Abstract
Purpose:
To estimate the economic impact of a TegadermTM chlorhexidine gluconate (CHG) gel dressing compared with a standard intravenous (i.v.) dressing (defined as non-antimicrobial transparent film dressing), used for insertion site care of short-term central venous and arterial catheters (intravascular catheters) in adult critical care patients using a cost-consequence model populated with data from published sources.
Material and Methods:
A decision analytical cost-consequence model was developed which assigned each patient with an indwelling intravascular catheter and a standard dressing, a baseline risk of associated dermatitis, local infection at the catheter insertion site and catheter-related bloodstream infections (CRBSI), estimated from published secondary sources. The risks of these events for patients with a Tegaderm CHG were estimated by applying the effectiveness parameters from the clinical review to the baseline risks. Costs were accrued through costs of intervention (i.e. Tegaderm CHG or standard intravenous dressing) and hospital treatment costs depended on whether the patients had local dermatitis, local infection or CRBSI. Total costs were estimated as mean values of 10,000 probabilistic sensitivity analysis (PSA) runs.
Results:
Tegaderm CHG resulted in an average cost-saving of £77 per patient in an intensive care unit. Tegaderm CHG also has a 98.5% probability of being cost-saving compared to standard i.v. dressings.
Conclusions:
The analyses suggest that Tegaderm CHG is a cost-saving strategy to reduce CRBSI and the results were robust to sensitivity analyses.
Keywords: Tegaderm chlorhexidine gluconate (CHG), cost-effectiveness, vascular access, bloodstream infection, catheter-related bloodstream infection (CRBSI), adult critical care, cost model, central venous catheter (CVC), arterial catheters, chlorhexidine dressing
Introduction
Short-term intravascular access devices, which include central venous and arterial catheters, are commonly used for the management of critically ill patients with the majority of patients receiving care that involves the use of these catheters (Curtis, 2009). The use of intravascular catheters is, however, associated with the risk of bloodstream infections (BSI); a leading cause of healthcare-associated infections (HCAI). In a recent survey reported by the Health Protection Agency (now Public Health England) blood stream infections accounted for 7.3% of all HCAI and of these 64% were in patients with a vascular access device (Health Protection Agency, 2011).
Infections associated with intravascular catheters are often categorised as either central-line associated bloodstream infection (CLABSI) or catheter-related bloodstream infection (CRBSI). The former term is often used in relation to epidemiological studies on the surveillance of bloodstream infections and the latter where a high degree of confidence in the origins of the infection is determined. The Centers for Disease Control and Prevention (CDC) recommends the CLABSI definition be used for surveillance of infections and defines it as: a confirmed BSI in any patient with a central venous catheter (CVC) present either at the time of or within a 48-h period before the detection of infection. CLABSI are likely therefore to overestimate the incidence of CRBSI, as they do not fully confirm the source of infection as the intravascular catheter. CRBSI is more frequently used in clinical research being a more precise and rigorous definition that requires isolation of the same microorganism from the catheter tip and peripheral blood or other validated methods including comparison of the numbers of microorganisms present in peripheral blood with samples obtained through the catheter or the differential time to positivity of blood obtained via the catheter versus peripheral blood (Chopra et al., 2013). For the purposes of this report we have used a single term and describe both CRBSI and CLABSI as CRBSI.
The risk of CRBSI is associated with a variety of factors including the site of insertion of the device and the severity of the patients underlying clinical condition. In 2009, the CDC estimated the number of CRBSI in intensive care units (ICUs) across the United States to be 18,000 (Srinivasan et al., 2011). In a pan-European survey of ICU patients, the incidence of CRBSI was in the range of 1–3.1 per 1000 patient days (Suetens et al., 2007). The reported incidence of CRBSI has decreased in more recently reported studies following various interventions including a care bundle approach. For example, in a national critical care programme in the UK, the incidence of CRBSI fell from 3.7 to 1.48 per 1000 patient days (Bion et al., 2013). However, CRBSI are still associated with attributable mortality rates of up to 11.5% and may extend ICU stay by up to 12 days (Renaud and Brun-Buisson, 2001; Soufir et al., 1999). Each CRBSI has been estimated to cost $16,550 and initiatives to reduce this complication raise the possibility of substantial savings (O’Grady et al., 2011).
