Abstract
Introduction
Study compliance is crucial when the study outcome is determined by an invasive procedure, such as prostate biopsy. To investigate predictors of compliance in study-mandated prostate biopsies, we analyzed demographic, clinical and reported lifestyle data from the REDUCE trial.
Methods
We retrospectively identified 8,025 men from REDUCE with at least 2-years of follow-up, and used multivariable logistic regression to test the association between baseline demographic and clinical characteristics and undergoing the study-mandated prostate biopsy at 2 years. We then examined whether missing any of these data was associated with undergoing a biopsy
Results
In REDUCE, 22% of men did not undergo a 2-year biopsy. On multivariable analysis, non-North American region was predictive of 42-44% increased likelihood of undergoing a 2-year biopsy (p≤0.001). Being enrolled at a center that enrolled >10 subjects (2nd and 3rd tertile) was associated with a 42-48% increased likelihood of undergoing a 2-year biopsy (p<0.001). Additionally, black race predicted 44% lower rate of on-study 2-year biopsy (OR=0.56; p=0.001). Finally, missing one or more baseline variables was associated with a 32% decreased likelihood of undergoing a 2-year biopsy (OR=0.68; p<0.001).
Conclusions
In REDUCE, men outside North America, those at higher volume centers, and those with complete baseline data were more likely to undergo study mandated 2-year biopsies. Given prostate biopsy is becoming increasingly utilized as an endpoint in trials that are often multi-national, regional differences in compliance should be considered when designing future trials. Likewise, efforts are needed to ensure compliance in low-volume centers or among subjects missing baseline data.
Introduction
Study adherence in trials is crucial for proper interpretation. However, only 43-78% of subjects comply with study requirements.(1) Poor adherence with the intervention can reduce the efficacy of the intervention. To overcome this, many trials have considered interventions to increase compliance with on-study medical therapy.(2) However, drug adherence is inherently different from compliance with a study-mandated invasive procedure such as prostate biopsy. The relevance is that when the study outcome is dependent on invasive procedures such as cancer prevention studies wherein the outcome is measured by a biopsy, poor compliance with the study mandated biopsy leads to non-interpretable data, reducing study power.
Recently, several studies examined factors influencing compliance to procedural interventions, such as prostate biopsies.(3-6) As prostate cancer screening and active surveillance are being actively studied,(7-11) on-study prostate biopsies are an increasingly necessary study end-point. Thus, it is crucial to understand factors that may influence procedure compliance when designing future studies requiring prostate biopsies, because if patients are noncompliant, results are non-interpretable.
To date, only one study examined prostate biopsy compliance in men in a randomized trial. In the Prostate Cancer Prevention Trial (PCPT), subjects were assessed for prostate cancer after 7 years by a study-mandated prostate biopsy. Factors predictive of biopsy compliance included compliance with on-study medical therapy a year prior, age <70, and never smoking.(5) However, this study examined factors at year-6 predicting compliance with the year-7 procedure, rather than baseline characteristics. Additionally, this trial was performed only in the U.S. and did not include men from other geographic regions. Regional differences in study compliance are important considerations given many phase 3 trials recruit subjects from multiple geographic locations.
To investigate predictors of compliance with study-mandated procedures in a clinical trial for prostate cancer risk, we analyzed baseline demographic, clinical and self-reported lifestyle data from REDUCE. REDUCE was a multi-national trial in which all men were screened for cancer and only enrolled if a baseline prostate biopsy was negative for prostate cancer. Men were then required to undergo study-mandated biopsies at 2 and 4 years after enrollment. We hypothesized that certain baseline factors would influence compliance with the study-mandated 2-year prostate biopsy. In secondary analysis, we explored whether missing any key data elements at baseline would predict not undergoing the 2-year biopsy.
Methods
Study Population
The REDUCE trial study design has been previously described.(12) Briefly, 8,122 men at risk of prostate cancer were randomized to dutasteride (0.5 mg/day; n=4,049) or placebo (n=4,073). Eligible men had a PSA of 2.5-10 ng/ml if aged 50-59 years or 3.0-10 ng/ml if aged 60-75 years. All men had a negative baseline biopsy within 6 months before enrollment. Subjects received PSA tests every 6 months and 10-core transrectal ultrasound (TRUS)-guided prostate biopsies at 2- and 4-years, regardless of PSA. Unscheduled biopsies were performed if clinically indicated and replaced protocol-mandated biopsies if performed during months 19-24 or 43-49.
At baseline, detailed medical histories were obtained including smoking history, alcohol use, medication use and medical comorbidities. Height and weight were measured and body mass index (BMI; kg/m2) was calculated. Race was self-reported. Digital rectal examination (DRE) findings and TRUS prostate volume were reported from the pre-study biopsy.
