence of the zinc concentration of sol-gel derived zinc substituted hydroxyapatite on cytokine production by human monocytes in vitro. Biomaterials. 2006;27(17):3195–3200. doi: 10.1016/j.biomaterials.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Kaully T., Kaufman-Francis K., Lesman A., Levenberg S. Vascularization—the conduit to viable engineered tissues. Tissue Engineering Part B: Reviews. 2009;15(2):159–169. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 16.Lovett M., Lee K., Edwards A., Kaplan D. L. Vascularization strategies for tissue engineering. Tissue Engineering Part B: Reviews. 2009;15(3):353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Q., Zhou F., Liu W. Bioinspired catecholic chemistry for surface modification. Chemical Society Reviews. 2011;40(7):4244–4258. doi: 10.1039/c1cs15026j. [DOI] [PubMed] [Google Scholar]
- 18.Lynge M. E., Van Der Westen R., Postma A., Städler B. Polydopamine—a nature-inspired polymer coating for biomedical science. Nanoscale. 2011;3(12):4916–4928. doi: 10.1039/c1nr10969c. [DOI] [PubMed] [Google Scholar]
- 19.Waite J. H., Qin X. X. Polyphosphoprotein from the adhesive pads of Mytilus edulis . Biochemistry. 2001;40(9):2887–2893. doi: 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- 20.Papov V. V., Diamond T. V., Biemann K., Waite J. H. Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel Mytilus edulis . The Journal of Biological Chemistry. 1995;270(34):20183–20192. doi: 10.1074/jbc.270.34.20183. [DOI] [PubMed] [Google Scholar]
- 21.Ku S. H., Lee J. S., Park C. B. Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine. Langmuir. 2010;26(19):15104–15108. doi: 10.1021/la102825p. [DOI] [PubMed] [Google Scholar]
- 22.Ku S. H., Ryu J., Hong S. K., Lee H., Park C. B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials. 2010;31(9):2535–2541. doi: 10.1016/j.biomaterials.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Ryu J., Ku S. H., Lee H., Park C. B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Advanced Functional Materials. 2010;20(13):2132–2139. doi: 10.1002/adfm.200902347. [DOI] [Google Scholar]
- 24.Rubinstein T. C., Giladi N., Hausdorff J. M. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson's disease. Movement Disorders. 2002;17(6):1148–1160. doi: 10.1002/mds.10259. [DOI] [PubMed] [Google Scholar]
- 25.Di Ciano P., Cardinal R. N., Cowell R. A., Little S. J., Everitt B. J. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. The Journal of Neuroscience. 2001;21(23):9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruhé H. G., Mason N. S., Schene A. H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Molecular Psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 27.Diehl D. J., Gershon S. The role of dopamine in mood disorders. Comprehensive Psychiatry. 1992;33(2):115–120. doi: 10.1016/0010-440x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- 28.Grace A. A., Floresco S. B., Goto Y., Lodge D. J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Lee H., Dellatore S. M., Miller W. M., Messersmith P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meredith P., Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Research. 2006;19(6):572–594. doi: 10.1111/j.1600-0749.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 31.Yu F., Chen S., Chen Y., et al. Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. Journal of Molecular Structure. 2010;982(1–3):152–161. doi: 10.1016/j.molstruc.2010.08.021. [DOI] [Google Scholar]