More recently, improvements in quality of care have focused on initiatives with a care bundle of evidence-based interventions. For example, the introduction of a central venous insertion bundle into multiple ICUs in Michigan, which included implementation and close monitoring of hand hygiene, the application of chlorhexidine skin preparations and the use of the subclavian vein resulted in a decrease of CR-BSI by 66% (Pronovost et al., 2006).
Many interventions are included in the current guidelines for insertion and care of CVCs. The guidelines available include those from the UK’s National Institute for Health and Care Excellence (NICE) relating to infections in primary care (NICE, 2012), epic3 which present national guidelines primarily for hospitals in UK (Loveday et al., 2014) and the Healthcare Infection Control Practices Advisory Committee (HICPAC) guidelines in US (O’Grady et al., 2011). The interventions recommended include: full barrier precautions during insertion; use of a 2% chlorhexidine gluconate in alcohol for care of the catheter insertion site; the application of sterile transparent dressings to cover the insertion site with replacement at least every 7 days; and the use of alcohol to disinfect hubs and ports prior to access.
Additional approaches to prevent CRBSI have included the introduction of new technological innovations, including antiseptic impregnated central catheters and chlorhexidine impregnated sponge dressings, which have also been associated with significant reductions in infection rates (Casey et al., 2008; Timsit et al., 2009). In certain healthcare institutions, where both bundle interventions and technological solutions have been implemented, the prospect of zero CRBSI is considered to be a potential goal. (Zingg et al., 2011).
More recently, the epic3 updated guideline included the recommendation to use a 2% chlorhexidine gel dressing for intravascular catheters (Loveday et al., 2015). The purpose of the current study was to develop a model to evaluate the economic value of implementing such a 2% chlorhexidine gel dressing in the care of insertion sites associated with CVC and arterial catheters in critically ill adult patients. There is a paucity of studies which have demonstrated cost-savings with individual infection prevention interventions. In the UK, NICE (NICE, 2011) uses cost-consequence analysis to evaluate medical devices. We therefore used a similar approach to estimate the cost impact of introducing Tegaderm chlorhexidine gluconate (CHG) dressing (3M Health Care, Loughborough, UK) into clinical practice in adult critical care. The Tegaderm CHG dressing delivers chlorhexidine over 7 days from an integrated gel pad placed over the point of insertion of an intravascular catheter. A previous study (Timsit et al., 2012) has suggested it is associated with significant reductions in CRBSI compared to standard, non-medicated, transparent film dressings (standard intravenous [i.v.] dressings). This paper describes a decision analytic model, informed by parameters derived from published data, which has been used to estimate costs associated with the use of Tegaderm CHG i.v. dressings compared with standard i.v. dressings.
Material and methods
A decision analytical model (Petrou et al., 2011) was developed using Microsoft Excel software (Microsoft Corporation) to estimate the economic impact of a Tegaderm CHG dressing when compared with standard i.v. dressings (defined as standard non-antimicrobial transparent film dressings) for patients admitted to an ICU requiring an intravascular catheter. The economic perspective of the model is the National Health Service (NHS) in England and Wales. The model used a short time horizon and the structure of the model is shown in Figure 1.
Figure 1.

Model structure.
The model assigned each patient with an indwelling intravascular catheter and a standard i.v. dressing, a baseline risk of associated dermatitis, local infection at the catheter insertion site and CRBSI. The risks of these events for patients with a Tegaderm CHG were estimated by applying the effectiveness parameters derived from the published literature to the baseline risks. Costs were accrued through costs of intervention (i.e. Tegaderm CHG or standard i.v. dressing) and hospital treatment costs depended on whether the patients had dermatitis, local infection or CRBSI. Results were estimated as mean values of 10,000 probabilistic sensitivity analysis (PSA) runs, each run with a different estimate for the risks, hazard ratios (HR) and costs sampled from probability distributions representing uncertainty in the parameter estimates.
Baseline risks
Baseline risks were estimated using data from existing medical literature. The outcomes included in the model were: (1) CRBSI; (2) local site infection; and (3) dermatitis.
The baseline primary outcome measure, CRBSI, was estimated from ‘Matching Michigan’: a 2-year study of central venous CRBSI in ICUs in England (Bion et al., 2013). This study reported an average rate of 1.48 catheter-related infections per 1000 adult catheter days, which is similar to the rate reported in other studies (Edwards et al., 2009; Timsit et al., 2012). The average length of stay in an ICU for a patient with a catheter has been estimated to be 10 days, a value that has been used in another economic analysis (Ye et al., 2011). In the economic model, CRBSI risk per patient was estimated by multiplying this length of stay by the CRBSI rate which resulted in a mean CRBSI risk of 0.0148 per patient.
