Abstract
The effect of glucose as a signaling molecule on induction of aliphatic glucosinolate biosynthesis was reported in our former study. Here, we further investigated the regulatory mechanism of indolic glucosinolate biosynthesis by glucose in Arabidopsis. Glucose exerted a positive influence on indolic glucosinolate biosynthesis, which was demonstrated by induced accumulation of indolic glucosinolates and enhanced expression of related genes upon glucose treatment. Genetic analysis revealed that MYB34 and MYB51 were crucial in maintaining the basal indolic glucosinolate accumulation, with MYB34 being pivotal in response to glucose signaling. The increased accumulation of indolic glucosinolates and mRNA levels of MYB34, MYB51, and MYB122 caused by glucose were inhibited in the gin2-1 mutant, suggesting an important role of HXK1 in glucose-mediated induction of indolic glucosinolate biosynthesis. In contrast to what was known on the function of ABI5 in glucose-mediated aliphatic glucosinolate biosynthesis, ABI5 was not required for glucose-induced indolic glucosinolate accumulation. In addition, our results also indicated that glucose-induced glucosinolate accumulation was due to enhanced sulfur assimilation instead of directed sulfur partitioning into glucosinolate biosynthesis. Thus, our data provide new insights into molecular mechanisms underlying glucose-regulated glucosinolate biosynthesis.
Glucosinolates (GS) are a group of nitrogen- and sulfur-containing secondary metabolites found throughout the family Crucifereae. Glucosinolates and their degradation products have a wide range of biological functions, including their well-known roles in plant defense against generalist herbivores, feeding or oviposition preference of crucifer-specialist herbivores1,2,3 as well as inhibition of microbial growth4,5. In addition, they also serve as important flavor components and anticarcinogenic agents6,7.
Glucosinolates are derived from amino acids, and can be grouped into aliphatic, aromatic, and indolic glucosinolates depending on the characteristic of the amino acids they originate from. The main biosynthetic pathway of glucosinolates has been elucidated in Arabidopsis. For example, in indolic glucosinolate biosynthesis, CYP79B2 and CYP79B3, two Arabidopsis cytochrome P450 enzymes, convert tryptophan to indole-3-acetaldoxime (IAOx), which is the common precursor of auxin, camalexin and indolic glucosinolates8,9,10, while another cytochrome P450, CYP83B1, controls the flux of IAOx to the indolic glucosinolate pathway11. In recent years, a group of MYB transcription factors belonging to subgroup 12 R2R3-MYB transcription factors were identified to regulate glucosinolate biosynthesis, among which MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis12,13,14,15,16,17,18. Furthermore, diverse environmental stimuli, including wounding, pathogens, insect herbivores as well as light and nutrition, have been shown to regulate glucosinolate metabolism through MYB transcription factors19,20,21,22,23,24,25,26,27.
Glucosinolate accumulation has been demonstrated to be enhanced by sulfur fertilization in some cases24,28,29,30. Approximately 6% of the total sulfur in the youngest leaves of oilseed rape is assimilated into glucosinolates under sufficient sulfur supply, and glucosinolates in vegetative tissues account for 2% to 8% of the total sulfur28,31. Inorganic sulfate is the main form of sulfur taken in by plants, and firstly activated by ATP sulfurylase (ATPS) with adenylation to adenosine 5′-phosphosulfate (APS). As a branching point of sulfate assimilation, APS can be reduced by APS reductase (APR) to sulfite, which is subsequently reduced to sulfide by sulfite reductase (SiR) and finally participates in the synthesis of cysteine and other sulfur-containing compounds. In addition, APS can also be phosphorylated by APS kinase (APK) to 3′-phosphoadenosine 5′-phosphosulfate (PAPS), which donates active sulfate to the sulfation of the desulfo-GS precursors or sulfation in other secondary metabolism by sulfotransferases (SOTs)32,33,34,35,36,37. Sulfur assimilation in plants is a complex process, and is regulated by numerous factors, such as nutrients including carbon, nitrogen and sulfur, environment conditions, and phytohormones38,39,40,41,42,43,44,45,46.
Glucose has fundamental and multiple effects on plant metabolism at different developmental stages47,48,49,50,51. Glucose signaling is one of the best elucidated signaling pathways in plant cells. Arabidopsis hexokinase 1 (HXK1), the conserved glucose sensor with uncoupled signaling activity and phosphorylation, mediates many glucose signaling events that control the daily life of plants48,52,53,54. Recently, several reports have illustrated that sugars modulate biosynthesis of plant secondary metabolites in Arabidopsis and Brassica crops55,56,57,58. Our former study has demonstrated that glucose positively regulated aliphatic glucosinolate biosynthesis by HXK1-mediated signaling via transcription factors MYB28, MYB29, and ABA-insensitive 5 (ABI5)59. As another major kind of glucosinolates in Arabidopsis, indolic glucosinolate is synthesized via a distinct pathway from that of aliphatic glucosinolate. Here, we investigated the regulatory mechanism of indolic glucosinolate by glucose signaling, and found that glucose promotes the accumulation of indolic glucosinolates through MYB34, MYB51, and MYB122, while MYB34 plays a key role. This process is mediated by HXK1, but not by ABI5, suggesting that the mechanism underlying glucose-regulated indolic glucosinolates is distinct from that of aliphatic ones. To date, little is known about the role of glucose in sulfur assimilation or sulfur partitioning between primary and secondary sulfur metabolism. In this study, we found that glucose-promoted accumulation of both aliphatic and indolic glucosinolates is associated with enhanced sulfur assimilation.
Results
Glucose boosts indolic glucosinolate biosynthesis in Arabidopsis
The contents of three individual (I3M, 3-indolylmethyl glucosinolate; 4MOI3M, 4-methoxy-3-indolylmethyl glucosinolate; 1MOI3M, 1-methoxy-3-indolylmethyl glucosinolate) and total indolic glucosinolates were analyzed in shoots of 10-day-old Ler and Col-0 seedlings treated by 3% (W/V) of glucose or sorbitol. It was reported that different kinds of abiotic stresses including osmotic stress could affect glucosinolate content60,61,62, so sorbitol was used as a osmotic stress control of glucose treatment. As shown in Table 1, significant increases in the content of I3M, the predominant indolic glucosinolate composition, as well as the total indolic glucosinolates were observed in glucose-treated shoots, compared with sorbitol-treated ones. Moreover, considering that indolic glucosinolates made up a high percentage of the total glucosinolates in roots63, we also detected the changes of total indolic glucosinolate content in roots of seedlings treated with glucose for 3 days. Similarly, their accumulation was enhanced by glucose treatment (Fig. S1). Detailed analysis of the total content of indolic glucosinolates showed that glucose treatment dramatically increased total indolic glucosinolate accumulation in a time dependent manner, compared with sorbitol treatment (Fig. S2). Since a small gene subfamily of cytochrome P450 monooxygenases, CYP81Fs, were reported to convert I3M to 4MOI3M (CYP81F2/3) and 1MOI3M (CYP81F4)64, we examined expression levels of CYP81F2/3/4 under glucose treatment. Consistently, transcripts of CYP81F2/3/4 were induced by glucose, particularly of CYP81F3 and CYP81F4. Taken together, our data suggest that glucose promotes the biosynthesis of indolic glucosinolates.