- 32.Bernsmann F., Ponche A., Ringwald C., et al. Characterization of dopamine−melanin growth on silicon oxide. The Journal of Physical Chemistry C. 2009;113(19):8234–8242. doi: 10.1021/jp901188h. [DOI] [Google Scholar]
- 33.Clark M. B., Jr., Gardella J. A., Jr., Schultz T. M., Patil D. G., Salvati L., Jr. Solid-state analysis of eumelanin biopolymers by electron spectroscopy for chemical analysis. Analytical Chemistry. 1990;62(9):949–956. doi: 10.1021/ac00208a011. [DOI] [Google Scholar]
- 34.Dreyer D. R., Miller D. J., Freeman B. D., Paul D. R., Bielawski C. W. Elucidating the structure of poly(dopamine) Langmuir. 2012;28(15):6428–6435. doi: 10.1021/la204831b. [DOI] [PubMed] [Google Scholar]
- 35.Dreyer D. R., Miller D. J., Freeman B. D., Paul D. R., Bielawski C. W. Perspectives on poly(dopamine) Chemical Science. 2013;4(10):3796–3802. doi: 10.1039/c3sc51501j. [DOI] [Google Scholar]
- 36.d'Ischia M., Napolitano A., Ball V., Chen C.-T., Buehler M. J. Polydopamine and eumelanin: from structure-property relationships to a unified tailoring strategy. Accounts of Chemical Research. 2014;47(12):3541–3550. doi: 10.1021/ar500273y. [DOI] [PubMed] [Google Scholar]
- 37.Ball V., Del Frari D., Toniazzo V., Ruch D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: insights in the polydopamine deposition mechanism. Journal of Colloid and Interface Science. 2012;386(1):366–372. doi: 10.1016/j.jcis.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Liebscher J., Mrówczyński R., Scheidt H. A., et al. Structure of polydopamine: a never-ending story? Langmuir. 2013;29(33):10539–10548. doi: 10.1021/la4020288. [DOI] [PubMed] [Google Scholar]
- 39.Peterson M. B., Le-Masurier S. P., Lim K., Hook J. M., Martens P., Granville A. M. Incorporation of 5-hydroxyindazole into the self-polymerization of dopamine for novel polymer synthesis. Macromolecular Rapid Communications. 2014;35(3):291–297. doi: 10.1002/marc.201300746. [DOI] [PubMed] [Google Scholar]
- 40.Pezzella A., Iadonisi A., Valerio S., et al. Disentangling Eumelanin ‘black chromophore’: visible absorption changes as signatures of oxidation state- and aggregation-dependent dynamic interactions in a model water-soluble 5,6-dihydroxyindole polymer. Journal of the American Chemical Society. 2009;131(42):15270–15275. doi: 10.1021/ja905162s. [DOI] [PubMed] [Google Scholar]
- 41.D'Ischia M., Napolitano A., Pezzella A. 5,6-dihydroxyindole chemistry: unexplored opportunities beyond eumelanin. European Journal of Organic Chemistry. 2011;(28):5501–5516. doi: 10.1002/ejoc.201100796. [DOI] [Google Scholar]
- 42.d'Ischia M., Napolitano A., Pezzella A., Meredith P., Sarna T. Chemical and structural diversity in eumelanins: unexplored bio-optoelectronic materials. Angewandte Chemie—International Edition. 2009;48(22):3914–3921. doi: 10.1002/anie.200803786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shalev T., Gopin A., Bauer M., Stark R. W., Rahimipour S. Non-leaching antimicrobial surfaces through polydopamine bio-inspired coating of quaternary ammonium salts or an ultrashort antimicrobial lipopeptide. Journal of Materials Chemistry. 2012;22(5):2026–2032. doi: 10.1039/c1jm13994k. [DOI] [Google Scholar]
- 44.Bernsmann F., Ponche A., Ringwald C., et al. Characterization of dopamine-melanin growth on silicon oxide. The Journal of Physical Chemistry C. 2009;113(19):8234–8242. doi: 10.1021/jp901188h. [DOI] [Google Scholar]
- 45.Della Vecchia N. F., Avolio R., Alfè M., Errico M. E., Napolitano A., D'Ischia M. Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Advanced Functional Materials. 2013;23(10):1331–1340. doi: 10.1002/adfm.201202127. [DOI] [Google Scholar]
