Abstract
BACKGROUND AND PURPOSE
The Albumin in Acute Stroke (ALIAS) Part 1 and 2 Trials evaluated whether 25% human serum albumin improves clinical outcomes after acute ischemic stroke above and beyond standard of care using similar protocols. The Part 1 Trial ended prematurely due to safety concerns, and the Part 2 Trial terminated early due to futility of finding a statistically significant effect of ALB over saline (control) administration. We combine the subject-level data of the Part 1 and 2 Trials to re-evaluate the efficacy and safety outcomes with the larger sample size.
METHODS
The combined data analyses closely follow those conducted in the Part 2 Trial. The primary outcome is the composite of the modified Rankin Scale and the NIH Stroke Scale defined as a composite of mRS 0-1 and/or NIHSS 0-1 at 90 days from randomization. The unadjusted analyses use a simple Chi-square test and those adjusting for baseline covariates use a generalized linear model with log link (to obtain relative risks).
RESULTS
The participant characteristics at baseline were generally similar between the treatment groups and between Trials; however, thrombolysis use was greater in Part 2 (84% vs 75%), and the upper age limit imposed in Part 2 resulted in a younger sample (mean age in Part 1 was 69 vs 64 in Part 2). In the combined sample the proportions of good outcome in the two treatment groups were identical (41%). Similar results were observed in all secondary efficacy outcomes. Pulmonary edema was a consistent safety concern, with a six-fold increase in the ALB arm (13%) compared to Saline (2%) (RR=7.76, 95% CI 3.87-15.57).
CONCLUSIONS
Treatment with intravenous albumin 25% at 2 g/kg was not associated with improved outcome at 90 days and was associated with increased rates of intracerebral hemorrhage and pulmonary edema.
Keywords: acute stroke, randomized trial, albumin
INTRODUCTION
In preclinical studies 1-3, high dose human albumin has been shown to be highly neuroprotective by reducing infarct volume and cerebral edema and improving behavioral function. To address whether this concept of neuroprotection could be conveyed in humans, two large, multicenter, randomized, placebo-controlled Phase III trials were initiated to ascertain whether 25% human serum albumin (ALB) confers benefit (relative to saline (SAL)) on 90-day neurological and/or functional outcome for patients with acute ischemic stroke treated within 5 hours of symptom onset.
The Albumin in Acute Stroke (ALIAS) Part 1 Trial enrolled its first subject in July 2006. In December 2007, the Data and Safety Monitoring Board (DSMB) established by the sponsor, the National Institute of Neurological Disorders and Stroke (NINDS), recommended that the Trial be suspended, after a total of 434 subjects were enrolled at 62 sites, due to concerns about the safety of ALB4-6. The study was subsequently redesigned based on an extensive unblinded safety analysis7, and the ALIAS Part 2 Trial began enrolling in February 2009 as an independent study. In September 2012, the pre-specified futility boundary was crossed during interim analysis of 732 subjects enrolled at 84 sites, and the study was terminated. Details of the design of ALIAS Part 1 and the redesign of ALIAS Part 2 have been published previously5-7.
The ALIAS investigators have previously reported the primary results of Part 2 of the ALIAS Trial8-10. Briefly, there was insufficient evidence to conclude that ALB increased the proportion of subjects with a favorable outcome (defined as a composite of the modified Rankin Scale (mRS) score 0-1 and/or National Institutes of Health Stroke Scale (NIHSS) Score 0-1 at 90 days) as compared with saline alone (adjusted relative risk 0.96, 95% confidence interval (CI) 0.84 to 1.10). This result was consistent across all predefined secondary outcomes, as well as the analysis of those treated with thrombolysis (adjusted relative risk 0.95, 99% CI 0.79 to 1.15) and those not treated with thrombolysis (adjusted relative risk 1.09, 99% CI 0.53 to 2.22).
The analysis of outcomes using subject-level data from the Part 1 and Part 2 Trials reported herein was a pre-specified secondary analysis of the Part 2 Trial.
METHODS
STUDY DESIGN
Both Part 1 and Part 2 were designed as saline-controlled, blinded multicenter trials to ascertain the effectiveness of ALB in acute ischemic stroke. The eligibility criteria were similar in the two trials and detailed in the respective publications4-6. Eligible patients were randomly assigned in a 1:1 ratio to either ALB or saline, via an algorithm of minimization and biased-coin accounting for clinical center in a step-forward randomization process11. The primary efficacy outcome of functional independence was defined as a composite of mRS 0-1 and/or NIHSS 0-1, both assessed at 90 days from randomization.