Table 1. Individual and total indolic glucosinolate contents (nmol/mg FW) in Ler and Col-0 treated with glucose or sorbitol.
| Ler |
Col-0 |
|||||
|---|---|---|---|---|---|---|
| water | sorbitol | glucose | water | sorbitol | glucose | |
| I3M | 0.072 ± 0.014b | 0.069 ± 0.006b | 0.191 ± 0.023a | 0.070 ± 0.005b | 0.079 ± 0.005b | 0.165 ± 0.027a |
| 4MOI3M | 0.028 ± 0.014b | 0.035 ± 0.008b | 0.059 ± 0.004a | 0.007 ± 0.001c | 0.010 ± 0.002b | 0.012 ± 0.002a |
| 1MOI3M | 0.030 ± 0.006a | 0.040 ± 0.005a | 0.032 ± 0.007a | 0.025 ± 0.003a | 0.021 ± 0.006a | 0.027 ± 0.004a |
| Total indolic GS | 0.130 ± 0.057b | 0.144 ± 0.034b | 0.282 ± 0.037a | 0.102 ± 0.003b | 0.110 ± 0.007b | 0.204 ± 0.028a |
Ten-day-old Arabidopsis seedlings were treated with 3% glucose or sorbitol. Shoots were collected after treatment for 3 days. Each data represents the mean of six independent biological replicates per treatment (mean ± SE). Values not sharing a common letter are significantly different at P < 0.05.
Glucose induces the expression of genes related to indolic glucosinolate biosynthesis
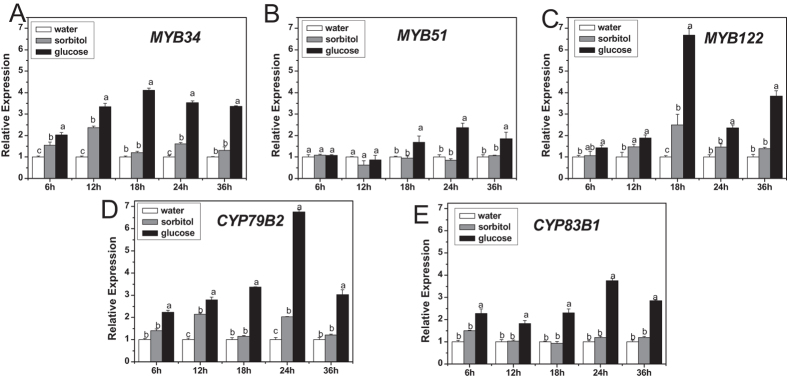
The response of three vital transcription factors (MYB34, MYB51, and MYB122) and two important biosynthetic genes (CYP79B2, CYP83B1) to glucose was analyzed. Expression of all these five genes was induced by exogenous glucose (Fig. 1). Glucose-induced mRNA levels of MYB34, MYB122, CYP79B2, and CYP83B1 were detected as early as 6 h after glucose treatment and subsequently increased steadily until reaching a peak at 18 h (MYB34, MYB122) or 24 h (CYP79B2, CYP83B1). MYB51 responded to glucose more slowly and mildly than the other two transcription factors. The mRNA levels of MYB34, MYB51, MYB122, CYP79B2, and CYP83B1 under glucose treatment accumulated ~3.40-, 1.78-, 2.68-, 2.45-, and 2.92-folds of those in sorbitol treatment, respectively, at 18 h. Thus, plants were sampled at this time point for the following analyses of gene expression.
Figure 1. Relative expression levels of MYB34 (A), MYB51 (B), MYB122 (C), CYP79B2 (D), and CYP83B1 (E) in young seedlings treated with glucose or sorbitol for indicated times.
The expression level was measured in 10-day-old Arabidopsis seedlings treated with 3% glucose or sorbitol, and then the whole plants were collected 6, 12, 18, 24, and 36 h after treatment, respectively. Each data point represents the mean of five independent biological replicates per treatment (mean ± SE). Expression level of genes in water-treated seedlings was set to 1. The gene transcription levels upon three treatments were compared for each time point and values not sharing a common letter are significantly different at P < 0.05.
Glucose-induced biosynthesis of indolic glucosinolates is affected in myb loss-of-function mutants
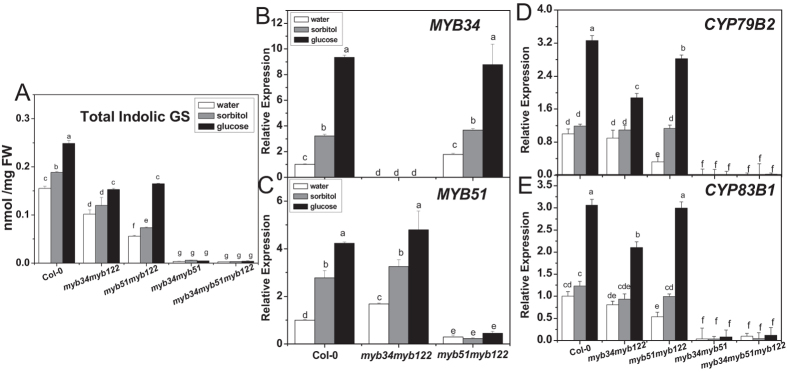
The content of total indolic glucosinolates in myb34myb122, myb51myb122, myb34myb51 double and myb34myb51myb122 triple mutants was measured with or without glucose treatment. As shown in Fig. 2A, these mutants produced less indolic glucosinolates compared with the wild type under the condition without glucose. The level of indolic glucosinolates was significantly lower in myb51myb122 than myb34myb122, and was almost undetected in myb34myb51 and myb34myb51myb122. However, the content of total indolic glucosinolates increased by 28% in myb34myb122 and 125% in myb51myb122 after glucose treatment compared with sorbitol treatment, whereas glucose had no such an effect on indolic glucosinolate accumulation in myb34myb51 and myb34myb51myb122 mutants.
Figure 2. Total indolic glucosinolate content and relative expression levels of genes related to glucosinolate biosynthesis in double and triple myb mutant seedlings treated with glucose or sorbitol.
Effect of glucose on accumulation of total indolic glucosinolates (A) was measured in myb34myb122, myb51myb122, myb34myb51 double and myb34myb51myb122 triple mutants, as well as expression levels of MYB34 (B), MYB51(C), CYP79B2 (D), and CYP83B1 (E) in these mutants. Ten-day-old Arabidopsis seedlings were treated with 3% glucose or sorbitol, and then the whole plants were collected 3 days (A) or 18 h (B–E) after treatment. Each data point represents the mean of three to five (for qPCR assay) or six (for glucosinolate assay) independent biological replicates per treatment (mean ± SE). Expression level of genes in water-treated Col-0 seedlings was set to 1. Values not sharing a common letter are significantly different at P < 0.05.
Furthermore, transcript levels of MYB34, MYB51, CYP79B2, and CYP83B1 in myb mutants were analyzed. The expression levels of MYB34 in myb51myb122 and MYB51 in myb34myb122 were induced by glucose treatment, which was similar to that in wild type. Notably, the steady-state levels of two biosynthetic genes, CYP79B2 and CYP83B1 were in line with the increased levels of indolic glucosinolates (Fig. 2D,E). In addition, the expression of CYP79B2 and CYP83B1 in myb51myb122 was induced almost as strong as in wild type by glucose, which was not the case for myb34myb122, myb34myb51, and the triple myb mutant. This observation pointed out the importance of three MYBs and especially of MYB34 in glucose-induced indolic glucosinolate biosynthesis.