- 46.Patil A. O., Pennington W. T., Desiraju G. R., Curtin D. Y., Paul I. C. Recent studies on the formation and properties of quinhydrone complexes. Molecular Crystals and Liquid Crystals. 1986;134:279–304. [Google Scholar]
- 47.Scheffer J. R., Wong Y.-F., Patil A. O., Curtin D. Y., Paul I. C. CPMAS 13C NMR spectra of quinones, hydroquinones, and their complexes. Use of CMR to follow a reaction in the solid state. Journal of the American Chemical Society. 1985;107(17):4898–4904. doi: 10.1021/ja00303a014. [DOI] [Google Scholar]
- 48.He J., Zhang A., Zhang Y., Guan Y. Novel redox hydrogel by in situ gelation of chitosan as a result of template oxidative polymerization of hydroquinone. Macromolecules. 2011;44(7):2245–2252. doi: 10.1021/ma1029532. [DOI] [Google Scholar]
- 49.Zhang A., He J., Guan Y., Li Z., Zhang Y., Zhu J. X. Oxidative polymerization of hydroquinone using deoxycholic acid supramolecular template. Science China Chemistry. 2012;55(5):830–835. doi: 10.1007/s11426-012-4504-2. [DOI] [Google Scholar]
- 50.Mrówczyński R., Turcu R., Leostean C., Scheidt H. A., Liebscher J. New versatile polydopamine coated functionalized magnetic nanoparticles. Materials Chemistry and Physics. 2013;138(1):295–302. doi: 10.1016/j.matchemphys.2012.11.059. [DOI] [Google Scholar]
- 51.Terrill H. C. Optimization of Polydopamine Coatings. Akron, Ohio, USA: University of Akron; 2015. (Hornors Research Projects). [Google Scholar]
- 52.Zhou P., Deng Y., Lyu B., et al. Rapidly-deposited polydopamine coating via high temperature and vigorous stirring: formation, characterization and biofunctional evaluation. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0113087.e113087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang J.-H., Zhu L.-P., Li X.-L., Xu Y.-Y., Zhu B.-K. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. Journal of Membrane Science. 2010;364(1-2):194–202. doi: 10.1016/j.memsci.2010.08.017. [DOI] [Google Scholar]
- 54.Chen S., Chen Y., Lei Y., Yin Y. Novel strategy in enhancing stability and corrosion resistance for hydrophobic functional films on copper surfaces. Electrochemistry Communications. 2009;11(8):1675–1679. doi: 10.1016/j.elecom.2009.06.021. [DOI] [Google Scholar]
- 55.Sugumaran M., Dali H., Semensi V. Chemical- and cuticular phenoloxidase-mediated synthesis of cysteinyl-catechol adducts. Archives of Insect Biochemistry and Physiology. 1989;11(2):127–137. doi: 10.1002/arch.940110206. [DOI] [Google Scholar]
- 56.Burzio L. A., Waite J. H. Cross-linking in adhesive quinoproteins: studies with model decapeptides. Biochemistry. 2000;39(36):11147–11153. doi: 10.1021/bi0002434. [DOI] [PubMed] [Google Scholar]
- 57.Lee H., Rho J., Messersmith P. B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Advanced Materials. 2009;21(4):431–434. doi: 10.1002/adma.200801222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou W.-H., Tang S.-F., Yao Q.-H., Chen F.-R., Yang H.-H., Wang X.-R. A quartz crystal microbalance sensor based on mussel-inspired molecularly imprinted polymer. Biosensors and Bioelectronics. 2010;26(2):585–589. doi: 10.1016/j.bios.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Ren Y., Rivera J. G., He L., Kulkarni H., Lee D.-K., Messersmith P. B. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnology. 2011;11, article 63 doi: 10.1186/1472-6750-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Q., Wang X., Yu B., Zhou F., Xue Q. Self-healing surface hydrophobicity by consecutive release of hydrophobic molecules from mesoporous silica. Langmuir. 2012;28(13):5845–5849. doi: 10.1021/la300187q. [DOI] [PubMed] [Google Scholar]