We excluded 97 subjects who were diagnosed with prostate cancer at least 6 months prior to the 2-year biopsy leaving 8,025 men available for primary analysis. We grouped subjects based on whether or not they had the 2-year biopsy. Men who were missing any baseline data were still included in primary analysis as we sought to address whether missing data influenced undergoing a 2-year biopsy. However, on secondary analysis, we excluded 691 men who were missing ≥1 data field.
Statistical Analysis
Our primary outcome was undergoing a 2-year biopsy. As we sought to identify the pre-trial/baseline data that predict increased study-mandated biopsy compliance, only data obtained at baseline were used for analysis. In order to test whether missing data fields predicted undergoing the 2-year biopsy, all variables were categorized with missing as a separate category. For variables with well-established cutoffs (age, BMI, IPSS), we used those cut-points. For variables without clearly defined cut-points, we grouped subjects into tertiles for continuous variables (center volume, prostate volume and PSA), used convenience cut-points for ordinal variables (number of medications) or cut-points previously used in REDUCE (alcohol use).(13) While many PSA cut-points exist, there are no universally used cut-points within the study inclusion criterion of PSA 2.5-10ng/ml and thus tertiles were used.
Characteristics of subjects who underwent a 2-year biopsy versus those who did not were compared using chi-squared test. Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between baseline demographic and clinical characteristics vs. undergoing 2-year biopsy (underwent vs. did not) were estimated using logistic regression. Variables of interest included baseline age (<65 vs. 65-69 vs. ≥70 years); race (white vs. black vs. other); region (North America vs. Europe vs. Other); center volume (0-10 vs. 11-25 vs. >25 subjects enrolled); BMI (<25 vs. 25-29.9 vs. ≥30 kg/m2 vs. missing); PSA (<4.8 vs. 4.8-6.6 vs. ≥6.7 ng/ml vs. missing); prostate volume (<37 vs. 37-52 vs. ≥53 cc vs. missing); DRE (normal/enlarged vs. abnormal vs. missing); IPSS score (0-7 vs. 8-19 vs. 20-35 vs. missing); diabetes mellitus (yes vs. no vs. missing); hypertension (yes vs. no vs. missing); coronary artery disease (yes vs. no); number of medications the subject reported taking (none vs. 1-5 vs. 6-10 vs. >10; not including study medication); smoking status (never vs. former vs. current vs. missing); alcoholic drinks (0 vs. 0-7/wk vs. >7/wk); family history of prostate cancer (yes vs. no vs. missing); or family history of breast cancer (yes vs. no vs. missing).
We performed a locally weighted scatterplot smoothing (LOWESS) regression to visually demonstrate the relationship between center volume and likelihood of undergoing the 2-year biopsy.
We used logistic regression to test the association between missing any baseline demographic or clinical characteristic and undergoing a 2-year biopsy (underwent vs. did not). There were insufficient men missing >1 baseline variables (n=31) to test the association between number of missing variables and undergoing a 2-year biopsy. This analysis includes only univariable calculations, as it is not possible to adjust for other data (i.e. multivariable regression) when patients are missing the data fields to be adjusted for.
Finally, to determine whether treatment arm assignment influenced the results of our analysis, we performed a subsequent analysis including a variable for treatment arm (placebo vs. dutasteride).
All statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX). Nominal statistical significance was defined as p<0.05. However, due to multiple comparisons, we also performed a Bonferroni correction defining statistical significance as p<0.05/number of comparisons (Table 1: 18 comparisons, p<0.003; Table 2: 38 comparisons, p<0.0013; Table 3: 1 comparison, p<0.05).
Table 1.