HXK1 is involved in glucose-induced indolic glucosinolate biosynthesis
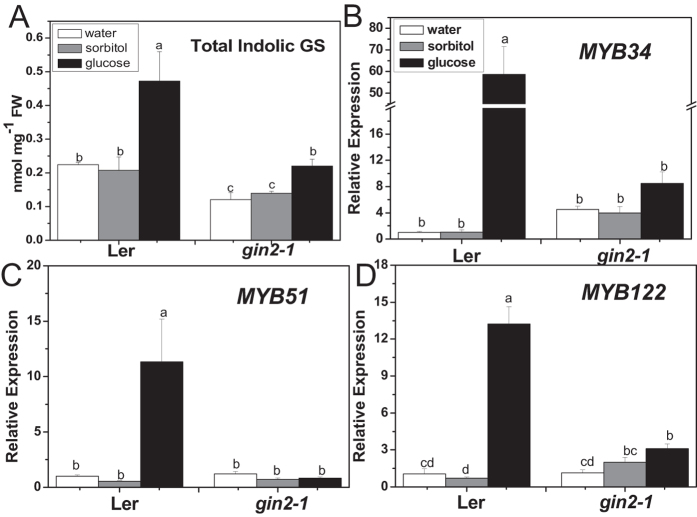
The gin2-1 is a HXK1 null mutant, which is insensitive to glucose54. As shown in Fig. 3A, gin2-1 mutant contained a significantly reduced level of total indolic glucosinolates in comparison with the wild type. The increased accumulation of total indolic glucosinolates resulted from glucose treatment in wild type was also inhibited in this mutant. Consistently, over-expressing HXK1 in gin2-1 plants recovered the deficiency of glucosinolates (Fig. S4). Similarly, the induced expressions of MYB34, MYB51, and MYB122 by glucose were almost vanished in the gin2-1 mutant (Fig. 3B–D). All above results suggest that HXK1 is important in glucose-induced indolic glucosinolate biosynthesis.
Figure 3. Total indolic glucosinolate content and relative expression levels of MYB34, MYB51, and MYB122 in glucose- or sorbitol-treated gin2-1 seedlings.
Ten-day-old Arabidopsis seedlings were treated with 3% glucose or sorbitol, and then the whole plants were collected 3 days (A) or 18 h (B–D) after treatment. Each data point represents the mean of five (for qPCR assay) or six (for glucosinolate assay) independent biological replicates per treatment (mean ± SE). Values not sharing a common letter are significantly different at P < 0.05. Relative expression values are given compared with Ler seedlings treated by water.
The role of ABI5 in glucose-induced indolic glucosinolate biosynthesis
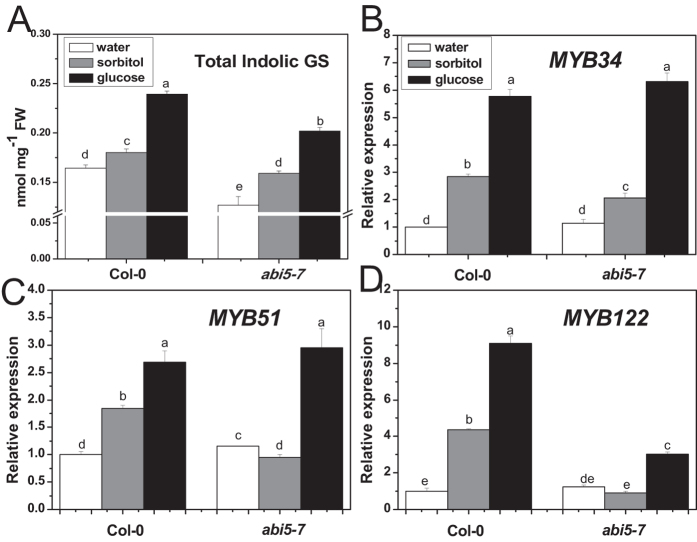
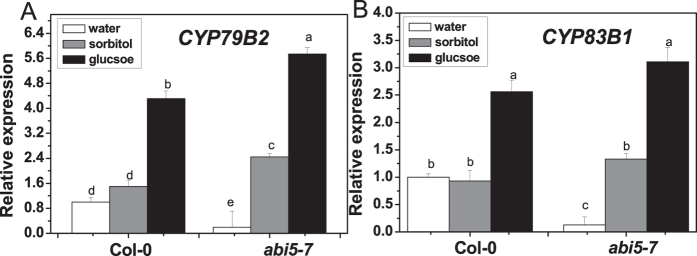
The abi5-7 mutant produced notable decreased total indolic glucosinolate content, compared with the wild type, in the presence or absence of glucose (Fig. 4A). However, glucose treatment raised the accumulation of total indolic glucosinolates in abi5-7 by 27%, compared with sorbitol treatment, which was comparable to that in wild type (33%). To our surprise, the transcription levels of MYB34 and MYB51 in abi5-7 were the same as in the wild type (Fig. 4B,C). We then checked transcription levels of CYP79B2 and CYP83B1 in the abi5-7 mutant. Quantitative PCR (qPCR) analysis showed that relative expression levels of CYP79B2 and CYP83B1 were much lower in abi5-7 than in the wild type (Fig. 5). However, glucose treatment dramatically enhanced the expression of these two genes in abi5-7, which was similar to that in the wild type, compared with sorbitol treatment.
Figure 4. Total indolic glucosinolate content and relative expression levels of MYB34, MYB51, and MYB122 in abi5-7 treated with glucose or sorbitol.
Ten-day-old Arabidopsis seedlings were treated with 3% glucose or sorbitol, and then the whole plants were collected 3 days (A) or 18 h (B–D) after treatment. Each data point represents the mean of five (for qPCR assay) or six (for glucosinolate assay) independent biological replicates per treatment (mean ± SE). Values not sharing a common letter are significantly different at P < 0.05. Relative expression values are given compared with Col-0 seedlings treated by water.
Figure 5. Relative expression levels of CYP79B2 (A) and CYP83B1 (B) in abi5-7 treated with glucose or sorbitol.
Ten-day-old Arabidopsis seedlings were treated with 3% glucose or sorbitol, and then the whole plantswere harvested 18 h after treatment. Each data point represents the mean of five independent biological replicates per treatment (mean ± SE). Values not sharing a common letter are significantly different at P < 0.05. Relative expression values are given compared with Col-0 seedlings treated by water.
Glucose induces the expression of sulfate metabolic genes
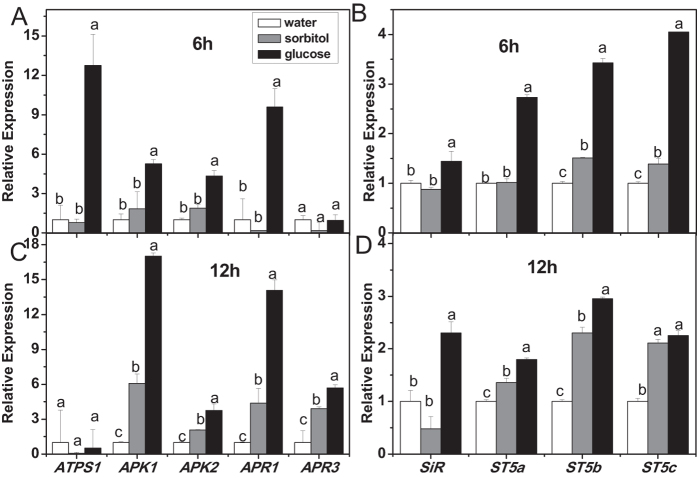
Most sulfur is absorbed by plants in inorganic sulfate, and then, plants either reduce sulfate and incorporate it into cysteine and other sulfur-containing compounds of primary metabolism, or take it into the secondary metabolism to synthesize various sulfated compounds, including glucosinolates33,35. The response of sulfate metabolic genes to glucose treatment was analyzed to verify whether glucose exerted an effect on sulfate assimilation (Fig. 6). The results showed that ATPS1 responded to glucose treatment quickly, representing a high induced expression level at 6 h, and then went back to the control level 12 h after glucose treatment. In addition, relative expression levels of APK1, APK2, APR1, SiR, and SOTs (ST5a, ST5b, ST5c) were increased by glucose as early as 6 h after treatment and maintained at a high level until 12 h. It seemed that sorbitol treatment stimulated expression of some genes, such as ARP1 and ST5b, more slowly than glucose treatment (Fig. 6C,D), indicating that glucose signaling is switched on rapidly in regulating sulfate assimilation.
