Abstract
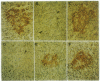
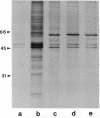
The autoimmune phenomena associated with destruction of the beta cell in pancreatic islets and development of type 1 (insulin-dependent) diabetes mellitus (IDDM) include circulating islet cell antibodies. We have immortalized peripheral blood lymphocytes from prediabetic individuals and patients with newly diagnosed IDDM by Epstein-Barr virus transformation. IgG-positive cells were selected by anti-human IgG-coupled magnetic beads and expanded in cell culture. Supernatants were screened for cytoplasmic islet cell antibodies using the conventional indirect immunofluorescence test on cryostat sections of human pancreas. Six islet cell-specific B-cell lines, originating from a patient with newly diagnosed IDDM, could be stabilized on a monoclonal level. All six monoclonal islet cell antibodies (MICA 1-6) were of the IgG class. None of the MICA reacted with human thyroid, adrenal gland, anterior pituitary, liver, lung, stomach, and intestine tissues but all six reacted with pancreatic islets of different mammalian species and, in addition, with neurons of rat cerebellar cortex. MICA 1-6 were shown to recognize four distinct antigenic epitopes in islets. Islet cell antibody-positive diabetic sera but not normal human sera blocked the binding of the monoclonal antibodies to their target epitopes. Immunoprecipitation of 35S-labeled human islet cell extracts revealed that a protein of identical size to the enzyme glutamate decarboxylase (EC 4.1.1.15) was a target of all MICA. Furthermore, antigen immunotrapped by the MICA from brain homogenates showed glutamate decarboxylase enzyme activity. MICA 1-6 therefore reveal glutamate decarboxylase as the predominant target antigen of cytoplasmic islet cell autoantibodies in a patient with newly diagnosed IDDM.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. A., Maclaren N. K., Scharp D. W., Lacy P. E., Riley W. J. 64,000 Mr autoantibodies as predictors of insulin-dependent diabetes. Lancet. 1990 Jun 9;335(8702):1357–1360. doi: 10.1016/0140-6736(90)91241-2. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Landin M., Kristensen J. K., Srikanta S., Bruining G. J., Mandrup-Poulsen T., de Beaufort C., Soeldner J. S., Eisenbarth G., Lindgren F. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest. 1987 Mar;79(3):926–934. doi: 10.1172/JCI112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Doniach D. Islet-cell antibodies (ICA) in diabetes mellitus (evidence of an autoantigen common to all cells in the islet of Langerhans). Ric Clin Lab. 1978 Jan-Jun;8(1-2):29–38. [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30;2(7892):1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Pipeleers D. G., Lernmark A., Baekkeskov S. Cellular and subcellular localization of an Mr 64,000 protein autoantigen in insulin-dependent diabetes. J Biol Chem. 1990 Jan 5;265(1):376–381. [PubMed] [Google Scholar]
- Colman P. G., Nayak R. C., Campbell I. L., Eisenbarth G. S. Binding of cytoplasmic islet cell antibodies is blocked by human pancreatic glycolipid extracts. Diabetes. 1988 May;37(5):645–652. doi: 10.2337/diab.37.5.645. [DOI] [PubMed] [Google Scholar]
- Dean B. M., Bottazzo G. F., Cudworth A. G. IgG subclass distribution in organ specific autoantibodies. The relationship to complement fixing ability. Clin Exp Immunol. 1983 Apr;52(1):61–66. [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Linnenbach A., Jackson R., Scearce R., Croce C. M. Human hybridomas secreting anti-islet autoantibodies. Nature. 1982 Nov 18;300(5889):264–267. doi: 10.1038/300264a0. [DOI] [PubMed] [Google Scholar]