Baseline characteristics of patients
| Did not undergo 2yr Biopsy |
Underwent 2 yr Biopsy | p-value | |
|---|---|---|---|
| Number of Patients, n (%) | 1,599 (20) | 6,463 (80) | - |
| Baseline Age, mean (SD) | 62.8 (6.3) | 62.7 (6.0) | - |
| <65, n (%) | 924 (19) | 3849 (81) | 0.065† |
| 65-69, n (%) | 407 (19) | 1681 (81) | |
| ≥70, n (%) | 268 (22) | 933 (78) | |
| Race, n (%) | <0.001† | ||
| White | 1432 (19) | 5911 (81) | |
| Black | 59 (32) | 122 (68) | |
| Other | 107 (20) | 430 (80) | |
| Missing | 1 (100) | 0 (0) | |
| Region, n (%) | <0.001† | ||
| North America | 485 (23) | 1596 (77) | |
| Europe | 929 (19) | 3951 (81) | |
| Other | 185 (17) | 916 (83) | |
| Center Volume, n (%) | <0.001† | ||
| 1st tertile (0-10 subjects) | 614 (25) | 1872 (75) | |
| 2nd tertile (11-25 subjects) | 489 (18) | 2271 (82) | |
| 3rd tertile (>25 subjects) | 496 (18) | 2320 (82) | |
| BMI, n (%) | 0.063† | ||
| <25 | 394 (19) | 1726 (81) | |
| 25-29.9 | 845 (20) | 3333 (80) | |
| ≥30 | 324 (20) | 1309 (80) | |
| Missing | 36 (27) | 95 (70) | |
| PSA, n (%) | 0.026† | ||
| 1st tertile | 551 (21) | 2066 (79) | |
| 2nd tertile | 501 (19) | 2195 (81) | |
| 3rd tertile | 540 (20) | 2191 (80) | |
| Missing | 7 (39) | 11 (61) | |
| Prostate Volume, n (%) | 0.004† | ||
| 1st tertile | 533 (20) | 2119 (80) | |
| 2nd tertile | 503 (19) | 2149 (81) | |
| 3rd tertile | 528 (20) | 2125 (80) | |
| Missing | 35 (33) | 70 (67) | |
| DRE, n (%) | 0.032† | ||
| Normal/enlarged | 1535 (20) | 6213 (80) | |
| Abnormal | 57 (19) | 242 (81) | |
| Missing | 7 (47) | 8 (53) | |
| IPSS Score, n (%) | 0.004† | ||
| 0-7 (“Mild”) | 701 (19) | 3066 (81) | |
| 8-19 (“Moderate”) | 733 (20) | 2840 (80) | |
| 20-35 (“Severe”) | 91 (26) | 258 (74) | |
| Missing | 74 (20) | 299 (80) | |
| # of Medications Reported, n (%) | 0.041† | ||
| No medications | 507 (22) | 1838 (78) | |
| 1-5 | 949 (19) | 4077 (81) | |
| 6-10 | 139 (21) | 527 (79) | |
| >10 | 4 (16) | 21 (84) | |
| Diabetes Mellitus Reported, n (%) | 0.070† | ||
| No | 1440 (20) | 5933 (80) | |
| Yes | 159 (23) | 529 (77) | |
| Missing | 0 (0) | 1 (100) | |
| Hypertension Reported, n (%) | 0.780† | ||
| No | 989 (20) | 3999 (80) | |
| Yes | 610 (20) | 2469 (80) | |
| Missing | 0 (0) | 2 (100) | |
| CAD Reported, n (%) | 0.644† | ||
| No | 1464 (20) | 5946 (80) | |
| Yes | 135 (21) | 515 (79) | |
| Missing | 0 (0) | 2 (100) | |
| Smoking Status, n (%) | 0.214† | ||
| Never | 718 (19) | 2971 (81) | |
| Former | 612 (19) | 2540 (81) | |
| Current | 268 (22) | 947 (78) | |
| Missing | 1 (17) | 5 (83) | |
| Reported Alcohol Use, n (%) | 0.001† | ||
| Non-drinker | 471 (22) | 1624 (78) | |
| Moderate Drinker (0-7/wk) | 705 (18) | 3151 (82) | |
| Heavy Drinker (>7/wk) | 410 (20) | 1653 (80) | |
| Missing | 13 (27) | 35 (73) | |
| FHx Prostate Cancer, n (%) | 0.203† | ||
| No | 1410 (20) | 5607 (80) | |
| Yes | 187 (18) | 852 (82) | |
| Missing | 2 (33) | 4 (67) | |
| FHx Breast Cancer, n (%) | 0.117† | ||
| No | 1424 (20) | 5740 (80) | |
| Yes | 173 (19) | 722 (81) | |
| Missing | 2 (67) | 1 (33) |
Abbreviations: BMI, body mass index (kg/m2); CAD, coronary artery disease; DRE, digital rectal exam; FHx, reported family history of; IPSS, International Prostate Symptom Score.
P-value (bold)=Bonferroni corrections applied for statistically significant P-values (P=0.05/18=0.003).
P-value (italics)=Nominally statistically significant P-values (P<0.05).
Pearson Chi2.
Table 2.