Figure 6. Relative expression levels of genes related to sulfate metabolism in glucose- or sorbitol-treated seedlings.
Ten-day-old Arabidopsis seedlings were treated with 3% glucose or sorbitol, and then the whole plants were harvested 6 h (A,B) or 12 h (C,D) after treatment. Each data point represents the mean of five independent biological replicates per treatment (mean ± SE). Values not sharing a common letter are significantly different at P < 0.05. Relative expression values are given compared with seedlings treated by water.
Glucose increases glucosinolate accumulation and thiol content in plants cultured under different sulfate concentrations
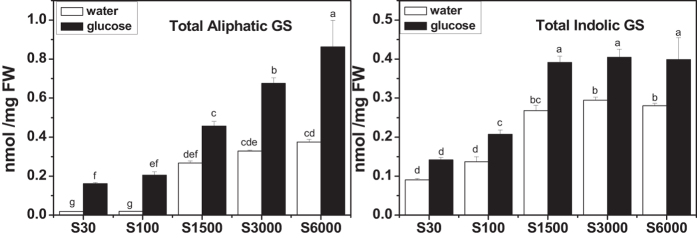
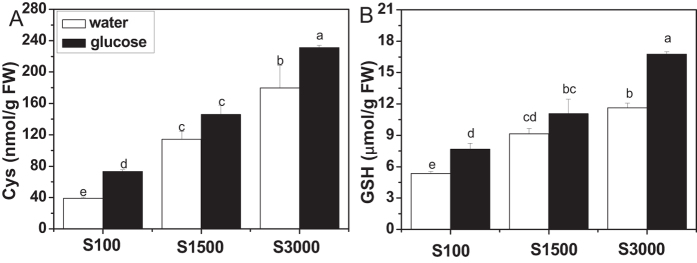
To investigate whether glucose-regulated glucosinolate accumulation was associated with the concentration of sulfate, we measured the content of glucosinolates in Arabidopsis cultured with different concentrations of sulfate. As shown in Fig. 7, plants accumulated more glucosinolates along with the increased concentration of sulfate in culture medium in the absence of glucose. When the sulfate concentration was increased to 1500 μM, the accumulation of both aliphatic and indolic glucosinolates reached saturation. However, their levels were differentially affected by glucose. More aliphatic glucosinolates accumulated with the increased sulfate concentration. Conversely, plants synthesized almost the same amount of indolic glucosinolates when sulfate concentration reached 1500 μM or even higher. This observation suggests differential roles of both glucose and sulfur in the production of aliphatic and indolic glucosinolates. In addition, the effect of glucose on cysteine and GSH levels were assessed. As shown in Fig. 8, plants accumulated more thiols when they were supplied with more sulfate, and glucose treatment promoted thiol accumulation at all tested sulfate concentrations, especially when the concentration was slightly low (100 μM) or high (3000 μM).
Figure 7. Glucosinolate contents in glucose-treated seedlings grown at different concentrations of sulfate.
Arabidopsis seedlings (wild type Col-0) were cultured at different concentrations of sulfate for 10 days and subsequently treated with 3% glucose. The whole plants were harvested 3 days after glucose treatment. Total aliphatic and indolic glucosinolate contents were measured. Each data point represents the mean of six independent biological replicates per treatment (mean ± standard error). Values not sharing a common letter are significantly different at P < 0.05.
Figure 8. Cys and GSH contents in glucose-treated seedlings grown at different concentrations of sulfate.
Arabidopsis seedlings (wild type Col-0) were cultured at different concentrations of sulfate for 10 days and then treated with 3% glucose. The whole plants were harvested 3 days after treatment, and then cysteine and GSH contents were measured. Each data point represents the mean of five independent biological replicates per treatment (mean ± standard error). Values not sharing a common letter are significantly different at P < 0.05.
Discussion
Glucose promotes indolic glucosinolate biosynthesis via indolic MYB transcription factors
Photosynthetic plants rely on sugars throughout their entire life, and glucose signaling is one of the vital mechanisms that control almost all phases of the plant life cycle48,50. Up to now, the mechanism of sugar-regulated anthocyanin biosynthesis has been well illustrated, while knowledge about sugar-regulated glucosinolate biosynthesis is limited55,56,57,58,65,66,67. Our former work indicated a positive role of glucose in promoting aliphatic glucosinolate biosynthesis59. In the present study, we found that glucose enhanced indolic glucosinolate accumulation in different parts (shoots and roots) of Arabidopsis seedlings with different genetic backgrounds (Col-0 and Ler) (Table 1, Fig. S1). Besides, glucose treatment could induce the expression of CYP81Fs (Fig. S3), indicating that glucose promoted the conversion of I3M to both 1MOI3M and 4MOI3M. Thus, our data reveals that glucose has a broad influence on glucosinolate biosynthesis.
MYB28 and MYB29 are two major transcription factors controlling aliphatic glucosinolate biosynthesis in Arabidopsis, of which MYB28 plays a key role14,17,18,68. Similarly, MYB28 acts predominantly in glucose-induced aliphatic glucosinolate accumulation59. Frerigmann and Gigolashvili (2014) recently addressed the distinct functions of MYB34, MYB51, and MYB122 in indolic glucosinolate biosynthesis13. In the present study, the expression of MYB34, MYB51, and MYB122 was induced by glucose, with that of MYB34 being the highest, compared with sorbitol treatment (Fig. 1). Both myb34myb51 double and myb34myb51myb122 triple mutants (with the absence of MYB34 and MYB51) produced only traces of indolic glucosinolates even in the presence of glucose (Fig. 2), indicating that MYB34 and MYB51 were crucial for the basal indolic glucosinolate biosynthesis. Interestingly, although myb51myb122 contained less indolic glucosinolates than myb34myb122, a much stronger induction of indolic glucosinolate biosynthesis occurred in myb51myb122 upon glucose treatment. This finding substantiated a special role of MYB34 in glucosinolate biosynthesis in response to glucose. Remarkably, the specific response of various MYB transcription factors to environmental stimuli is a universal phenomenon. Among aliphatic MYBs, MYB28 is vital in glucose signaling, whereas MYB29 plays an important role in response to jasmonic acid (JA) and salicylic acid (SA)17,18. As for indolic glucosinolate, MYB51 is known to be crucial in ethylene and SA-mediated induction of their biosynthesis in addition to the transient response to the mechanical stimulus13,15. MYB34 is known as the key regulator in response to abscisic acid (ABA)13, as well as glucose signaling as shown by our present study. Taken together, we have demonstrated that glucose enhanced indolic glucosinolate biosynthesis through indolic MYBs, in which MYB34 and MYB51 were crucial in maintaining the basal indolic glucosinolate accumulation, while MYB34 was pivotal in response to glucose signaling.