Because of the clinical potential for interaction between ALB and thrombolysis, the ALIAS Part 1 Trial was designed to test the efficacy of ALB independently in each of two cohorts (those who received thrombolysis and those who did not) and was effectively two parallel trials in a single design. For the Part 1 trial, the maximum sample size of 900 subjects in each of the two cohorts was calculated to detect an absolute treatment difference of 10% in the proportion of subjects with favorable outcome at 90 days, assuming a proportion of 40% in the saline arm, two-sided Type I error probability of 0.05, 80% power, 1:1 randomization, and an inflation factor of 1.11 to account for approximately 5% potential lost to follow up. These calculations took into account three planned interim efficacy analyses conducted according to the O'Brien and Fleming type alpha spending function for efficacy and beta spending function for futility, intended to be conducted when 225, 450, and 675 subjects had completed the primary 90 day follow-up period. The planned primary efficacy analysis was a Z-test for binomial proportions with normal approximation. Since each cohort would be treated as a separate independent study group, the two-sided alpha level of 0.05 was specified for each primary analysis. An unfavorable outcome was imputed for all subjects with the primary outcome missing or obtained outside of the specified time window.
For the ALIAS Part 2 trial, several key statistical assumptions differed from Part 1. ALIAS Part 2 was a single trial and considered thrombolysis treatment status (defined by administration of IV tPA, endovascular procedure, or both) as a stratifying variable for randomization. An interaction term between thrombolysis treatment status and study treatment was a pre-specified covariate in the analysis model. The primary statistical model for Part 2 also included adjustments for stroke severity via the NIHSS. The Type I and Type II error probabilities were revised to be 0.025 (one-sided) and 0.10, respectively. This resulted in an intended sample size of 1,100 subjects for an absolute treatment difference of 10% in the proportion of subjects with favorable outcome at 90 days, assuming a proportion of 40% in the saline arm, 1:1 randomization, and an inflation factor of 1.11 to account for approximately 5% lost to follow up. The effect size was justified based upon the review of the part 1 study when new part 2 criteria were applied6-7. These calculations took into account three planned interim efficacy analyses conducted according to the O'Brien and Fleming type alpha spending function for efficacy and beta spending function for futility, intended to be conducted when 275, 550, and 825 subjects had completed the primary 90 day follow-up period. The planned primary efficacy analysis was to first test for interaction between study treatment and thrombolysis. However, because of insufficient numbers of non-thrombolysis subjects and a resulting decreased power to test the interaction effect, this interaction test was dropped from the primary efficacy analysis. An unfavorable outcome was imputed for all subjects with the primary outcome missing or obtained outside of the specified window.
The Data Coordination Unit at the Medical University of South Carolina was the Statistics and Data Management Center (SDMC) for both ALIAS Trials. The case report forms (CRFs) remained consistent between the two Trials.
The data from the two Trials were concatenated to create the ALIAS Trial combined data set with a sample size of 1,275 (637 in the ALB group and 638 in the SAL group). Analyses on the primary, secondary and safety outcomes were conducted in the same manner as those for the Part 2 data. The effect size estimates were adjusted for the pre-specified covariates: baseline NIHSS (continuous), thrombolysis treatment status (defined by administration of IV tPA, endovascular procedure, or both), and the interaction between thrombolysis treatment status and study treatment. For the unadjusted analyses comparing baseline characteristics, a simple chi-square test or independent t-test were used as appropriate. For the adjusted analyses of the primary and secondary efficacy and safety outcomes, generalized linear models with the log link were applied in order to interpret the treatment effect estimate as a relative risk (or relative benefit). The safety outcome analyses used the safety population, defined a priori as all subjects who received at least 20% of the calculated dose of study drug.
RESULTS
Baseline characteristics were similar between the treatment groups within each Trial. Subjects who were randomized into Part 2 were about 5 years younger than in Part 1. Most notably, Part 2 had more subjects who received thrombolysis (84% vs 75%), which was associated with a slightly longer time from tPA initiation to study drug infusion.