Univariable and multivariable model examining characteristics associated with receipt vs. no receipt of a 2-year biopsy.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Baseline Age | ||||||
| <65 | Ref | ----- | ----- | Ref | ----- | ----- |
| 65-69 | 0.99 | 0.87-1.13 | 0.898 | 0.94 | 0.82-1.08 | 0.365 |
| ≥70 | 0.84 | 0.72-0.97 | 0.022 | 0.80 | 0.68-0.94 | 0.007* |
| Race | ||||||
| White | Ref | ----- | ----- | Ref | ----- | ----- |
| Black | 0.50 | 0.37-0.69 | <0.001 | 0.62 | 0.44-0.86 | 0.005* |
| Other | 0.97 | 0.78-1.21 | 0.811 | 0.99 | 0.77-1.28 | 0.956 |
| Region | ||||||
| North America | Ref | ----- | ----- | Ref | ----- | ----- |
| Europe | 1.29 | 1.14-1.46 | <0.001 | 1.35 | 1.16-1.57 | <0.001 |
| Other | 1.50 | 1.25-1.82 | <0.001 | 1.55 | 1.24-1.94 | <0.001 |
| Center Volume | ||||||
| 1st tertile (0-10 subjects) | Ref | ----- | ----- | Ref | ----- | ----- |
| 2nd tertile (11-25 subjects) | 1.52 | 1.33-1.74 | <0.001 | 1.49 | 1.30-1.70 | <0.001 |
| 3rd tertile (>25 subjects) | 1.53 | 1.34-1.75 | <0.001 | 1.40 | 1.22-1.62 | <0.001 |
| BMI | ||||||
| <25 | Ref | ----- | ----- | Ref | ----- | ----- |
| 25-29.9 | 0.90 | 0.79-1.03 | 0.122 | 0.89 | 0.78-1.02 | 0.093 |
| ≥30 | 0.92 | 0.78-1.09 | 0.332 | 0.94 | 0.79-1.12 | 0.482 |
| Missing | 0.60 | 0.40-0.91 | 0.013* | 0.66 | 0.44-1.01 | 0.055 |
| PSA | ||||||
| 1st tertile | Ref | ----- | ----- | Ref | ----- | ----- |
| 2nd tertile | 1.17 | 1.02-1.34 | 0.024* | 1.14 | 1.00-1.31 | 0.056 |
| 3rd tertile | 1.08 | 0.95-1.24 | 0.245 | 1.07 | 0.93-1.23 | 0.321 |
| Missing | 0.42 | 0.16-1.09 | 0.073 | 0.46 | 0.18-1.23 | 0.123 |
| Prostate Volume | ||||||
| 1st tertile | Ref | ----- | ----- | Ref | ----- | ----- |
| 2nd tertile | 1.07 | 0.94-1.23 | 0.299 | 1.07 | 0.93-1.23 | 0.347 |
| 3rd tertile | 1.01 | 0.88-1.16 | 0.858 | 1.03 | 0.89-1.18 | 0.686 |
| Missing | 0.50 | 0.33-0.76 | 0.001 | 0.58 | 0.38-0.90 | 0.015* |
| DRE | ||||||
| Normal/enlarged | Ref | ----- | ----- | Ref | ----- | ----- |
| Abnormal | 1.05 | 0.78-1.41 | 0.750 | 1.12 | 0.83-1.51 | 0.460 |
| Missing | 0.28 | 0.10-0.78 | 0.015* | 0.45 | 0.15-1.32 | 0.144 |
| IPSS Score | ||||||
| 0-7 (“Mild”) | Ref | ----- | ----- | Ref | ----- | ----- |
| 8-19 (“Moderate”) | 0.89 | 0.79-0.99 | 0.040* | 0.88 | 0.78-0.99 | 0.030* |
| 20-35 (“Severe”) | 0.65 | 0.50-0.83 | 0.001 | 0.64 | 0.49-0.83 | 0.001 |
| Missing | 0.92 | 0.71-1.21 | 0.561 | 0.87 | 0.65-1.15 | 0.319 |
| # of Medications Reported | ||||||
| No medications | Ref | ----- | ----- | Ref | ----- | ----- |
| 1-5 | 1.19 | 1.05-1.34 | 0.006* | 1.39 | 1.21-1.60 | <0.001 |
| 6-10 | 1.05 | 0.85-1.29 | 0.678 | 1.55 | 1.20-2.01 | 0.001 |
| >10 | 1.45 | 0.49-4.24 | 0.499 | 2.30 | 0.77-6.93 | 0.138 |
| Diabetes Mellitus Reported | ||||||
| No | Ref | ----- | ----- | Ref | ----- | ----- |
| Yes | 0.81 | 0.67-0.97 | 0.025* | 0.80 | 0.66-0.98 | 0.029* |
