Abstract
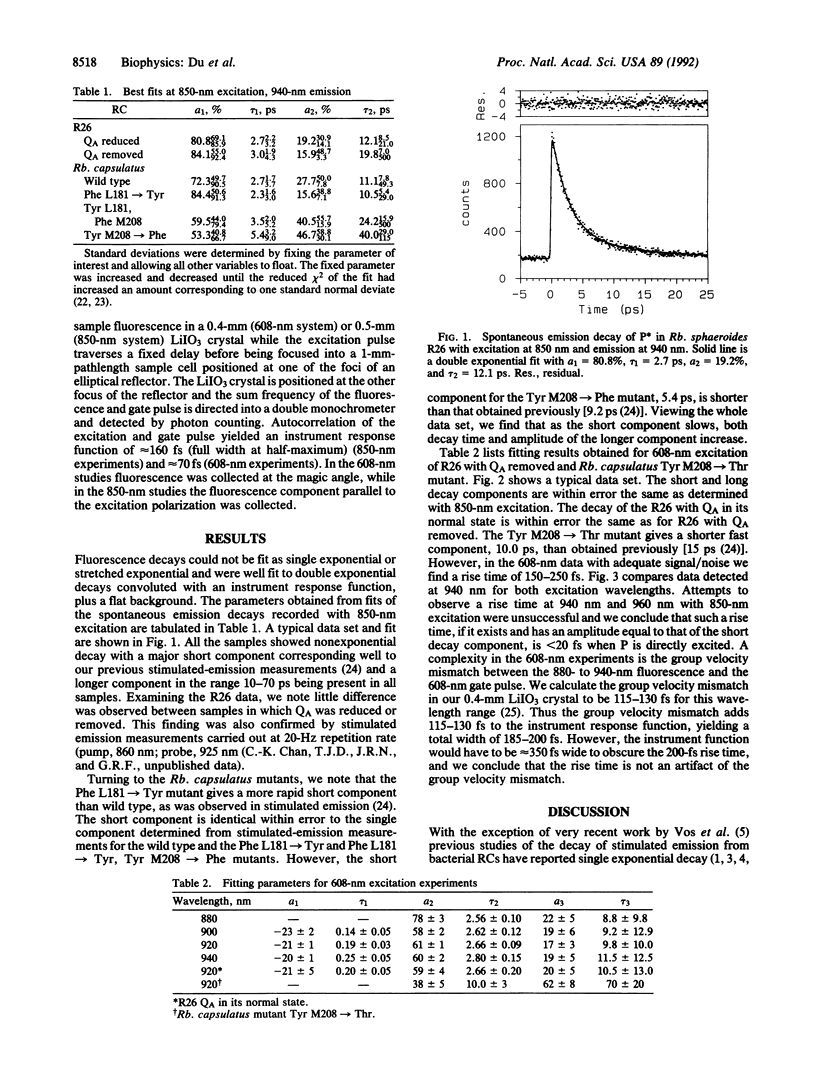
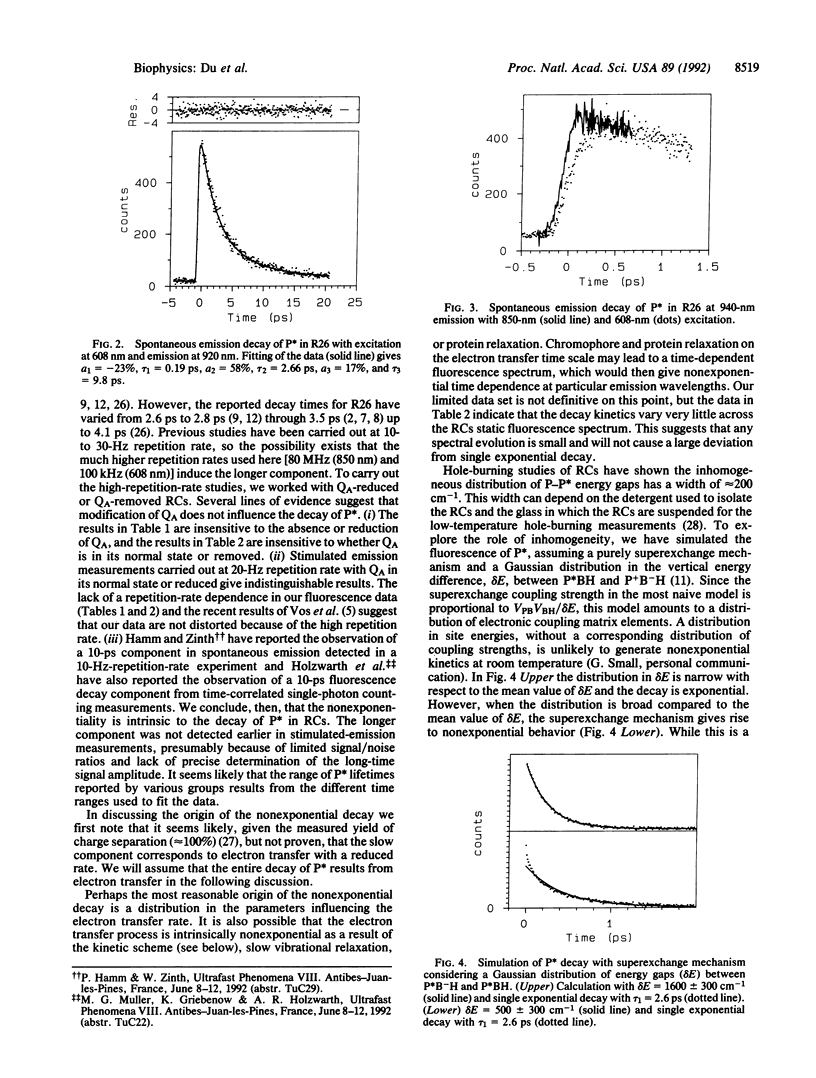
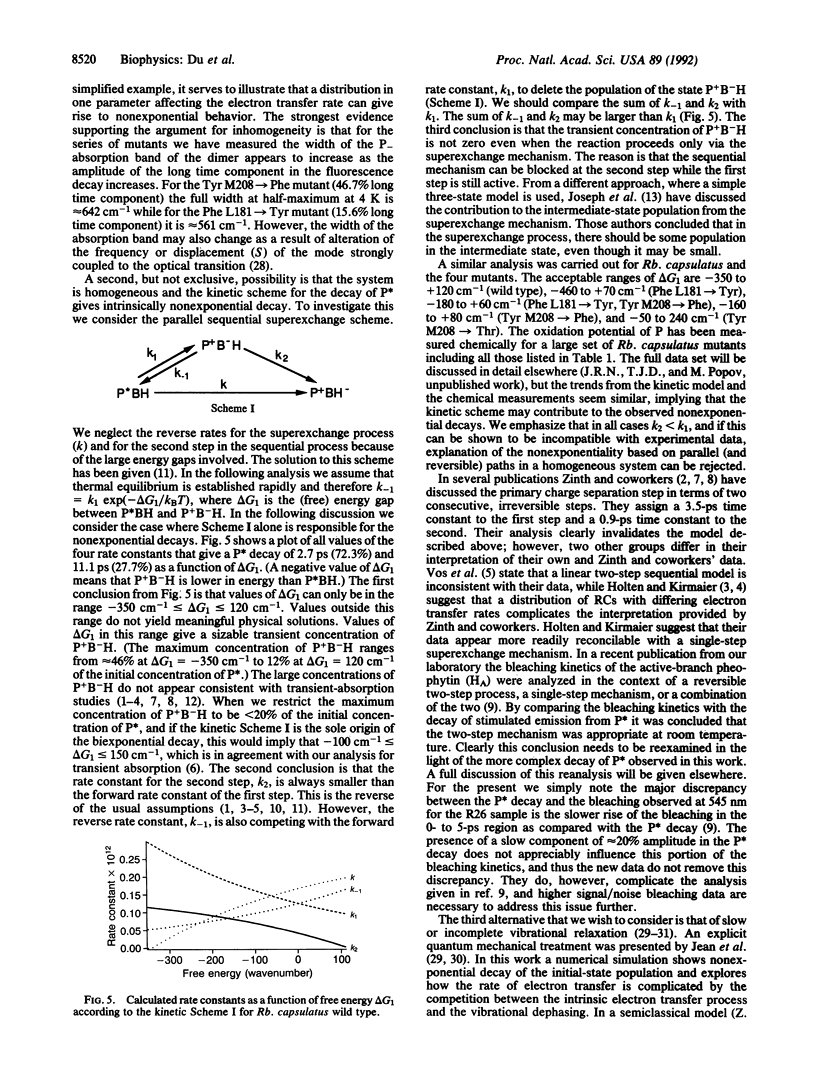
Spontaneous emission from reaction centers of photosynthetic bacteria has been recorded with a time resolution of 50 fs. Excitation was made directly into both the special-pair band (850 nm) and the Qx band of bacteriochlorophylls (608 nm). Rhodobacter sphaeroides R26, Rhodobacter capsulatus wild type, and four mutants of Rb. capsulatus were studied. In all cases the fluorescence decay was not single exponential and was well fit as a sum of two exponential decay components. The short components are in excellent agreement with the single component detected by measurements of stimulated emission. The origin of the nonexponential decay is discussed in terms of heterogeneity, the kinetic scheme, and the possibility of slow vibrational relaxation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan C. K., DiMagno T. J., Chen L. X., Norris J. R., Fleming G. R. Mechanism of the initial charge separation in bacterial photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11202–11206. doi: 10.1073/pnas.88.24.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. G., Pollard K. M., Webb J. Antibodies to histones in systemic lupus erythematosus: prevalence, specificity, and relationship to clinical and laboratory features. Ann Rheum Dis. 1992 Jan;51(1):61–66. doi: 10.1136/ard.