A key secondary outcome measure of interest was the local site infection rate. There are substantially different estimates of incidence reported in various sources, with incidence in the range of 10–30%. Crawford et al. (2004) used a local infection rate of 18.1% in their economic analysis and Vokurka et al. (2009) reported a rate of local site infection of 30%. However, Pemberton et al. (1996) reported the rate of local site infection as 10% when using non-antimicrobial transparent film dressings. This figure was also used in the economic analysis by Ye et al. (2011) concerning an antimicrobial patch. In this current economic model, a conservative estimate of a mean local site infection rate of 10% was used as shown in Table 1 for the base case analysis.
Table 1.
Summary of model parameters.
| Parameter | Mean | Distribution | Source |
|---|---|---|---|
| Average length of catheterisation | 10 days | Normal (10, 2) | Timsit et al., 2012 |
| Baseline risks | |||
| CRBSI risk (per 1000 catheter days) Local site infection risk (per patient) Dermatitis risk (per catheter) |
1.48/1000 catheter days 0.1 0.0026 |
Normal (1.48, 0.075) Normal (0.1, 1) Normal (0.0026, 0.0002) |
Bion et al., 2013 Ye et al., 2011 Schwebel et al., 2012, Timsit et al., 2012 |
| HRs for Tegaderm CHG | |||
| CRBSI Local site infection Dermatitis (as RR) |
0.402 0.402 4.4 |
Lognormal (–0.911, 0.393) Lognormal (–0.911, 0.393) Lognormal (1.482, –0.489) |
Timsit et al., 2012
Timsit et al., 2012 Timsit et al., 2012 |
| Costs (in £) | |||
| Unit cost of standard i.v. dressings Unit cost of Tegaderm CHG dressings CRBSI Local site infection Dermatitis |
£1.34 £6.21 £9900 £250 £150 |
Fixed Fixed Gamma (198, 50) Gamma (50, 5) Gamma (30, 5) |
3M 3M Hockenhull et al., 2008 Saint et al., 2000 Schwebel et al., 2012 |
Normal distribution is represented with mean and SD, with 95% of the values in the distribution lying between 2 SDs on either side of the mean, e.g. normal (10, 2) implies that 95% of the samples lie between 6 and 14. Lognormal distribution is a continuous probability distribution whose logarithm is normally distributed. Gamma (a, b) distribution, where a is the shape parameter and b is the scale parameter, is typically used for skewed distributions and has a mean expected value of a × b, e.g. the average value of the samples of distribution Gamma (198, 50) is 9900 (i.e. 198 × 50).
Another outcome of interest was dermatitis. A dermatitis rate of 1.1% was associated with the use of Tegaderm CHG as compared to 0.29% for standard dressings (Timsit et al., 2012). However, this study was undertaken using a first generation version of the Tegaderm CHG dressing that appears to have a higher occurrence of this type of adverse event compared to the current version of the dressing that utilises a higher permeability film backing. Indeed, the introduction of a second generation Tegaderm CHG dressing was associated with a reduction in the number of skin reactions reported in Manufacturer and User Facility Device Experience from 2010 to 2012, and in numbers that were comparable to CHG sponge dressings (Jenks et al., 2015). In a study by Schwebel et al. (2012) on the clinical use of a CHG sponge dressing, a rate of device-related dermatitis of 1.1 and 4.1/1000 catheters for a 3 and 7 days CHG dressing change protocol, respectively, was reported. The rate of dermatitis in the current model was assumed to be the average of these two dressing change frequencies. In this current study, a mean dermatitis rate of 2.6/1000 catheters associated with the use of Tegaderm CHG dressing was therefore used as shown in Table 1.
Effectiveness of Tegaderm CHG
The effectiveness parameters for Tegaderm CHG dressing were estimated from Timsit et al. (2012), the only relevant study identified in a systematic review which was undertaken in accordance with recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement. The purpose of the review was to identify studies evaluating the effectiveness and safety of 3M Tegaderm CHG dressings compared to standard i.v. dressings used in routine care of patients (age >18 years) admitted to a critical care setting such as an ICU who required intravascular access—via an arterial catheter or CVC or both—for at least 24 h. The details of the search strategy used are provided in the appendix.