| Hypertension Reported | ||||||
| No | Ref | ----- | ----- | Ref | ----- | ----- |
| Yes | 1.00 | 0.89-1.12 | 0.975 | 0.96 | 0.84-1.09 | 0.521 |
| CAD Reported | ||||||
| No | Ref | ----- | ----- | Ref | ----- | ----- |
| Yes | 0.94 | 0.77-1.14 | 0.535 | 0.92 | 0.75-1.13 | 0.423 |
| Smoking Status | ||||||
| Never | Ref | ----- | ----- | Ref | ----- | ----- |
| Former | 1.00 | 0.89-1.13 | 0.961 | 0.97 | 0.86-1.10 | 0.635 |
| Current | 0.85 | 0.73-1.00 | 0.051 | 0.83 | 0.71-0.98 | 0.025* |
| Missing | 1.21 | 0.14-10.36 | 0.863 | 1.66 | 0.18-15.55 | 0.656 |
| Reported Alcohol Use | ||||||
| Non-drinker | Ref | ----- | ----- | Ref | ----- | ----- |
| Moderate Drinker | 1.30 | 1.14-1.48 | <0.001 | 1.23 | 1.08-1.41 | 0.002* |
| Heavy | 1.17 | 1.01-1.36 | 0.040* | 1.05 | 0.90-1.23 | 0.515 |
| Missing | 0.78 | 0.41-1.49 | 0.452 | 0.74 | 0.38-1.47 | 0.417 |
| FHx of Prostate Cancer | ||||||
| No | Ref | ----- | ----- | Ref | ----- | ----- |
| Yes | 1.15 | 0.97-1.36 | 0.114 | 1.20 | 1.00-1.42 | 0.044* |
| Missing | 0.50 | 0.09-2.75 | 0.428 | 0.99 | 0.10-9.99 | 0.993 |
| FHx of Breast Cancer | ||||||
| No | Ref | ----- | ----- | Ref | ----- | ----- |
| Yes | 1.04 | 0.87-1.23 | 0.698 | 1.04 | 0.87-1.24 | 0.693 |
| Missing | 0.12 | 0.01-1.37 | 0.088 | 0.11 | 0.01-2.04 | 0.139 |
Abbreviations: BMI, body mass index (kg/m2); CAD, coronary artery disease; CI, confidence interval; DRE, digital rectal exam; FHx, reported family history of; IPSS, International Prostate Symptom Score; OR, odds ratio.
P-value (bold)=Bonferroni corrections applied for statistically significant P-values (P=0.05/38=0.0013).
Nominally statistically significant P-values (P<0.05).
Table 3.
Univariate analysis of number of missing variables predicting compliance
| Number of Missing Variables n (%) |
OR | CI | p-value |
|---|---|---|---|
|
0
7,419 (92%) |
Ref. | ----- | ----- |
|
≥1
643 (8%) |
0.75 | 0.62-0.90 | 0.002 |
Abbreviations: CI, confidence interval; OR, odds ratio. P<0.05 significant in bold.
Results
Of the 8,025 men analyzed, 1,733 men (22%) did not undergo a 2-year on-study biopsy. Baseline characteristics of men who did and did not undergo the 2-year biopsy are summarized in Table 1. After Bonferroni correction, men who underwent a 2-year biopsy were less likely to be black (p<0.001), were more likely to be non-North American (p<0.001), seen at higher volume centers (p<0.001), and more likely to have an IPSS <20 (p=0.002). Supplementary Table 1 reflects the individual countries represented by “other” in our analysis. Also, missing data for alcohol intake (p<0.001), IPSS score (p=0.002) and prostate volume (p=0.001) was associated with lower rates of 2-year biopsy. While missing PSA data was nominally associated with not undergoing the 2-year biopsy, the association lost significance after Bonferroni adjustment.