Regulation of indolic glucosinolate biosynthesis by glucose is HXK1-dependant
Arabidopsis HXK1, the intracellular glucose sensor, functions in various glucose-regulated processes. The role of HXK1 in sensing glucose signal was uncoupled from glucose metabolism54,69,70,71,72. It has been known that the repression of glucose on ethylene response factor 1 (ERF1) and activation on biosynthetic gene ABA2, transcription factor ABI4 are HXK1 dependent52,70,73. In the present study, expression of ABI5 greatly induced by glucose in the wild type disappeared in gin2-1 (Fig. S5), which was similar to the observation by Cho et al.70, suggesting that induction of ABI5 by glucose is HXK1-dependent. Yanagisawa et al. (2003) reported that the degradation of ethylene-insensitive3 (EIN3), a key transcriptional regulator in ethylene signaling, was enhanced by glucose through HXK172. Meanwhile, there also exists HXK1-independent glucose signaling pathways73,74,75. Our previous study has proved that the regulation of aliphatic glucosinolate biosynthesis by glucose was HXK1-dependent. In current survey, a notable deficiency of indolic glucosinolates was observed in gin2-1 in comparison with the wild type, and the significant increase in indolic glucosinolate accumulation upon glucose treatment in the wild type was also inhibited in gin2-1 (Fig. 3A), which could be rescued by overexpression of AtHXK1 (Fig. S4). These results suggest the involvement of HXK1 in glucose-enhanced indolic glucosinolate biosynthesis. Furthermore, the increased expression of MYB34, MYB51, and MYB122 in response to glucose was disturbed by the absence of HXK1 (Fig. 3B–D), suggesting that HXK1 also modulates the glucose-activated indolic MYBs expression. Taken together, HXK1 plays an important role in glucose-induced indolic glucosinolate biosynthesis.
ABI5 participates in indolic glucosinolate biosynthesis, but is not required for glucose-induced indolic glucosinolate biosynthesis
ABI5 encodes a transcription factor belonging to a large basic leucine zipper (bZIP) domain family, and plays a crucial role in ABA signal transduction, especially during seed development and germination76,77,78,79. The abi5-7 mutant is originally discovered for its insensitivity to high level of exogenous ABA (3 μM), which is normally inhibitory for seed germination and seedling development of the wild type80. However, it is also found to be insensitive to glucose, revealing a function of ABI5 in glucose signaling76,81,82. Furthermore, the expression of ABI5 is greatly increased by low concentrations of glucose in the wild type, but not in gin2-1, indicating that the regulation of ABI5 by glucose is HXK1-dependent70 (Fig. S5). In our former survey, ABI5 is proved to be involved in the regulation of aliphatic glucosinolate biosynthesis as a mediator in glucose signaling59. However, it seems that ABI5 acts in a different way in regulation of indolic glucosinolate biosynthesis. Undoubtedly, ABI5 participates in indolic glucosinolate biosynthesis based on the decreased constitutive indolic glucosinolate content and decreased expression levels of biosynthetic genes (CYP79B2, CYP83B1) in the abi5-7 mutant (Figs 4A and 5). Nevertheless, the absence of ABI5 did not interfere with the glucose-triggered increase in indolic glucosinolate biosynthesis and expression of biosynthetic genes (Figs 4A and 5), suggesting that ABI5 was not required for glucose-induced indolic glucosinolate biosynthesis. In addition, both the basal mRNA level of indolic MYB transcription factors and the inducing effect of glucose on their expression were not disturbed in abi5-7 (Fig. 4B–D). This observation points out the possibility that regulation occurs at a protein level or with other factors. Recently, MYC2, MYC3, and MYC4 were shown to form complexes with MYBs to modulate glucosinolate biosynthesis83,84. The possibility of existing ABI5-MYB complexes in regulating indolic glucosinolate biosynthesis or post-transcriptional control of indolic MYBs by ABI5 remains to be verified. Further studies are needed to elucidate the regulatory mechanism and to identify the yet unknown factors involved in the regulation of indolic glucosinolate biosynthesis by glucose signaling.
Glucose enhances indolic glucosinolate accumulation without weakening primary sulfate assimilation
Sulfur is an essential nutrient for all higher plants, and plants reduce sulfate to sulfide and incorporate it into organic metabolites85. The backbone of glucosinolates contains from two to three S atoms, with one originating from 3′-phosphoadenosine 5′-phosphosulfate, the second one from glutathione, and the third being present in methionine derived aliphatic glucosinolates86. Sulfur assimilation is therefore crucial for glucosinolate biosynthesis35,36. Up to now, great progress has been achieved in illustrating the regulation of sulfur metabolism32,33,87,88. The effect of glucose on sulfur assimilation has also been investigated38,41,42. Hesse et al. (2003) reported that 0.5% (w/v) glucose induced an increase in the APR level and incorporation of S in Arabidopsis38. There are also reports demonstrating that the addition of sucrose or glucose led to a rise in the mRNA level and enzyme activity of APR41,42. In the present study, our results showed that glucose treatment increased the mRNA levels of sulfate metabolism related genes, such as ATPS1, APK1, APK2, APR1, SiR, ST5a, ST5b, and ST5c (Fig. 6), indicating a positive effect of glucose on sulfur assimilation. As shown in Fig. 7, an increase in glucosinolate content was observed in plants grown in higher sulfate concentrations, which was accordant with descriptions of other reports24,28,29,30. The glucosinolate accumulation reached saturation when sufficient sulfur (about 1500 μM) was given to the plants. Interestingly, addition of glucose to the media with sufficient sulfur could further enhance glucosinolate biosynthesis, which could be explained by the simultaneous activation of sulfur assimilation and glucosinolate biosynthesis59 (Figs 1 and 6). However, the accumulation of aliphatic and indolic glucosinolate in response to glucose induction was under different mechanism. As shown in Fig. 7, the biosynthesis of aliphatic glucosinolates could be further induced by adding both glucose and sulfate in the media while indolic glucosinolate biosynthesis could not be further stimulated by the addition of glucose or sulfate when its content reached about 0.4 nmol/mg fresh weight (FW). Aliphatic glucosinolates can be synthesized from methionine, the production of sulfate assimilation. Hence there are sufficient precursors for aliphatic glucosinolate biosynthesis as sulfate assimilation in plants can be enhanced by glucose treatment. In contrast, the precursor of indolic glucosinolate is tryptophan. It has been report that, in case of S starvation, the pools of reduced sulfur containing organic molecules (such as cysteine and GSH) decreased, while the precursors (O-acetylserine and serine) accumulated32,89,90. Moreover, as serine is directly involved in tryptophan biosynthesis91, and tryptophan showed evident elevated levels under sulfur-deficient growth conditions89,90,92. Plant usually delicately balances the nutrients or metabolites. When S is sufficient, serine might be utilized for biosynthesis of immediate products, resulting in a competition with the conversion of serine to tryptophan. Therefore, indolic glucosinolates could not be constantly accumulated. The analysis of thiol content showed that glucose treatment also enhanced the synthesis of cysteine and GSH, the productions of primary sulfate assimilation (Fig. 8A,B). Hence, the induction of glucosinolate accumulation by glucose should be mainly derived from the enhanced sulfate assimilation, but not the sulfur partitioning into glucosinolate biosynthesis. As glucose could activate sulfur assimilation and enhance thiol synthesis, we further investigated the role of HXK1, the receptor of glucose signaling in this process. Interestingly, glucose-modulated expressions of APK1, SiR, and ST5c were strongly inhibited in gin2-1 mutant (Fig. S6B,D,E). However, a strong induction of ATPS1 expression by glucose was also observed in this mutant, and no significant difference existed in expression of APR1 between gin2-1 mutant and the related wild type plants (Fig. S6A,C). These results demonstrated that glucose-regulated sulfur assimilation is only partially HXK1-dependent. The regulation of glucose in primary sulfur assimilation is complicated, and further studies are needed to elucidate the mechanism.