HRs estimated from Timsit et al. (2012) were used as effectiveness parameters in the model for Tegaderm CHG dressing. As used in Ye et al. (2011), the HR for local site infection was assumed to be the same as the HR for CRBSI. The probabilities of developing a CRBSI and local infections for patients given Tegaderm CHG dressing were estimated by applying the HRs to the baseline parameters. For dermatitis, the effectiveness of Tegaderm CHG dressing was modelled as a relative risk (RR) and the probability of dermatitis for patients with Tegaderm CHG dressing were estimated by applying the RR to the baseline dermatitis probability.
Costs
The costs included in the model are the costs of the dressings and those associated with the treatment, where appropriate, for any local infections at the catheter insertion site, CRBSI and dermatitis. It was assumed that all other initial treatment costs were the same and were not included in the model. The Tegaderm CHG dressing is readily adopted into clinical practice and can directly replace standard i.v. dressing in the care pathways for arterial and CVCs. Therefore, no additional staff costs were included in the model.
The cost of standard care was estimated as the number of standard i.v. dressings required multiplied by the unit cost of this standard i.v. dressing. The number of dressings required was estimated by dividing the average duration of stay of a patient with a catheter on ICU in situ by the average dressing duration. The average length of stay for a patient with an intravascular catheter in situ on ICU was estimated to be 10 days (Ye et al., 2011) and with the prescribed time for standard i.v. dressing being between 3 and 7 days, this resulted in three standard i.v. dressings required per patient. The unit cost of a standard i.v. dressing was £1.34, as shown in Table 1 and thus, in the model, the average cost of standard i.v. dressings per patient was calculated to be £4.02.
The cost of using Tegaderm CHG dressings per patient was estimated as the number of Tegaderm CHG dressings required multiplied by the unit cost of the Tegaderm CHG dressing. The unit cost of Tegaderm CHG dressing was £6.21 for the NHS as shown in Table 1. Again, the number of dressings required was estimated by dividing the average length of stay of a patient on an ICU with a catheter by the average dressing duration. The average length of stay of a patient on an ICU with an indwelling intravascular catheter was taken as 10 days (Ye et al., 2011) and an estimate of three dressings per patient resulted in the average cost of Tegaderm CHG dressings per patient being £18.63.
The main cost driver included in the model was the cost of CRBSI, which included costs of diagnosis, catheter replacement and the costs associated with increased length of stay. Schwebel et al. (2012) reported that the mean additional hospital length of stay for patients with CRBSI is 11 days, which resulted in a reported cost estimate of CRBSI in excess of $25,000 (Schwebel et al., 2012; Ye et al., 2011). In Europe, additional length of stay in ICU has been reported as 9–10 days (Timsit et al., 2012). However, this was regarded as longer than that seen in many ICUs in the UK by our clinical experts (the co-authors of this manuscript), who considered that the average length of stay for a CRBSI patient will vary between 6 days (first 2 days in ICU and the remaining 4 days in a general medical ward) and 10 days (first 3 days in ICU and the remaining 7 days in a general medical ward). In a UK hospital, the cost of an average day in the ICU has been determined as being in the range of £1800–2400 with an additional £100 for the consultant time and consumables. This figure, in combination with the extra ward based costs of £480 per day, results in an average extra total cost of around £9750. Also, it is estimated that in clinical practice approximately 50% of intravascular catheters are removed due to suspected CRBSI and if they are subsequently replaced the cost is estimated to be £140 (acquisition cost of catheter £35; X-ray for confirming position of catheter £50 plus consumables of £15 plus staff costs to carry out the procedure at £40). In view of this, a figure of £140 for catheter replacement has been included in the total cost of CRBSI, which results in the overall CRBSI cost of £9890. This figure is very similar to that used in a Health Technology Assessment (HTA) (Hockenhull et al., 2008) who reported a mean CRBSI cost of £9148, that when inflated to present day costs using the hospital and community health services (HCHS) pay and price index (Curtis, 2013) is £9905. Thus, the cost of CRBSI used in the model is £9900 as shown in Table 1.
The cost of treatment for a local site infection was reported as $400 (Saint et al., 2000) and it was considered that a cost of £250 for treatment of local infection in UK was a reasonable estimate and was therefore adopted in the model as shown in Table 1.