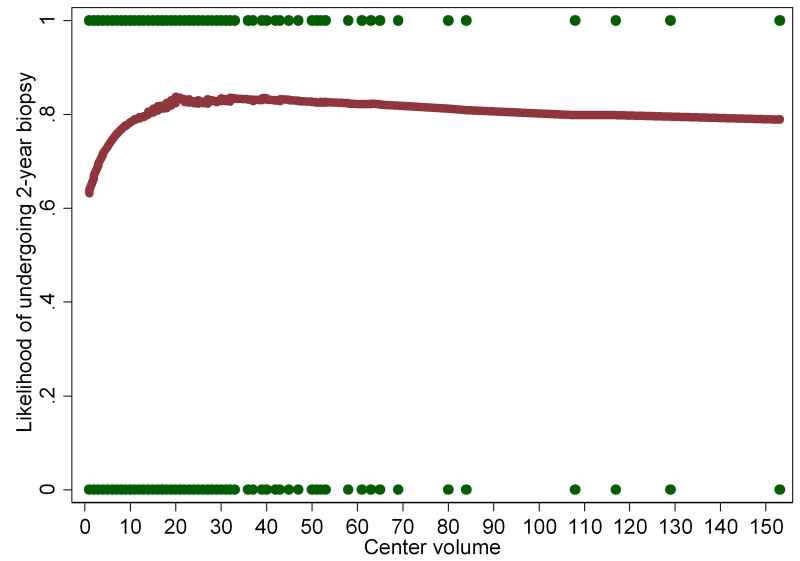
Table 2 summarizes the association between baseline demographic and clinical characteristics and undergoing an on-study 2-year biopsy. On multivariable analysis, while black race predicted 44% lower rate of on-study 2-year biopsy (OR=0.56; p=0.001), non-North American region was significantly predictive of 42-44% increased likelihood of undergoing a 2-year biopsy (OR=1.42; p<0.001; OR=1.44; p=0.001 for Europe and Other, respectively). Center volume >10 subjects was significantly predictive of 42-48% increased likelihood of undergoing a 2-year biopsy (OR=1.48; p<0.001; OR=1.42; p<0.001 for 11-25 and >25 subjects, respectively). The association between center volume as a continuous variable and undergoing the 2-year prostate biopsy is shown in Figure 1. High IPSS (≥20) was associated with a 37% decreased likelihood of undergoing a 2-year biopsy (OR=0.63, p<0.001).
Figure 1.
While missing data was generally associated with lower likelihood of undergoing a 2-year biopsy regardless of the data field missing, after Bonferroni correction this only reached nominal significance for missing prostate volume (OR=0.52; p=0.002). Similarly, taking 1-10 medications relative to no medications was associated with a 33-43% increased likelihood of biopsy regardless of the number of medications taken (all p≤0.005), though after Bonferroni adjustment, this only reached significance in subjects taking 1-5 medications (OR=1.33, p<0.001).
Although current smoking status was associated with not undergoing a 2-year biopsy, and reported moderate alcohol use and family history of prostate cancer were associated with increased likelihood of undergoing the 2-year biopsy (all p≤0.017), the effects of these predictors were modest and not statistically significant after Bonferroni corrections were applied.
Though the number of men missing data was small, missing data for any one of several variables (i.e. PSA, prostate volume, DRE, IPSS score, alcohol use, and family history of breast cancer) all were associated with a trend toward decreased likelihood of undergoing a 2-year biopsy. To better test whether missing any data was associated with undergoing the 2-year biopsy, we created a composite variable to categorize subjects as missing no data (n=7,387; 92%) or missing any variable (n=638, 8%). When this was done (Table 3), missing any data was associated with a 32% decreased likelihood of undergoing a 2-year biopsy (OR=0.68; p<0.001).
Finally, treatment arm (placebo vs. dutasteride) was not a significant predictor of undergoing a 2-year biopsy and its inclusion in the multivariable model did not change the results of the other risk factors (data not shown).
Discussion
Compliance in clinical trials is imperative for accurate assessment of study outcomes, especially in studies where endpoints require data from invasive procedures. To assess whether baseline subject characteristics and demographic factors predict future compliance with a study-mandated invasive procedure, we examined baseline predictors of undergoing a 2-year study-mandated prostate biopsy in REDUCE. We found 22% did not undergo the 2-year on-study biopsy. Those who did not undergo the 2-year biopsy were more likely to be North American, enrolled at low-volume sites, or have missing baseline data. If validated in other studies, these findings suggest that, in a multi-national trial, region may affect compliance with study-mandated invasive procedures such as prostate biopsy. Additionally, enrollment at low-volume centers or missing baseline data, whether due to failed subject report or collection error, may be associated with decreased compliance.
The only other study examining compliance with study-mandated prostate biopsy in a clinical trial is from PCPT where among men with 6-year data, 37% did not undergo the year-7 end-of-study biopsy.(5) However, this number does not include the additional 8% who were lost to follow-up or died prior to year-6. Together with our data, non-compliance with study-mandated prostate biopsy may range from 22%-45%.
In PCPT, study drug (finasteride) adherence at year-6 was associated with 84% compliance with year-7 biopsy vs. 47% for those who were non-adherent to study drug at year-6 (p<0.0001).(5) We did not examine on-study medication adherence predicting biopsy compliance because we sought to test only baseline factors predicting the 2-year biopsy. However, we did find biopsy compliance was similar in dutasteride vs. placebo arms. Of note, the withdrawal rate due to adverse events, while higher in the dutasteride arm, was low in both arms (≤4.3%).(12) Whether similar results would be seen with interventions having greater side effects is unknown. Interestingly, we found men who reported taking any medications at baseline were ~30% more likely to undergo the 2-year biopsy. While we do not have data on adherence to baseline pre-study medications, it is possible self-report of taking medications in itself suggests increased responsiveness to medical recommendations. Viewed alternatively, men taking no baseline medications were possibly less familiar with needing to follow medical advice leading to less compliance with the 2-year biopsy. If confirmed in future studies, this suggests subjects taking no baseline medications may need closer attention and/or education to increase study compliance.