Pre-specified primary and secondary efficacy outcomes showed a consistent lack of treatment effect between the two groups, with the relative risk (RR) ranging from 0.87 to 1.07 for all outcomes. There were differences in the absolute rates of good outcome in Part 1 and Part 2, likely due to the change in inclusion and exclusion criteria (eg. age limits) that were implemented in the Part 2 trial. The temporal trends in the trial evolution (Figure 1) show that early on in the Part 1 trial with only 25% of the subjects enrolled, the ALB group fared poorly compared to the saline group and in the Part 2 trial, the ALB group fared much better compared to the saline group. In both trials, the favorable outcome rates had converged at the time the trials were halted. In hindsight, this initial planned interim analysis with only 25% of subjects enrolled may have been too early to make an informed decision due to the fluctuating cumulative outcome at that point in the trial.
Figure 1.
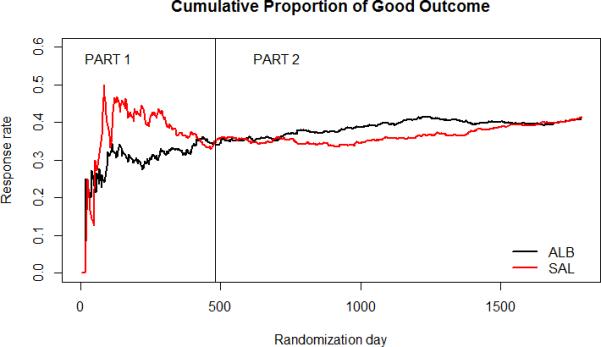
Cumulative Efficacy: Parts 1 and 2 Combined
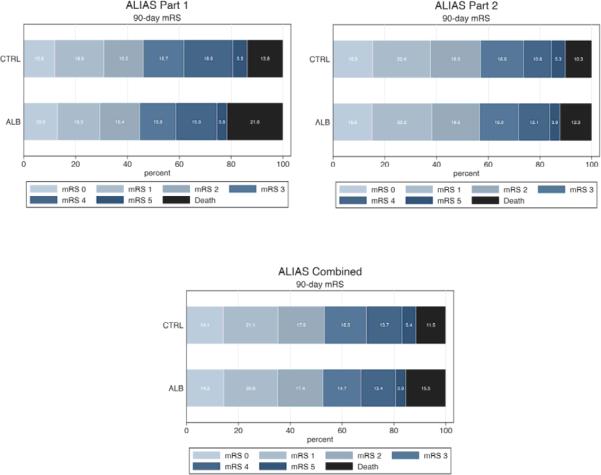
Figure 2 presents the distribution of the full scale mRS scores by treatment for each part separately and combined. No significant differences were observed between the treatment groups (Van Elteren test p-value=0.5016). In general, the combined analysis confirmed that, in the ischemic stroke populations enrolled, intravenous ALB at 2g/kg provided no added benefit over routine standard of care plus saline control.
Figure 2.
Distribution of 90-day MRS Scores by Treatment
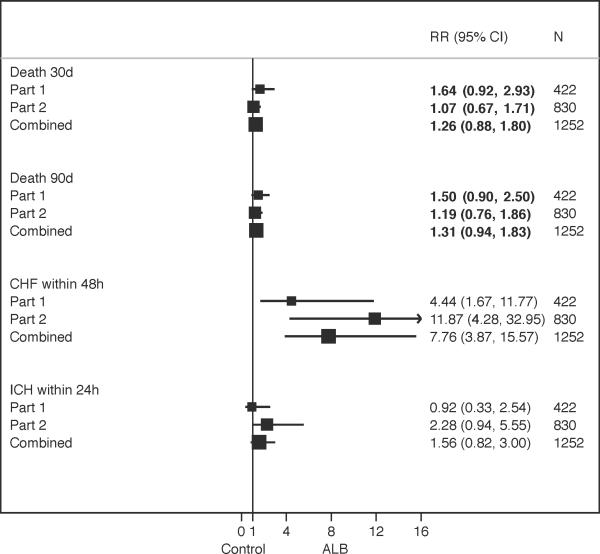
Figure 3 presents the safety outcomes for Part 1 and Part 2 and the combined cohort using the safety sample (i.e., those that received at least 20% of their weight-based dose). The RRs for pulmonary edema/congestive heart failure (PE/CHF) were significant for both Parts and for the combined analysis, despite more vigilant monitoring of the fluids and diuretic treatment in Part 2. Recurrent stroke occurred more frequently in the ALB group, with a non-significant RR of 1.99 and a very wide 95% CI. Although not statistically significant, there was an increased risk of symptomatic intracerebral hemorrhage with ALB in Part 2. As demonstrated in Figure 3, the RRs for deaths within 30 days and 90 days of randomization were reduced in Part 2 and were not significantly different in the combined analysis.