The costs of treating dermatitis were taken from Schwebel et al. (2012), who reported that contact dermatitis requires four standard i.v. dressings, removal of the catheter and insertion of a new catheter. They used a micro-costing approach to estimate the total costs as $228. We considered that the equivalent cost of £150 for treatment of dermatitis was acceptable and this was used in the model as shown in Table 1.
Summary of model parameters
The decision analytic model assigned a baseline risk of different events for each patient with standard i.v. dressing. The risks of these events for patients with Tegaderm CHG dressing were estimated by applying the HRs from published literature to the baseline risks. A summary of the model parameters is provided in Table 1, along with their sources. The scope of this study was adult intensive care patients.
Results
First, we estimated the mean event rates that would be expected in a typical patient if Tegaderm CHG dressing were implemented instead of standard care. Tegaderm CHG dressing reduces the all event rates per patient except for dermatitis (Table 2). It is of note that the formulation of the Tegaderm CHG dressing was changed in 2011 to include a more permeable film on the back. The introduction of second generation Tegaderm CHG dressing was associated with a reduction in the number of skin reactions reported in Manufacturer and User Facility Device from 2010 to 2012 (FDA, 2014).
Table 2.
Summary of clinical risks associated with either the standard i.v. dressing or Tegaderm CHG.
| Clinical risk | Standard i.v. dressing | Tegaderm CHG |
|---|---|---|
| CRBSI risk | 1.48 per 1000 catheter days | 0.6 per 1000 catheter days |
| Local site infection risk (per patient) | 10% | 4.25% |
| Dermatitis risk (per patient) | 0.26% | 1.11% |
We then estimated the costs that would be expected in a typical service for 1000 adult patients who require a short-term intravascular catheter located in an ICU where Tegaderm CHG dressing was the routine dressing instead of a standard i.v. dressing. Results were estimated as mean values of 1000 probabilistic sensitivity analysis runs, each run with a different estimate for the risks, HRs and costs sampled from the probability distributions reported in Table 1. The model showed use of Tegaderm CHG dressing results in an overall saving of £77,427 per 1000 patients, i.e. an average cost saving of £77 (95% CI, £76.70–78.10) per adult patient. The Tegaderm CHG dressing therefore has a 98.5% probability of being cost-saving compared to standard i.v. dressings.
The total cost-savings is provided as a breakdown of the individual cost differences as shown in Table 3. Most of the savings described are due to a reduction in suspected and confirmed CRBSI. There are higher costs associated with the acquisition of CHG dressings but the savings in reduced event rates more than offset these costs associated with the technology, as seen in Figure 2.
Table 3.
Breakdown of cost results.
| Standard i.v. Dressing | Tegaderm CHG | Difference | |
|---|---|---|---|
| Dressing costs | £4021 | £18,631 | £14,610 |
| CRBSI | £146,457 | £63,603 | −£82,854 |
| Local site infection | £24,997 | £11,153 | −£13,844 |
| Dermatitis | £1166 | £5826 | £4660 |
| Total costs | £176,639 | £99,212 | −£77,427 |
Figure 2.
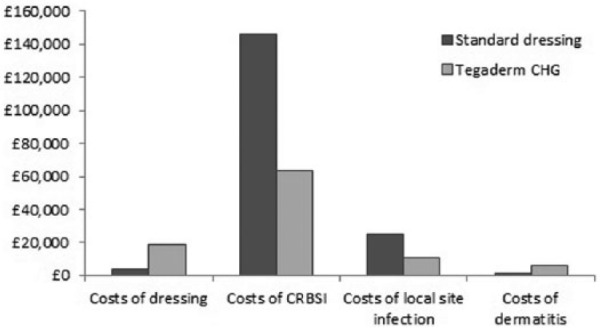
Breakdown of the different costs for standard and Tegaderm CHG dressings (for a cohort of 10,000 patients).
One-way sensitivity analysis was performed on all the model parameters. The baseline CRBSI risks and unit cost of CRBSI were identified as the key cost drivers while the rest of the parameters did not have an impact on the results or conclusions. Thus, only the results of sensitivity analysis for CRBSI risks and costs are presented here. At a lower CRBSI baseline risk of 0.5 per 1000 catheter days, the mean cost savings were £22,747 and at a higher estimate of 2.5 per 1000 catheter days, the mean cost savings were £133,591. At a lower cost estimate of CRBSI of £5000, the mean cost savings were £36,174 while the cost savings were £119,040 at a higher estimate of £15,000. Thus the lower the CRBSI baseline risk and cost of CRBSI, the lower the cost savings. Similarly, the higher the baseline risk of CRBSI and the cost of CRBSI, the higher the average cost savings. Threshold analysis conducted suggested that the baseline CRBSI risk has to be lower than 0.1 per 1000 catheter days for Tegaderm CHG dressing to not be cost-saving.