It has been reported that glucose activates ABA biosynthesis and signal transduction via transcription factor52,70,73. The function of ABA in promoting indolic glucosinolate accumulation13 and enhancing cysteine and GSH synthesis has also been demonstrated39,93. Therefore, whether the effect of glucose on glucosinolate and thiol synthesis is ABA-dependent needs to be verified in the future.
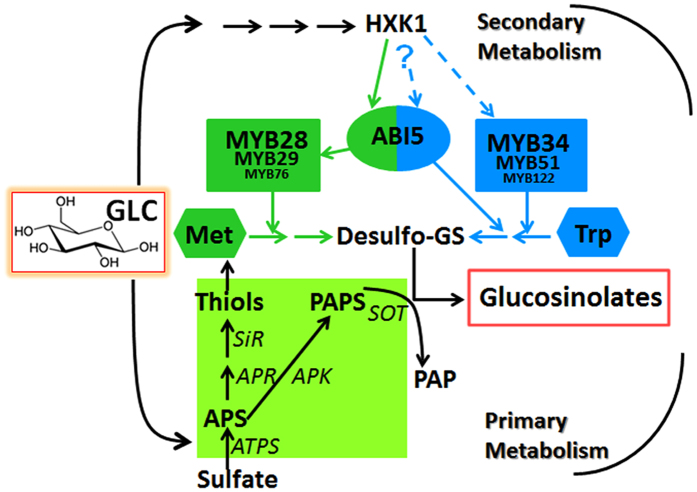
We summarize our present and former work59 as Fig. 9, which suggests a positive role of glucose in both glucosinolate biosynthesis and sulfur assimilation. Glucose promotes glucosinolate biosynthesis mainly through MYB28 and MYB34 in a HXK1 dependent manner. However, the regulation by ABI5 in glucose signaling is distinct between aliphatic and indolic glucosinolate biosynthesis. ABI5 seems to be involved in biosynthesis of both aliphatic and indolic glucosinolates, as well as in glucose-induced aliphatic rather than indolic glucosinolate biosynthetic pathway. The diverse biological function of aliphatic and indolic glucosinolates in plant development and stress resistance might explain the significance of their biosynthesis under different regulatory mechanisms by the same glucose signaling. In addition, glucose enhances glucosinolate biosynthesis by strengthening sulfur assimilation instead of simply redirecting sulfur partitioning into glucosinolate biosynthesis.
Figure 9. A model for glucose-regulated glucosinolate biosynthesis in Arabidopsis.
Glucose plays a key and positive role in both primary and secondary metabolism. Symbols with light green color represent sulfate metabolism pathway. Symbols with dark green color represent aliphatic glucosinolate biosynthesis and regulation. Symbols with blue color represent indolic glucosinolate biosynthesis and regulation. Multiple enzymatic steps are indicated with interrupted arrows. GLC, glucose; Met, methionine; Trp, tryptophan; Desulfo-GS, Desulfo-glucosinolates; – – – –> possible activation.
Materials and Methods
Plants and Growth Conditions
The sterilized seeds were stratified for 3 days at 4 °C, and transferred into flasks (~50 seeds per flask) with 40 ml of liquid growth medium [full-strength sterilized Murashige–Skoog (MS) salt solution +0.5% glucose]. For assays in Arabidopsis cultured at different concentrations of sulfate, the liquid growth medium was modified Murashige–Skoog (MS) salt solution +0.5% glucose. S0 was prepared by complete replacement of SO42− to Cl−. S30, S100, S1500, S3000, and S6000 liquid media were prepared by adding MgSO4 to the S0 medium at final concentrations of 30 μM, 100 μM, 1500 μM, 3000 μM, and 6000 μM. Mg2+ concentration was adjusted to 1500 μM by adding MgCl294. Plants were grown under a photoperiod of 16 h light/8 h dark with gentle shaking (135 rpm) for 10 days in a plant growth chamber at 21 °C55. The gin2-1 and abi5-7 mutants were generously provided by Dr. Sheng Teng (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences). The double mutant myb34myb51, myb34myb122, myb51myb122, and triple mutant myb34myb51myb122 were isolated in previous study at University of Cologne, Germany13. The transgenic plants AtHXK1/gin2, over expression of AtHXK1 in gin2-1 background, is kindly gifted by Dr. Jen Sheen (Department of Molecular Biology and Centre for Computational and Integrative Biology, Massachusetts General Hospital, and Department of Genetics, Harvard Medical School). The genetic background of all mutants was Columbia (Col-0), except for gin2-1, which was Landsberg (Ler).
Glucose and Sorbitol Treatments
Sterilized glucose and sorbitol were added into the flasks after 10 days with water as a control. 3% (w/v) was selected as the final concentration for glucose and sorbitol treatments according to our previous study59. After treatments, plants were cultured in the same condition as before and were collected at different time points for analysis.
Glucosinolate Assay
The analysis of glucosinolate content was performed on a HPLC device (Shimadzu, Kyoto, Japan) as described recently59.
RNA Isolation and Expression Analysis
The isolation of RNA and expression analysis were performed as previously described59. The expression level of Arabidopsis ACTIN2 was used as an internal control and the expression of other genes was computed with the 2−ΔΔCT method95. Primers are listed in Table S1.
HPLC Analysis of Thiols
The analysis of cysteine and glutathione (GSH) was performed as described37. Approximately 100 mg of plant materials were homogenized in liquid nitrogen. After adding 1 ml of cold 0.1 M HCl, samples were mixed and agitated at room temperature at 300 rpm for 40 min. The suspension was centrifuged at 14000 rpm for 20 min at 4 °C. 120 μl of supernatant were mixed with 200 μl of 0.25 M CHES-NaOH, pH 9.4 and 70 μl of 10 mM dithiothreitol by vortexing, and then incubated at room temperature for 40 min. After that, 10 μl of 25 mM of monobromobimane were added and the derivatisation of thiols was conducted by incubating samples in the dark at room temperature for 15 min. Reaction was stopped by adding 220 μl of 100 mM methanesulfonic acid. After 30 min centrifugation at 14000 rpm, 4 °C, supernatants were collected for HPLC analysis.
The separation and analysis of the thiols were performed using reversed-phase (Hypersil C18 column, 5 μm particle size, 4.6 mm × 250 mm; Elite Analytical Instruments Co. Ltd, Dalian, China) HPLC system. 10% (v/v) methanol mixed with 0.25% (v/v) acetic acid (pH 3.9) was set as solvent A and 90% (v/v) methanol mixed with 0.25% (v/v) acetic acid (pH 3.9) as solvent B. The flow rate was kept constant at 1.0 ml min−1. The procedure employed isocratic elution with 100% solvent A for the first 2 min; a linear gradient to 92% over the next 10 min; followed by a linear gradient to 86% within 5 min. Bimanederivates were detected fluorimetrically (Shimadzu, Kyoto, Japan) with excitation at 390 nm and emission at 480 nm.
Statistical Analysis
Statistical analysis was performed using the SPSS package program version 11.5 (SPSS Inc., Chicago, IL, USA). Differences were analyzed by one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test at a 95% confidence level (P < 0.05). The values are reported as means with standard error for all results.