- 61.Harrington M. J., Masic A., Holten-Andersen N., Waite J. H., Fratzl P. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science. 2010;328(5975):216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H., Scherer N. F., Messersmith P. B. Single-molecule mechanics of mussel adhesion. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z., Jordan R. B. Kinetics of dissociation of iron(III) complexes of tiron in aqueous acid. Inorganic Chemistry. 1996;35(6):1571–1576. doi: 10.1021/ic941303v. [DOI] [PubMed] [Google Scholar]
- 64.Schmitt L., Ludwig M., Gaub H. E., Tampé R. A metal-chelating microscopy tip as a new toolbox for single-molecule experiments by atomic force microscopy. Biophysical Journal. 2000;78(6):3275–3285. doi: 10.1016/S0006-3495(00)76863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sever M. J., Weisser J. T., Monahan J., Srinivasan S., Wilker J. J. Metal-mediated cross-linking in the generation of a marine-mussel adhesive. Angewandte Chemie—International Edition. 2004;43(4):448–450. doi: 10.1002/anie.200352759. [DOI] [PubMed] [Google Scholar]
- 66.Holten-Andersen N., Mates T. E., Toprak M. S., Stucky G. D., Zok F. W., Waite J. H. Metals and the integrity of a biological coating: the cuticle of mussel byssus. Langmuir. 2009;25(6):3323–3326. doi: 10.1021/la8027012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ham H. O., Liu Z., Lau K. H. A., Lee H., Messersmith P. B. Facile DNA immobilization on surfaces through a catecholamine polymer. Angewandte Chemie—International Edition. 2011;50(3):732–736. doi: 10.1002/anie.201005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang S. M., Rho J., Choi I. S., Messersmith P. B., Lee H. Norepinephrine: material-independent, multifunctional surface modification reagent. Journal of the American Chemical Society. 2009;131(37):13224–13225. doi: 10.1021/ja905183k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu Y., Zhu Y., Zhou X., Ruan C., Pan H., Catchmark J. M. Bioabsorbable cellulose composites prepared by an improved mineral-binding process for bone defect repair. Journal of Materials Chemistry B. 2016;4(7):1235–1246. doi: 10.1039/c5tb02091c. [DOI] [PubMed] [Google Scholar]
- 70.Cai Y., Wang X., Poh C. K., et al. Accelerated bone growth in vitro by the conjugation of BMP2 peptide with hydroxyapatite on titanium alloy. Colloids and Surfaces B: Biointerfaces. 2014;116:681–686. doi: 10.1016/j.colsurfb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Chien C.-Y., Liu T.-Y., Kuo W.-H., Wang M.-J., Tsai W.-B. Dopamine-assisted immobilization of hydroxyapatite nanoparticles and RGD peptides to improve the osteoconductivity of titanium. Journal of Biomedical Materials Research—Part A. 2013;101(3):740–747. doi: 10.1002/jbm.a.34376. [DOI] [PubMed] [Google Scholar]
- 72.Chien C.-Y., Tsai W.-B. Poly(dopamine)-assisted immobilization of Arg-Gly-Asp peptides, hydroxyapatite, and bone morphogenic protein-2 on titanium to improve the osteogenesis of bone marrow stem cells. ACS Applied Materials & Interfaces. 2013;5(15):6975–6983. doi: 10.1021/am401071f. [DOI] [PubMed] [Google Scholar]
- 73.Sun K., Xie Y., Ye D., et al. Mussel-inspired anchoring for patterning cells using polydopamine. Langmuir. 2012;28(4):2131–2136. doi: 10.1021/la2041967. [DOI] [PubMed] [Google Scholar]
- 74.Sun K., Song L., Xie Y., et al. Using self-polymerized dopamine to modify the antifouling property of oligo(ethylene glycol) self-assembled monolayers and its application in cell patterning. Langmuir. 2011;27(10):5709–5712. doi: 10.1021/la2012099. [DOI] [PubMed] [Google Scholar]