Figure 3.
Impact of Treatment on Safety Events by Trial Part
DISCUSSION
This pre-planned pooled analysis of the ALIAS part 1 and 2 trials confirms the lack of benefit and shows greater adverse events with 25% albumin treatment. In addition, we show interesting temporal effects in the effect size over the course of the two trials.
Some of the described differences between the two trials were expected. An upper age limit was implemented in Part 2 which resulted in a higher average age for Part 1. The increased thrombolysis use in Part 2 reflects the evolution of the field of thrombolytics during the course of the study. PE/CHF was an expected adverse event associated with Albumin treatment and thus it was not surprising that the RRs were significant for both Part 1 and Part 2 and combined analyses. However, in Part 2 fluid management and diuretic treatment we demonstrated that PE/CHF was readily remedied through careful fluid management and diuretic treatment. There were very few cases where PE/CHF was a SAE resulting in, for example, intubation or an admission to ICU, and none were fatal. The modifications made to the protocol for Part 2 intended to address the mortality effect observed in Part 1, resulted in similar death rates between the two treatment arms, and the combined sample analysis reflects the reduced RRs of deaths.
The ALIAS Trials are the latest in a string of clinical trials of putative neuroprotection that have failed to demonstrate clinical efficacy, despite very strong pre-clinical evidence. The Phase I dose-finding study showed promising effects of ALB compared to the data from the controls in the NINDS rt-PA Stroke Study12. Over the last several decades there has been a decline in stroke case-fatality and gradual efforts to systematically improve stroke care. This has included the use of thrombolysis, as well as improved stroke unit care, early supported discharge and rehabilitation therapies. The use of non-concurrent control comparison likely led to an exaggerated treatment effect because of secular and gradual improvements in stroke care; the historical controls had poorer outcomes. The small number of subjects in the pilot study, from only two clinical sites, enabled close monitoring of the study implementation and protocol adherence, which allowed for better signal to noise ratio.
In contrast, in both the ALIAS Parts 1 and 2 Trials, over 114 sites enrolled 1,275 subjects. This order of magnitude increase in study size and number of sites likely introduced greater variability in the execution of the study protocol, which may have contributed to the dilution of the treatment effect. Nevertheless, from a practical perspective, the objective was to show that ALB would be effective across a wide variety of hospitals and clinical settings. The consistent analysis results on all efficacy outcomes suggest the clear lack of benefit of ALB treatment above and beyond the standard of care for acute ischemic stroke patients.
In the combined analysis, the 41.4 % good outcome rate in the control group was on target with our design assumption. In Part 2, the Saline group showed a temporal trend in the cumulative good outcome rate, from about 30% after approximately 200 subjects enrolled and steadily increased to about 60% by the end of the Trial with 419 subjects. In contrast, the control group in the Interventional Management of Stroke (IMS) III Trial13 that was implemented concurrently with ALIAS Parts 1 and 2 lacked this trend; the rates of good outcome in both the treatment and control arms became steady after about 150 subjects’ data were obtained. This stable outcome rate seen in each group in IMS III is what is generally anticipated from clinical trials.
In the case of the ALIAS Part 2 Trial, this unanticipated trend in the Saline group response remains unexplained. Changes in stroke care should have affected both groups. During the trial there was a rising use of both intravenous thrombolysis and endovascular stroke treatment, and although ALB was shown to increase the risk of symptomatic intracerebral hemorrhage in combination with thrombolysis (intravenous and/or endovascular), the absolute risk increase was too small to account for the difference between treatment groups. Because of this temporal trend, the study came close in the early phase to crossing an overwhelming efficacy boundary. The ALIAS Trial experience highlights the importance of monitoring temporal trends in effect size.
Finally, the pre-clinical data supporting neuroprotection remains strong and the apparent paradox of failed translation to humans remains a extreme challenge. With the evolution of endovascular treatment for stroke since the ALIAS trials were completed, there is now an opportunity to revisit adjuvant and neuroprotective therapies. The vast majority of pre-clinical models of so-called neuroprotective agents have shown efficacy in models of ischemiareperfusion. Yet, in the human case, on average, early reperfusion was achieved much less than 50% of the time, and commonly it has not been measured. This has now changed with the evolution of endovascular treatments, and we look forward to new studies which will re-examine the neuroprotection hypothesis in an era of proven early reperfusion.