Additional Information
How to cite this article: Miao, H. et al. Glucose enhances indolic glucosinolate biosynthesis without reducing primary sulfur assimilation. Sci. Rep. 6, 31854; doi: 10.1038/srep31854 (2016).
Supplementary Material
Acknowledgments
We would like to thank Dr. Chuanyou Li (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for helpful discussion, Dr. Zhixiang Chen (Department of Botany and Plant Pathology, Purdue University) for critical reading. This work was financially supported by grants from National Science Foundation of China (No. 31270343, 31300259, 31470385).
Footnotes
Author Contributions H.M. and C.C. equally contributed to the work. Q.W., J.H. and B.W. jointly designed the research. Q.W. supervised the project. H.M., C.C. and J.W. performed most of the experiments. H.M., C.C., J.C., H.Q. and X.Z. analyzed the data. H.M., C.C., Y.Z. and B.S. prepared the figures. H.M., C.C. and Q.W. wrote the manuscript. All authors reviewed and approved the final manuscript.
References
- Grubb C. D. & Abel S. Glucosinolate metabolism and its control. Trends Plant Sci 11, 89–100 (2006). [DOI] [PubMed] [Google Scholar]
- Kim J. H., Lee B. W., Schroeder F. C. & Jander G. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J 54, 1015–1026 (2008). [DOI] [PubMed] [Google Scholar]
- Kos M. et al. Herbivore-mediated effects of glucosinolates on different natural enemies of a specialist aphid. J Chem Ecol 38, 100–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P. et al. A Glucosinolate Metabolism Pathway in Living Plant Cells Mediates Broad-Spectrum Antifungal Defense. Science 323, 101–106 (2009). [DOI] [PubMed] [Google Scholar]
- Clay N. K., Adio A. M., Denoux C., Jander G. & Ausubel F. M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon J., Delage B., Williams D. & Dashwood R. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 55, 224–236 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B. & Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep 21, 425–447 (2004). [DOI] [PubMed] [Google Scholar]
- Hull A. K., Vij R. & Celenza J. L. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. P Natl Acad Sci USA 97, 2379–2384 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M. D., Hansen C. H., Wittstock U. & Halkier B. A. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem 275, 33712–33717 (2000). [DOI] [PubMed] [Google Scholar]
- Zhao Y. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Gene Dev 16, 3100–3112 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S., Tax F. E., Feldmann K. A., Galbraith D. W. & Feyereisen R. CYP83B1, a cytochrome P450 at the metabolic branch paint in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 13, 101–111 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L. The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol 137, 253–262 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H. & Gigolashvili T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol Plant 7, 814–828 (2014). [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Yatusevich R., Berger B., Müller C. & Flügge U.-I. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis inArabidopsis thaliana. Plant J 51, 247–261 (2007). [DOI] [PubMed] [Google Scholar]
- Gigolashvili T. et al. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 50, 886–901 (2007). [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Berger B. & Flügge U.-I. Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem Rev 8, 3–13 (2008). [Google Scholar]
- Gigolashvili T., Engqvist M., Yatusevich R., Müller C. & Flügge U.-I. HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis inArabidopsis thaliana. New Phytol 177, 627–642 (2008). [DOI] [PubMed] [Google Scholar]
- Hirai M. Y. et al. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. P Natl Acad Sci USA 104, 6478–6483 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D. J., Figuth A. & Mitchell-Olds T. Genetic architecture of plastic methyl jasmonate responses in Arabidopsis thaliana. Genetics 161, 1685–1696 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M. D. et al. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol 131, 298–308 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol 138, 1149–1162 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires A., Rosa E. & Carvalho R. Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var.italica). J Sci Food Agr 86, 1512–1516 (2006). [Google Scholar]
- Chen X.-j., Zhu Z.-j., Ni X.-l. & Qian Q.-q. Effect of nitrogen and sulfur supply on glucosinolates in Brassica campestris ssp. chinensis. Agr Sci China 5, 603–608 (2006). [Google Scholar]
- Falk K. L., Tokuhisa J. G. & Gershenzon J. The effect of sulfur nutrition on plant glucosinolate content: physiology and molecular mechanisms. Plant Biology 9, 573–581 (2007). [DOI] [PubMed] [Google Scholar]
- Chen Y., Yan X. & Chen S. Bioinformatic analysis of molecular network of glucosinolate biosynthesis. Comput Biol Chem 35, 10–18 (2011). [DOI] [PubMed] [Google Scholar]
- Liu M.-S., Li H.-C., Chang Y.-M., Wu M.-T. & Chen L.-F. O. Proteomic analysis of stress-related proteins in transgenic broccoli harboring a gene for cytokinin production during postharvest senescence. Plant Sci 181, 288–299 (2011). [DOI] [PubMed] [Google Scholar]
- Yan X. F. & Chen S. Regulation of plant glucosinolate metabolism. Planta 226, 1343–1352 (2007). [DOI] [PubMed] [Google Scholar]
- Blake-Kalff M. M. A., Harrison K. R., Hawkesford M. J., Zhao F. J. & McGrath S. P. Distribution of sulfur within oilseed rape leaves in response to sulfur deficiency during vegetative growth. Plant Physiol 118, 1337–1344 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Matsuo T., Watanabe M. & Watanabe Y. Effect of nitrogen and sulphur application on the glucosinolate content in vegetable turnip rape (Brassica rapa L.). Soil Sci Plant Nutr 48, 43–49 (2002). [Google Scholar]
- Van der Kooij T. A. W., DeKok L. J., Haneklaus S. & Schnug E. Uptake and metabolism of sulphur dioxide by Arabidopsis thaliana. New Phytol 135, 101–107 (1997). [DOI] [PubMed] [Google Scholar]
- Fieldsend J. & Milford G. F. J. Changes in glucosinolates during crop development in single- and double-low genotypes of winter oilseed rape (Brassica napus): I. Production and distribution in vegetative tissues and developing pods during development and potential role in the recycling of sulphur within the crop. Ann Appl Biol 124, 531–542 (1994). [Google Scholar]
- Davidian J. C. & Kopriva S. Regulation of sulfate uptake and assimilation–the same or not the same? Mol Plant 3, 314–325 (2010). [DOI] [PubMed] [Google Scholar]
- Kopriva S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann Bot-London 97, 479–495 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T., Martin M. N., Bick J. A. & Davies J. P. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Biol 51, 141–165 (2000). [DOI] [PubMed] [Google Scholar]
- Mugford S. G., Lee B.-R., Koprivova A., Matthewman C. & Kopriva S. Control of sulfur partitioning between primary and secondary metabolism. Plant J 65, 96–105 (2011). [DOI] [PubMed] [Google Scholar]
- Mugford S. G. et al. Disruption of adenosine-5′-phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell 21, 910–927 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatusevich R. et al. Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J 62, 1–11 (2010). [DOI] [PubMed] [Google Scholar]
- Hesse H. et al. Effect of glucose on assimilatory sulphate reduction in Arabidopsis thaliana roots. J Exp Bot 54, 1701–1709 (2003). [DOI] [PubMed] [Google Scholar]
- Jiang M. Y. & Zhang J. H. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42, 1265–1273 (2001). [DOI] [PubMed] [Google Scholar]
- Jost R. et al. Expression profiling of metabolic genes in response to methyl jasmonate reveals regulation of genes of primary and secondary sulfur-related pathways in Arabidopsis thaliana. Photosynth Res 86, 491–508 (2005). [DOI] [PubMed] [Google Scholar]
- Kopriva S. et al. Light regulation of assimilatory sulphate reduction in Arabidopsis thaliana. Plant J 20, 37–44 (1999). [DOI] [PubMed] [Google Scholar]