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel W., Finkele U., Kaiser W., Oesterhelt D., Scheer H., Stilz H. U., Zinth W. Initial electron-transfer in the reaction center from Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5168–5172. doi: 10.1073/pnas.87.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmaier C., Holten D. An assessment of the mechanism of initial electron transfer in bacterial reaction centers. Biochemistry. 1991 Jan 22;30(3):609–613. doi: 10.1021/bi00217a003. [DOI] [PubMed] [Google Scholar]
- Kirmaier C., Holten D. Evidence that a distribution of bacterial reaction centers underlies the temperature and detection-wavelength dependence of the rates of the primary electron-transfer reactions. Proc Natl Acad Sci U S A. 1990 May;87(9):3552–3556. doi: 10.1073/pnas.87.9.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D., Okamura M. Y., Feher G. Charge recombination kinetics as a probe of protonation of the primary acceptor in photosynthetic reaction centers. Biophys J. 1985 Nov;48(5):849–852. doi: 10.1016/S0006-3495(85)83844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp E. W., Fischer S. F., Zinth W., Sander M., Kaiser W., Deisenhofer J., Michel H. Analysis of optical spectra from single crystals of Rhodopseudomonas viridis reaction centers. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8463–8467. doi: 10.1073/pnas.82.24.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Vos M. H., Lambry J. C., Robles S. J., Youvan D. C., Breton J., Martin J. L. Direct observation of vibrational coherence in bacterial reaction centers using femtosecond absorption spectroscopy. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8885–8889. doi: 10.1073/pnas.88.20.8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M. H., Lambry J. C., Robles S. J., Youvan D. C., Breton J., Martin J. L. Femtosecond spectral evolution of the excited state of bacterial reaction centers at 10 K. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):613–617. doi: 10.1073/pnas.89.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Parson W. W., Gunner M. R., Prince R. C., Dutton P. L. Radical-pair energetics and decay mechanisms in reaction centers containing anthraquinones, naphthoquinones or benzoquinones in place of ubiquinone. Biochim Biophys Acta. 1986 Aug 13;851(1):6–22. doi: 10.1016/0005-2728(86)90243-4. [DOI] [PubMed] [Google Scholar]
- Wraight C. A. Electron acceptors of bacterial photosynthetic reaction centers. II. H+ binding coupled to secondary electron transfer in the quinone acceptor complex. Biochim Biophys Acta. 1979 Nov 8;548(2):309–327. doi: 10.1016/0005-2728(79)90138-5. [DOI] [PubMed] [Google Scholar]