Acknowledgments
FUNDING SOURCES: Supported by cooperative agreements from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NINDS grants U01NS040406 (University of Miami); U01NS054630 (Medical University of South Carolina); and U01NS056975 (University of Michigan))
Footnotes
CLINICALTRIALS.gov REGISTRATION: NCT00235495 (https://clinicaltrials.gov/ct2/show/NCT00235495?term=alias&rank=2)
DISCLOSURE STATEMENT:
Dr. Renee Martin is currently serving on a Biogen sponsored Phase II clinical trial DSMB. Dr. Yuko Palesch is currently serving on a Brainsgate, Ltd. Clinical trial DSMB, has participated as a consultant for Remedy, Inc. and has served on a Biogen sponsored Phase I clinical trial DSMB. Dr. Sharon Yeatts receives consultant fees from Genentech for her role on the PRISMS Trial Steering Committee. Dr. Hill reports an ownership interest (common shares) in Calgary Scientific Inc and Quikflo Health Inc. He is serving as a paid consultant outcomes adjudicator for clinical trials committee for Merck Inc.
REFERENCES
- 1.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: Marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg M, Belayev L, Bazan N, Marcheselli V, Hill M, Palesch Y, et al. Albumin-based neurotherapeutics for acute ischemic stroke: From bench to bedside. In: J K, S K, editors. Pharmacology of cerebral ischemia. Medpharm Scientific Publishers; Stuttgart: 2004. pp. 421–433. [Google Scholar]
- 3.Belayev L, Zhao W, Pattany PM, Weaver RG, Huh PW, Lin B, et al. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. doi: 10.1161/01.str.29.12.2587. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg MD, Palesch YY, Hill MD. The ALIAS (ALbumin In Acute Stroke) Phase III randomized multicentre clinical trial: design and progress report. Biochem Soc Trans. 2006;34:1323–6. doi: 10.1042/BST0341323. [DOI] [PubMed] [Google Scholar]
- 5.Hill MD, Moy CS, Palesch YY, Martin R, Dillon CR, Waldman BD, et al. for the ALIAS Investigators The albumin in acute stroke trial (ALIAS); design and methodology. Int J Stroke. 2007;2:214–9. doi: 10.1111/j.1747-4949.2007.00143.x. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg MD, Palesch YY, Martin RH, Hill MD, Moy CS, Waldman BD, et al. for the ALIAS Investigators The albumin in acute stroke (ALIAS) multicenter clinical trial: safety analysis of part 1 and rationale and design of part 2. Stroke. 2011;42:119–27. doi: 10.1161/STROKEAHA.110.596072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill MD, Martin RH, Palesch YY, Tamariz D, Waldman B, Ryckborst KJ, et al. on behalf of the ALIAS Investigators and the Neurological Emergencies Treatment Trials (NETT) Network The Albumin in Acute Stroke Part 1 Trial: an exploratory efficacy analysis. Stroke. 2011;42:1621–1625. doi: 10.1161/STROKEAHA.110.610980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg MD, Palesch YY, Hill MD, Martin RL, Moy CS, Barsan WG, et al. for the ALIAS and NETT Investigators High-dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12:1049–58. doi: 10.1016/S1474-4422(13)70223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg MD, Hill MD. Symptomatic intracranial hemorrhage in the ALIAS Multicenter Trial: relationship to endovascular thrombolytic therapy. International Journal of Stroke. 2015;10:494–500. doi: 10.1111/ijs.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill MD, Martin R, Palesch YY, Moy C, Tamariz D, Ryckborst R, et al. Congestive heart failure, intracerebral hemorrhage and albumin safety analysis of the ALIAS Part 2 multicenter trial. PLOS One. 2015;10:1371. doi: 10.1371/journal.pone.0131390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao W, Ciolino J, Palesch Y. Step-forward randomization in multi-site emergency treatment clinical trials. Acad Emerg Med. 2010;17:659–665. doi: 10.1111/j.1553-2712.2010.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD. The ALIAS Pilot Trial: A dose-escalation and safety study of albumin therapy for acute ischemic stroke –II: Neurological outcome and efficacy analysis. Stroke. 2006;37:2107–2114. doi: 10.1161/01.STR.0000231389.34701.b5. [DOI] [PubMed] [Google Scholar]
- 13.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khari P, Hill MD, et al. for the Interventional Management of Stroke III Investigators Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]