- Garzelli C., Taub F. E., Scharff J. E., Prabhakar B. S., Ginsberg-Fellner F., Notkins A. L. Epstein-Barr virus-transformed lymphocytes produce monoclonal autoantibodies that react with antigens in multiple organs. J Virol. 1984 Nov;52(2):722–725. doi: 10.1128/jvi.52.2.722-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerling I., Baekkeskov S., Lernmark A. Islet cell and 64K autoantibodies are associated with plasma IgG in newly diagnosed insulin-dependent diabetic children. J Immunol. 1986 Dec 15;137(12):3782–3785. [PubMed] [Google Scholar]
- Gilon P., Tappaz M., Remacle C. Localization of GAD-like immunoreactivity in the pancreas and stomach of the rat and mouse. Histochemistry. 1991;96(4):355–365. doi: 10.1007/BF00271357. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Chang Y. C., Schwob J. E. Monoclonal antibodies to glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8808–8812. doi: 10.1073/pnas.83.22.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Browning S. W., Kennedy S., Moore D. D. Immunodot assay for determining the isotype and light chain type of murine monoclonal antibodies in unconcentrated hybridoma culture supernates. J Immunol Methods. 1983 Oct 28;63(3):281–290. doi: 10.1016/s0022-1759(83)80001-5. [DOI] [PubMed] [Google Scholar]
- Nayak R. C., Omar M. A., Rabizadeh A., Srikanta S., Eisenbarth G. S. "Cytoplasmic" islet cell antibodies. Evidence that the target antigen is a sialoglycoconjugate. Diabetes. 1985 Jun;34(6):617–619. doi: 10.2337/diab.34.6.617. [DOI] [PubMed] [Google Scholar]
- Richter W., Eiermann T. H., Graf G., Glück M., Scherbaum W. A., Pfeiffer E. F. Isolation of IgG islet cell autoantibody-producing B lymphocytes from the peripheral blood of type 1 diabetic patients and an ICA-positive non-diabetic individual. Horm Metab Res. 1989 Dec;21(12):686–688. doi: 10.1055/s-2007-1009321. [DOI] [PubMed] [Google Scholar]
- Richter W., Eiermann T. H., Scherbaum W. A. Effect of cytokines on proliferation of Epstein Barr virus-transformed B lymphocytes. Hybridoma. 1990 Feb;9(1):1–8. doi: 10.1089/hyb.1990.9.1. [DOI] [PubMed] [Google Scholar]
- Ricordi C., Finke E. H., Lacy P. E. A method for the mass isolation of islets from the adult pig pancreas. Diabetes. 1986 Jun;35(6):649–653. doi: 10.2337/diab.35.6.649. [DOI] [PubMed] [Google Scholar]
- Satoh J., Prabhakar B. S., Haspel M. V., Ginsberg-Fellner F., Notkins A. L. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med. 1983 Jul 28;309(4):217–220. doi: 10.1056/NEJM198307283090405. [DOI] [PubMed] [Google Scholar]
- Schatz D. A., Barrett D. J., Maclaren N. K., Riley W. J. Polyclonal nature of islet cell antibodies in insulin-dependent diabetes. Autoimmunity. 1988;1(1):45–50. doi: 10.3109/08916938808997175. [DOI] [PubMed] [Google Scholar]
- Seissler J., Hering B., Richter W., Glück M., Yassin N., Bretzel R. G., Boehm B. O., Federlin K., Scherbaum W. A. Antibodies to the M(r) 64,000 (64K) protein in islet cell antibody positive non-diabetic individuals indicate high risk for impaired beta-cell function. Diabetologia. 1992 Jun;35(6):550–554. doi: 10.1007/BF00400483. [DOI] [PubMed] [Google Scholar]
- Sigurdsson E., Baekkeskov S. The 64-kDa beta cell membrane autoantigen and other target molecules of humoral autoimmunity in insulin-dependent diabetes mellitus. Curr Top Microbiol Immunol. 1990;164:143–168. doi: 10.1007/978-3-642-75741-9_8. [DOI] [PubMed] [Google Scholar]
- Tuson J. R., Pascoe E. W., Jacob D. A. A novel immunohistochemical technique for demonstration of specific binding of human monoclonal antibodies to human cryostat tissue sections. J Histochem Cytochem. 1990 Jul;38(7):923–926. doi: 10.1177/38.7.2355173. [DOI] [PubMed] [Google Scholar]