Discussion
Our analyses suggest that Tegaderm CHG dressing is a cost-saving strategy when implemented for care of adult patients with intravascular catheters in ICU. Tegaderm CHG dressing results in an overall savings of £77,427 per 1000 adult patients, i.e. an average cost saving of £77 per patient compared to standard care with a 98.5% probability of being cost-saving compared to standard i.v. dressings. A 12-bed ICU is estimated to admit approximately 770 patients each year, the majority of whom will receive an intravascular catheter. The estimated cost savings for replacement of standard i.v. dressings with Tegaderm CHG dressing in such a unit is £59,600 per annum. The results were robust to sensitivity analyses performed on the baseline CRBSI risks and unit cost of CRBSI. These average cost-savings reported are for units that follow the English national trend for incidence of CRBSI reported by Bion et al. (2013). Interestingly, the model also indicates that cost-savings can be found even in units with levels of CRBSI as low as 0.33 per 1000 catheter days.
Dermatitis has been identified as an adverse event that has a higher risk for CHG containing dressings compared to standard i.v. dressings. The results from this study indicate that the costs associated with the management of the related dermatitis per patient has a mean of £4.66. Another, albeit uncommon but potentially serious, adverse event associated with the use of chlorhexidine containing products is anaphylaxis (MHRA, 2012). However, no systemic adverse events associated with use of Tegaderm CHG dressing were reported during a comparative study including 1879 adult patients (Timsit et al., 2012) and the potential costs associated with anaphylaxis were not included in the model. It is of note that Tegaderm CHG dressing is not indicated for use in patients aged under 2 months and our study only included adult patients.
There are other published analyses studies of CHG impregnated devices including CHG dressings, but to date none of them included Tegaderm CHG gel dressing. The analysis reported by Veenstra et al. (1999) compared the number of CRBSIs associated with CHG impregnated and standard intravascular catheters and suggested that antiseptic impregnated catheters are cost-saving. Crawford et al. (2004) performed a trial based evaluation and concluded that CHG sponge dressings would reduce costs, local infections and CRBSIs, and associated mortality. Similarly, Hockenhull et al. (2008) developed a decision analytic model for patients requiring CVCs in the UK and suggested that antimicrobial CVCs are cost-saving. More recently, Ye et al. (2011) suggested that a CHG dressing is also a cost-effective CRBSI prevention treatment option. Furthermore, Schwebel et al. (2012) performed a trial based evaluation comparing both 3- and 7-day CHG dressing with standard dressings and concluded that CHG dressings are associated with financial savings. Our findings for Tegaderm CHG dressing are, therefore, in line with those reported in previous studies for antimicrobial devices used to prevent catheter-related infections.
The analysis reported in this paper has some limitations. Any modelling process involves simplifications and assumptions that may not accurately reflect local clinical practice. Owing to the lack of detail of cost estimates in research studies included in the analysis, scenarios were developed and their costs were independently reviewed by members of the research team. The uncertainties about the assumptions made in the estimation of these costs (especially CRBSI costs) were tested using scenario analysis and the conclusion that Tegaderm CHG dressing is cost-saving remains valid in these analyses. Threshold analysis performed suggested that the findings are robust and that large fluctuations in the CRBSI rates and cost parameters are needed before Tegaderm CHG dressing becomes cost incurring.
The results of the current analysis have important implications for the healthcare systems facing rising demand from emergency admissions into ICUs. The evidence suggests that the use of Tegaderm CHG dressing may substantially reduce the incidence of CRBSI and in doing so reduce the length of stay on an adult ICU and in subsequent clinical areas. This would also reduce the need for the use of antibiotic therapy and the risk of emergence of antimicrobial resistant microorganisms. The introduction of Tegaderm CHG dressings may also alleviate some pressure on acute beds and have associated consequent cost-savings. In concurrence with these findings, NICE has recently issued guidance suggesting that Tegaderm CHG dressing is cost-saving for the NHS in England and Wales (NICE, 2015).