- Kopriva S. et al. Interaction of sulfate assimilation with carbon and nitrogen metabolism in Lemna minor. Plant Physiol 130, 1406–1413 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A., Suter M., Op den Camp R., Brunold C. & Kopriva S. Regulation of sulfate assimilation by nitrogen in Arabidopsis. Plant Physiol 122, 737–746 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A., North K. A. & Kopriva S. Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol 146, 1408–1420 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander U., Suter M. & Brunold C. Regulation of Sulfate Assimilation by Light and O-Acetyl-l-Serine in Lemna minor L. Plant Physiol 97, 253–258 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H. et al. Regulation of sulfur assimilation in higher plants: A sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. P Natl Acad Sci USA 94, 11102–11107 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastdrager J., Hanson J. & Smeekens S. Sugar signals and the control of plant growth and development. J Exp Bot 65, 799–807 (2014). [DOI] [PubMed] [Google Scholar]
- Rolland F., Baena-Gonzalez E. & Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu Rev Plant Biol 57, 675–709 (2006). [DOI] [PubMed] [Google Scholar]
- Rolland F., Moore B. & Sheen, J. Sugar sensing and signaling in plants. Plant Cell 14, S185–S205 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Master regulators in plant glucose signaling networks. J Plant Biol 57, 67–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Ma J., Hanson J. & Rolland F. Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13, 274–279 (2010). [DOI] [PubMed] [Google Scholar]
- León P. & Sheen, J. Sugar and hormone connections. Trends Plant Sci 8, 110–116 (2003). [DOI] [PubMed] [Google Scholar]
- Gibson S. I. Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8, 93–102 (2005). [DOI] [PubMed] [Google Scholar]
- Moore B. et al. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300, 332–336 (2003). [DOI] [PubMed] [Google Scholar]
- Loreti E. et al. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes inArabidopsis. New Phytol 179, 1004–1016 (2008). [DOI] [PubMed] [Google Scholar]
- Teng S., Keurentjes J., Bentsink L., Koornneef M. & Smeekens S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 139, 1840–1852 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Yuan G. & Wang Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem 129, 1080–1087 (2011). [DOI] [PubMed] [Google Scholar]
- Wei J., Miao H. & Wang Q. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Sci Hortic-Amsterdam 129, 535–540 (2011). [Google Scholar]
- Miao H. et al. Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. J Exp Bot 64, 1097–1109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A. M., Ulrichs C. & Mewis I. Influence of water stress on the glucosinolate profile of Brassica oleracea var. italica and the performance of Brevicoryne brassicae and Myzus persicae. Entomol Exp Appl 137, 229–236 (2010). [Google Scholar]
- López-Berenguer C., Martínez-Ballesta M. C., García-Viguera C. & Carvajal M. Leaf water balance mediated by aquaporins under salt stress and associated glucosinolate synthesis in broccoli. Plant Sci 174, 321–328 (2008). [Google Scholar]
- Yuan G., Wang X., Guo R. & Wang Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem 121, 1014–1019 (2010). [Google Scholar]
- Brown P. D., Tokuhisa J. G., Reichelt M. & Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochem 62, 471–481 (2003). [DOI] [PubMed] [Google Scholar]
- Marina P. et al. Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell 23, 716–729 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Oki K., Hoshino K. & Kuboi T. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci 164, 259–265 (2003). [Google Scholar]
- Larronde F., Krisa S., Decendit A., Cheze C. & Merillon J. M. Regulation of polyphenol production in Vitis vinifera cell suspension cultures by sugars. Plant Cell Rep 17, 946–950 (1998). [DOI] [PubMed] [Google Scholar]
- Solfanelli C., Poggi A., Loreti E., Alpi A. & Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140, 637–646 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderby I. E. et al. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS One 2, e1322 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. H., Yoo S. D. & Sheen J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 127, 579–589 (2006). [DOI] [PubMed] [Google Scholar]
- Cho Y. H., Sheen J. & Yoo S. D. Low glucose uncouples hexokinase1-dependent sugar signaling from stress and defense hormone abscisic acid and C2H4 responses in Arabidopsis. Plant Physiol 152, 1180–1182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W. Y., Sheen J. & Jang J. C. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44, 451–461 (2000). [DOI] [PubMed] [Google Scholar]
- Yanagisawa S., Yoo S. D. & Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425, 521–525 (2003). [DOI] [PubMed] [Google Scholar]
- Ramon M., Rolland F. & Sheen J. Sugar sensing and signaling. Arabidopsis Book 6, e0117 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. G. & Jones A. M. AtRGS1 function in Arabidopsis thaliana. Regulators of G-Protein Signaling, Part A 389, 338–350 (2004). [DOI] [PubMed] [Google Scholar]
- Cho Y. H. & Yoo S. D. Signaling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. Plos Genet 7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard I. M. Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response. Plant Physiol 129, 1533–1543 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles C. et al. Regulation of Arabidopsis thaliana Em genes: role of ABI5. Plant J 30, 373–383 (2002). [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R. & Lynch T. J. The arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12, 599–609 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand B., McLachlin D. T., Chait B. T. & Chua N. H. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32, 317–328 (2002). [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R. Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J 5, 765–771 (1994). [Google Scholar]
- Arenas-Huertero F., Arroyo A., Zhou L., Sheen J. & Leon P. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Gene Dev 14, 2085–2096 (2000). [PMC free article] [PubMed] [Google Scholar]
- Laby R. J., Kincaid M. S., Kim D. G. & Gibson S. I. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23, 587–596 (2000). [DOI] [PubMed] [Google Scholar]
- Schweizer F. et al. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25, 3117–3132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H., Berger B. & Gigolashvili T. bHLH05 is an interaction partner of MYB51 and a novel regulator of glucosinolate biosynthesis in Arabidopsis. Plant Physiol 166, 349–369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Kopriva S., Giordano M., Saito K. & Hell R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. In Annu Rev Plant Biol Vol 62 (eds Merchant S. S., Briggs W. R. & Ort D.) 157–184 (2011). [DOI] [PubMed] [Google Scholar]
- Frerigmann H. & Gigolashvili T. Update on the role of R2R3-MYBs in the regulation of glucosinolates upon sulfur deficiency. Front Plant Sci 5, 626–626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T. Molecular genetics of sulfate assimilation in plants. Physiol Plantarum 97, 411–419 (1996). [Google Scholar]
- Takahashi H. Regulation of sulfate transport and assimilation in plants. In International Review of Cell and Molecular Biology Vol 281 (ed. Jeon K. W.) 129–159 (2010). [DOI] [PubMed] [Google Scholar]
- Nikiforova V., Freitag J. S., Adamik M., Hesse H. & Hoefgen R. Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. Plant J 33, 633–650 (2003). [DOI] [PubMed] [Google Scholar]
- Nikiforova V. J. et al. Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiol 138, 304–318 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanski E. R. & Last R. L. Tryptophan biosynthesis and molecular genetics biochemical and molecular genetics. Plant Cell 7, 921–934 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova V. J. et al. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids 30, 173–183 (2006). [DOI] [PubMed] [Google Scholar]
- Barroso C., Romero L. C., Cejudo F. J., Vega J. M. & Gotor C. Salt-specific regulation of the cytosolic O-acetylserine(thiol)lyase gene from Arabidopsis thaliana is dependent on abscisic acid. Plant Mol Biol 40, 729–736 (1999). [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A., Nakamura Y., Tohge T., Saito K. & Takahashi H. Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 18, 3235–3251 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.