In PCPT, biopsy compliance varied by study site. Larger sites, those receiving recruitment and adherence grants, and those with increased study form submission had 8-14% increased biopsy rates at year-7 (all p<0.0001).(5) Similarly, we found higher volume sites (>10 subjects enrolled) had 42-48% increased compliance with the 2-year biopsy. However, PCPT did not include non-North American men, which is a strength of REDUCE.(12) Our analysis demonstrated a significant 42-44% increased likelihood of undergoing the 2-year biopsy in non-North American men. While the exact reasons for this are unknown, we speculate this may result from cultural attitudes towards medical care, differences in perception of risks from prostate biopsy, or site-specific variations in how subjects are informed about the necessity of complying with study-mandated procedures. Regardless of the reason, our findings suggest that regional differences in study compliance are important considerations when designing future clinical trials.
Regional differences in study compliance have influenced the endpoints of recent randomized trials for prostate cancer. In a multi-national phase 3 trial comparing atrasentan to placebo for the treatment of non-metastatic hormone-resistant prostate cancer, large regional differences in treatment compliance were noted, with discontinuation rates twice as high in the U.S. compared with non-U.S. regions.(14) A more recent trial studying ipilimumab in metastatic castrate-resistant prostate cancer found ipilimumab significantly improved survival in non-North American (HR=0.79, 95%CI=0.66-0.96) but not North American men (HR=0.99, 95%CI=0.69-1.42).(15) Whether or not these latter findings are due to compliance is unclear; however given our data and the data from the atrasentan trial, it is reasonable to suggest that these differences in outcomes in the ipilimumab trial may relate to regional differences in compliance rather than regional variations in disease biology, though this remains speculative. Certainly, based on the atrasentan trial, it is clear that regional differences in study compliance may affect study outcome. Given our findings that non-North American region was associated with 42-44% increased compliance with study-mandated biopsy, future multi-national studies may benefit from including targeted measures to ensure increased compliance at North American sites.
Another factor that predicted lower likelihood of undergoing the 2-year biopsy was missing data at baseline. Men missing data for even a single variable at baseline had a 32% decreased chance of undergoing the study-mandated 2-year biopsy. Missing data may result from multiple factors, including not reported by subjects (subject-driven), not properly recorded by the study site (site-driven), or a combination of both. Prior studies have indeed shown that increased subject engagement during studies is associated with increased compliance in clinical trials, highlighting the importance of subject-driven factors.(2, 16-19) Also, the analysis of PCPT showed that larger sites, those that received additional financial resources, and well performing sites were all associated with compliance with the end-of-study biopsy(5), highlighting the importance of site-specific factors. Regardless, our findings suggest that missing data are a red-flag that merits attention to avoid non-compliance.
Besides prostate biopsies, other invasive procedures, such as colonoscopy, are integral components of cancer screening and prevention trials.(20) However, few studies have examined factors influencing compliance with invasive procedures in clinical trials. In the Aspirin/Folate Polyp Prevention study, North American subjects were randomized to receive aspirin or folate, with a study-mandated colonoscopy within 34-40 months of enrollment. Compliance with study-mandated colonoscopy was 97%.(21) In a multi-national randomized trial examining celecoxib in colon polyp prevention, 89% and 79% of subjects had study-mandated colonoscopies at 1 and 3-years after randomization, respectively, though data were not separated by region.(22) In summary, non-compliance with colonoscopy in these trials ranged from 3%-20%, compared to 22%-45% in on-study prostate biopsy compliance for REDUCE and PCPT. The exact reason for these differences is unclear. One possible reason is that colonoscopy is routinely recommended for all older subjects (i.e. is part of routine standard of care) and thus may be more acceptable to participants vs. prostate biopsy, which is only done for cause and may carry higher risks including sepsis leading to poorer compliance. Also, the colon studies included both men and women and perhaps men are less compliant contributing to lower compliance in prostate biopsy studies.