Acknowledgments
The authors thank Ruth Wong for conducting the searches for the systematic review of Tegaderm CHG dressing studies. The authors also thank Steph Haslam and Vanessa Wright for administrative support.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PT, EP and MStJ are employees of ScHARR who received an unrestricted grant from 3M concerning aspects of this publication. MA and SF are full time employees of 3M United Kingdom PLC, a supplier of vascular access care products to the NHS. JA is a former employee of ScHARR who received an unrestricted grant from 3M concerning aspects of this publication. TE has received unrestricted research grants from 3M and Carefusion companies. TE has also served as a speaker in symposia and has participated in scientific boards for 3M and Carefusion companies in the past 5 years. TW has accepted an invitation to speak at a symposium on behalf of Astellas in 2015.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statement: Not commissioned; blind peer-reviewed.
References
- Bion J, Richardson A, Hibbert P, Beer J, Abrusci T, McCutcheon M, Cassidy J, Eddleston J, Gunning K, Bellingan G, Patten M, Harrison D; Matching Michigan Collaboration & Writing Committee. (2013) ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Quality & Safety 22: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey AL, Mermel LA, Nightingale P, Elliott TS. (2008) Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet Infectious Diseases 8: 763–776. [DOI] [PubMed] [Google Scholar]
- Chopra V, Krein SL, Olmsted RN, Safdar N, Sanjay S. (2013) Prevention of Central Line-Associated Bloodstream Infections: Brief Update Review in Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. AHRQ Publication No. 13-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Crawford AG, Fuhr JP, Jr, Rao B. (2004) Cost-benefit analysis of chlorhexidine gluconate dressing in the prevention of catheter-related bloodstream infections. Infection Control and Hospital Epidemiology 25: 668–674. [DOI] [PubMed] [Google Scholar]
- Curtis L. (2013) Unit Costs of Health & Social Care 2013. Canterbury: Personal Social Services Research Unit. [Google Scholar]
- Curtis RL. (2009) Catheter-related bloodstream infection in the intensive care unit. Journal of the Intensive Care Society 10: 102–108. [Google Scholar]
- Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, Dudeck MA, Pollock DA, Horan TC. (2009) National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. American Journal of Infection Control 37: 783–805. [DOI] [PubMed] [Google Scholar]
- FDA. (2014) Manufacturer and User Facility Device (MAUDE), Manufacturer: 3m Brand Name: tegaderm chg Report Date From: 07/01/2000 Report Date To: 07/29/2013. 2014. Ref Type: Online Source. Available at: http://www.fda.gov/.
- Health Protection Agency. (2011) English National Point Prevalence Survey on Healthcare-associated Infections and Antimicrobial Use, 2011. London: Health Protection Agency. [Google Scholar]
- Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, Gamble C, McLeod C, Walley T, Dickson R. (2008) The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technology Assessment 12: iii–xii, 1–154. [DOI] [PubMed] [Google Scholar]
- Jenks M, Craig J, Green W, Hewitt N, Arber M, Sims A. (2016) Tegaderm CHG IV securement dressing for central venous and arterial catheter insertion sites: A NICE medical technology guidance. Applied Health Economics and Health Policy 14: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, Browne J, Prieto J, Wilcox M. and UK Department of Health. (2014) epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. Journal of Hospital Infection 86 (Suppl. 1): S1–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday HP, Wilson JA, Prieto J, Wilcox M. (2016) epic3: revised recommendation for intravenous catheter and catheter site care. Journal of Hospital Infection 92: 346–348. [DOI] [PubMed] [Google Scholar]
- MHRA. (2012) Medical Device Alert, MDA/2012/075 All medicinal products containing chlorhexidine. Issued: 25 October 2012. Available at: https://www.gov.uk/drug-device-alerts/medical-device-alert-all-medical-devices-and-medicinal-products-containing-chlorhexidine-risk-of-anaphylactic-reaction-due-to-chlorhexidine-allergy (accessed 17 February 2016).