- 75.Tsai W.-B., Chien C.-Y., Thissen H., Lai J.-Y. Dopamine-assisted immobilization of poly(ethylene imine) based polymers for control of cell-surface interactions. Acta Biomaterialia. 2011;7(6):2518–2525. doi: 10.1016/j.actbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Chien H.-W., Tsai W.-B. Fabrication of tunable micropatterned substrates for cell patterning via microcontact printing of polydopamine with poly(ethylene imine)-grafted copolymers. Acta Biomaterialia. 2012;8(10):3678–3686. doi: 10.1016/j.actbio.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 77.Luo R., Tang L., Zhong S., et al. In vitro investigation of enhanced hemocompatibility and endothelial cell proliferation associated with quinone-rich polydopamine coating. ACS Applied Materials and Interfaces. 2013;5(5):1704–1714. doi: 10.1021/am3027635. [DOI] [PubMed] [Google Scholar]
- 78.Nijhuis A. W. G., van den Beucken J. J. J. P., Boerman O. C., Jansen J. A., Leeuwenburgh S. C. G. 1-Step versus 2-step immobilization of alkaline phosphatase and bone morphogenetic protein-2 onto implant surfaces using polydopamine. Tissue Engineering Part C: Methods. 2013;19(8):610–619. doi: 10.1089/ten.tec.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanderleyden E., Van Bael S., Chai Y. C., Kruth J.-P., Schrooten J., Dubruel P. Gelatin functionalised porous titanium alloy implants for orthopaedic applications. Materials Science and Engineering C. 2014;42:396–404. doi: 10.1016/j.msec.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 80.Hong S., Kim K. Y., Wook H. J., et al. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine. 2011;6(5):793–801. doi: 10.2217/nnm.11.76. [DOI] [PubMed] [Google Scholar]
- 81.Wei Q., Li B., Yi N., et al. Improving the blood compatibility of material surfaces via biomolecule-immobilized mussel-inspired coatings. Journal of Biomedical Materials Research Part A. 2011;96(1):38–45. doi: 10.1002/jbm.a.32956. [DOI] [PubMed] [Google Scholar]
- 82.Liu H., Cui J., Feng W., et al. Local administration of calcitriol positively influences bone remodeling and maturation during restoration of mandibular bone defects in rats. Materials Science and Engineering C. 2015;49:14–24. doi: 10.1016/j.msec.2014.12.064. [DOI] [PubMed] [Google Scholar]
- 83.Ge L., Liu L., Wei H., et al. Preparation of a small intestinal submucosa modified polypropylene hybrid mesh via a mussel-inspired polydopamine coating for pelvic reconstruction. Journal of Biomaterials Applications. 2016;30(9):1385–1391. doi: 10.1177/0885328216628469. [DOI] [PubMed] [Google Scholar]
- 84.Madhurakkat Perikamana S. K., Lee J., Ahmad T., et al. Effects of immobilized BMP-2 and nanofiber morphology on in vitro osteogenic differentiation of hMSCs and in vivo collagen assembly of regenerated bone. ACS Applied Materials and Interfaces. 2015;7(16):8798–8808. doi: 10.1021/acsami.5b01340. [DOI] [PubMed] [Google Scholar]
- 85.Li W., Zheng Y., Zhao X., et al. Osteoinductive effects of free and immobilized bone forming peptide-1 on human adipose-derived stem cells. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150294.e0150294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao X., Song J., Ji P., et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Applied Materials & Interfaces. 2016;8(5):3499–3515. doi: 10.1021/acsami.5b12413. [DOI] [PubMed] [Google Scholar]