One limitation of our study is the self-reported nature of many baseline characteristics. That being said, previous studies have demonstrated self-reported information to be >97% accurate when assessed clinically.(23) Other factors that were not analyzed that could influence biopsy compliance include socioeconomic status, barriers to health care (distance, costs, etc.), cultural and religious characteristics related to delivery of health care. We also did not have data on patient satisfaction or complication rates with the initial pre-study biopsy, which may influenced compliance with the subsequent 2-year biopsy. While it would be informative to examine whether specific characteristics of high versus low enrollment sites could predict compliance, these data were unfortunately unavailable for analysis. Additionally, given that we examined a large amount of characteristics, our results are subject to multiple comparisons and type 1 errors. To account for this, we used a Bonferroni correction to define the level of significance and noted that many associations remained significant.
Despite these limitations, a strength of our study is that we were able to examine baseline characteristics in a clinical trial setting and demonstrate associations with compliance to a study-mandated prostate biopsy 2 years later. The only other study examining subject features predicting compliance with on-study prostate biopsy in a clinical trial examined factors at year-6 of the study influencing undergoing biopsy at year-7, rather than baseline characteristics. Thus our study uniquely examined how characteristics of subjects identifiable at trial onset may influence future compliance with on-study procedures. Additionally, our study is the first to demonstrate a significant regional difference in compliance with prostate biopsy in a multi-national phase 3 clinical trial.
In summary, men outside of North America were significantly more likely to undergo an on-study 2-year biopsy in REDUCE. Our findings suggest that regional differences can play a role in whether or not study participants comply with study-mandated invasive procedures such as prostate biopsy. Additionally, missing data even a single data field at baseline predicted decreased likelihood of undergoing a study-mandated biopsy. Finally, enrollment at a higher volume center correlated with improved compliance. Future clinical trials should consider these regional and other features including missing baseline data and enrollment at a low-volume center that predict non-compliance to identify study populations at risk of non-compliance and implement interventions to increase compliance.
Supplementary Material
Acknowledgments
Disclosure of Potential Conflicts of Interest: This study was supported by GlaxoSmithKline (GSK). Dr. Andriole is a consultant to GSK. Dr. Castro-Santamaria is an employee of GSK. Dr. Freedland received research support from GSK and NIH K24CA160653
Financial support: Supported by GlaxoSmithKline and NIH 1K24CA160653
Footnotes
ClinicalTrials.gov Identifier: NCT00056407
References
- 1.Osterberg L, Blaschke T. Adherence to medication. The New England journal of medicine. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. The Cochrane database of systematic reviews. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. Journal of the National Cancer Institute. 2012;104(2):125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery KN, Metcalfe C, Vedhara K, Lane JA, Davis M, Neal DE, et al. Predictors of attendance for prostate-specific antigen screening tests and prostate biopsy. European urology. 2012;62(4):649–55. doi: 10.1016/j.eururo.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 5.Gritz ER, Arnold KB, Moinpour CM, Burton-Chase AM, Tangen CM, Probstfield JF, et al. Factors associated with adherence to an end-of-study biopsy: lessons from the prostate cancer prevention trial (SWOG-Coordinated Intergroup Study S9217) Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(8):1638–48. doi: 10.1158/1055-9965.EPI-14-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus PM, Ogden SL, Gren LH, Childs JC, Pretzel SM, Lamerato LE, et al. Non-compliance with the initial screening exam visit in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Preventive medicine. 2014;67:82–8. doi: 10.1016/j.ypmed.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. The New England journal of medicine. 2014;370(10):932–42. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. The New England journal of medicine. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 9.Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. European urology. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. European urology. 2014;65(1):124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early detection of prostate cancer: AUA Guideline. The Journal of urology. 2013;190(2):419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. The New England journal of medicine. 2010;362(13):1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 13.Fowke JH, Howard L, Andriole GL, Freedland SJ. Alcohol Intake Increases High-grade Prostate Cancer Risk Among Men Taking Dutasteride in the REDUCE Trial. European urology. 2014 doi: 10.1016/j.eururo.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113(9):2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer J, Rosenheck R, Kirk G, Krol W, Krystal J, Group VANS Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2003;6(5):566–73. doi: 10.1046/j.1524-4733.2003.65269.x. [DOI] [PubMed] [Google Scholar]
- 17.Besch CL. Compliance in clinical trials. Aids. 1995;9(1):1–10. doi: 10.1097/00002030-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. Journal of clinical pharmacy and therapeutics. 2001;26(5):331–42. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 19.Fouad MN, Johnson RE, Nagy MC, Person SD, Partridge EE. Adherence and retention in clinical trials: a community-based approach. Cancer. 2014;120(Suppl 7):1106–12. doi: 10.1002/cncr.28572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled clinical trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 21.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. The New England journal of medicine. 2003;348(10):891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 22.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. The New England journal of medicine. 2006;355(9):885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 23.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of clinical epidemiology. 2004;57(10):1096–103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.