- NICE. (2011) Medical Technologies Evaluation Programme Methods guide. London: NICE; Available at: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-medical-technologies/Medical-technologies-evaluation-programme-methods-guide.pdf. [PubMed] [Google Scholar]
- NICE. (2012) Clinical Guideline 139, Infection: prevention and control of healthcare-associated infections in primary and community care. London: NICE; Available at: https://www.nice.org.uk/guidance/cg139. [PubMed] [Google Scholar]
- NICE. (2015) The 3M Tegaderm CHG IV securement dressing for central venous and arterial catheter insertion sites. NICE medical technology guidance [MTG25] London: NICE; Available at: https://www.nice.org.uk/guidance/mtg25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S. (2011) Guidelines for the prevention of intravascular catheter-related infections. Clinical Infectious Diseases 52: e162–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton LB, Ross V, Cuddy P, Kremer H, Fessler T, McGurk E. (1996) No difference in catheter sepsis between standard and antiseptic central venous catheters. A prospective randomized trial. Archives of Surgery 131: 986–989. [DOI] [PubMed] [Google Scholar]
- Petrou S, Gray A. (2011) Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ 342: d1766. [DOI] [PubMed] [Google Scholar]
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. New England Journal of Medicine 355: 2725–2732. [DOI] [PubMed] [Google Scholar]
- Renaud B, Brun-Buisson C. (2001) Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. American Journal of Respiratory and Critical Care Medicine 163: 1584–1590. [DOI] [PubMed] [Google Scholar]
- Saint S, Veenstra DL, Lipsky BA. (2000) The clinical and economic consequences of nosocomial central venous catheter-related infection: are antimicrobial catheters useful? Infection Control and Hospital Epidemiology 21: 375–380. [DOI] [PubMed] [Google Scholar]
- Schwebel C, Lucet JC, Vesin A, Arrault X, Calvino-Gunther S, Bouadma L, Timsit JF. (2012) Economic evaluation of chlorhexidine-impregnated sponges for preventing catheter-related infections in critically ill adults in the Dressing Study. Critical Care Medicine 40: 11–17. [DOI] [PubMed] [Google Scholar]
- Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. (1999) Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infection Control and Hospital Epidemiology 20: 396–401. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Wise M, Bell M, Cardo D, Edwards J, Fridkin S, Jernigan J, Kallen A, McDonald LC, Patel PR, Pollock D; Centers for Disease Control and Prevention (CDC). (2011) Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morbidity and Mortality Weekly Report 60: 243–248. [PubMed] [Google Scholar]
- Suetens C, Morales I, Savey A, Palomar M, Hiesmayr M, Lepape A, Gastmeier P, Schmit JC, Valinteliene R, Fabry J. (2007) European surveillance of ICU-acquired infections (HELICS-ICU): methods and main results. Journal of Hospital Infection 65 (Suppl. 2): 171–173. [DOI] [PubMed] [Google Scholar]
- Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S, Plantefeve G, Bronchard R, Troche G, Gauzit R, Antona M, Canet E, Bohe J, Lepape A, Vesin A, Arrault X, Schwebel C, Adrie C, Zahar JR, Ruckly S, Tournegros C, Lucet JC. (2012) Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. American Journal of Respiratory and Critical Care Medicine 186: 1272–1278. [DOI] [PubMed] [Google Scholar]
- Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, Pease S, Herault MC, Haouache H, Calvino-Gunther S, Gestin B, Armand-Lefevre L, Leflon V, Chaplain C, Benali A, Francais A, Adrie C, Zahar JR, Thuong M, Arrault X, Croize J, Lucet JC. (2009) Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. Journal of the American Medical Association 301: 1231–1241. [DOI] [PubMed] [Google Scholar]
- Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. (1999) Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. Journal of the American Medical Association 281: 261–267. [DOI] [PubMed] [Google Scholar]
- Vokurka S, Kabatova-Maxova K, Skardova J, Bystricka E. (2009) Antimicrobial chlorhexidine/silver sulfadiazine-coated central venous catheters versus those uncoated in patients undergoing allogeneic stem cell transplantation. Supportive Care in Cancer 17: 145–151. [DOI] [PubMed] [Google Scholar]
- Ye X, Rupnow M, Bastide P, Lafuma A, Ovington L, Jarvis WR. (2011) Economic impact of use of chlorhexidine-impregnated sponge dressing for prevention of central line-associated infections in the United States. American Journal of Infection Control 39: 647–654. [DOI] [PubMed] [Google Scholar]
- Zingg W, Walder B, Pittet D. (2011) Prevention of catheter-related infection: Toward zero risk? Current Opinion in Infectious Diseases 24: 377–384. [DOI] [PubMed] [Google Scholar]