- 87.Li P., Kangasniemi I., de Groot K., Kokubo T. Bonelike hydroxyapatite induction by a gel-derived titania on a titanium substrate. Journal of the American Ceramic Society. 1994;77(5):1307–1312. doi: 10.1111/j.1151-2916.1994.tb05407.x. [DOI] [Google Scholar]
- 88.Yu X., Walsh J., Wei M. Covalent immobilization of collagen on titanium through polydopamine coating to improve cellular performances of MC3T3-E1 cells. RSC Advances. 2014;4(14):7185–7192. doi: 10.1039/c3ra44137g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poh C. K., Shi Z., Lim T. Y., Neoh K. G., Wang W. The effect of VEGF functionalization of titanium on endothelial cells in vitro. Biomaterials. 2010;31(7):1578–1585. doi: 10.1016/j.biomaterials.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 90.Makadia H. K., Siegel S. J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan H., Zheng Q., Yang S., Guo X. Effects of functionalization of PLGA-[Asp-PEG]n copolymer surfaces with Arg-Gly-Asp peptides, hydroxyapatite nanoparticles, and BMP-2-derived peptides on cell behavior in vitro. Journal of Biomedical Materials Research—Part A. 2014;102(12):4526–4535. doi: 10.1002/jbm.a.35129. [DOI] [PubMed] [Google Scholar]
- 92.Ko E., Yang K., Shin J., Cho S.-W. Polydopamine-assisted osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromolecules. 2013;14(9):3202–3213. doi: 10.1021/bm4008343. [DOI] [PubMed] [Google Scholar]
- 93.Lee Y. J., Lee J.-H., Cho H.-J., Kim H. K., Yoon T. R., Shin H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials. 2013;34(21):5059–5069. doi: 10.1016/j.biomaterials.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 94.Kim T.-H., Yun Y.-P., Park Y.-E., et al. In vitro and in vivo evaluation of bone formation using solid freeform fabrication-based bone morphogenic protein-2 releasing PCL/PLGA scaffolds. Biomedical Materials. 2014;9(2) doi: 10.1088/1748-6041/9/2/025008.025008 [DOI] [PubMed] [Google Scholar]
- 95.Cho H.-J., Madhurakkat Perikamana S. K., Lee J.-H., et al. Effective immobilization of BMP-2 mediated by polydopamine coating on biodegradable nanofibers for enhanced in vivo bone formation. ACS Applied Materials and Interfaces. 2014;6(14):11225–11235. doi: 10.1021/am501391z. [DOI] [PubMed] [Google Scholar]
- 96.Jun D.-R., Moon S.-K., Choi S.-W. Uniform polydimethylsiloxane beads coated with polydopamine and their potential biomedical applications. Colloids and Surfaces B: Biointerfaces. 2014;121:395–399. doi: 10.1016/j.colsurfb.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 97.Judeinstein P., Sanchez C. Hybrid organic-inorganic materials: a land of multidisciplinarity. Journal of Materials Chemistry. 1996;6(4):511–525. doi: 10.1039/jm9960600511. [DOI] [Google Scholar]
- 98.Liu Y., Ai K., Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chemical Reviews. 2014;114(9):5057–5115. doi: 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- 99.Shen W., Cai K., Yang Z., Yan Y., Yang W., Liu P. Improved endothelialization of NiTi alloy by VEGF functionalized nanocoating. Colloids and Surfaces B: Biointerfaces. 2012;94:347–353. doi: 10.1016/j.colsurfb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 100.Kang K., Choi I. S., Nam Y. A biofunctionalization scheme for neural interfaces using polydopamine polymer. Biomaterials. 2011;32(27):6374–6380. doi: 10.1016/j.biomaterials.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 101.Kim E., Lee S., Hong S., et al. Sticky ‘delivering-from’ strategies using viral vectors for efficient human neural stem cell infection by bioinspired catecholamines. ACS Applied Materials and Interfaces. 2014;6(11):8288–8294. doi: 10.1021/am5011095. [DOI] [PubMed] [Google Scholar]