Abstract
Background
Elevated lipoprotein(a) [Lp(a)] is a prevalent, independent cardiovascular risk factor but the underlying mechanisms responsible for its pathogenicity are poorly defined. Since Lp(a) is the prominent carrier of pro-inflammatory oxidized phospholipids (OxPL), part of its atherothrombosis might be mediated through this pathway.
Methods
In vivo imaging techniques MR imaging, 18F-FDG-PET/CT and SPECT/CT were used to measure subsequently atherosclerotic burden, arterial wall inflammation and monocyte trafficking to the arterial wall. Ex vivo analysis of monocytes was performed using FACS analysis, inflammatory stimulation assays and transendothelial migration assays. In vitro studies to the pathophysiology of Lp(a) on monocytes were performed using an in vitro model for trained immunity.
Results
We show that subjects with elevated Lp(a) (108 [50–195] mg/dL; n=30) have increased arterial inflammation and enhanced PBMCs trafficking to the arterial wall, compared with subjects with normal Lp(a) (7 [2–28] mg/dL; n=30). In addition, monocytes isolated from subjects with elevated Lp(a) remain in a long-lasting primed state, as evidenced by an increased capacity to transmigrate and produce pro-inflammatory cytokines upon stimulation (n=15). In vitro studies show that Lp(a) contains OxPL and augments the pro-inflammatory response in monocytes derived from healthy controls (n=6). This effect was markedly attenuated by inactivating OxPL on Lp(a) or removing OxPL on apo(a).
Conclusions
These findings demonstrate that Lp(a) induces monocyte trafficking to the arterial wall and mediates pro-inflammatory responses through its OxPL content. These findings provide a novel mechanism by which Lp(a) mediates cardiovascular disease.
Clinical Trial Registration
URL: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5006 Unique Identifier: NTR5006 (VIPER study)
Keywords: lipoprotein (a), atherosclerosis, arterial wall inflammation, monocytes, oxidized phospholipids
Introduction
Lipoprotein(a) [Lp(a)] is a plasma lipoprotein composed of apolipoprotein(a) [apo(a)] covalently bound to apolipoprotein B-100 (apoB) of a low density lipoproteins (LDL)-like particle (Figure S1). A role for Lp(a) in atherogenesis has now been established by both meta-analyses of epidemiological studies, as well as by genome-wide association and Mendelian randomization studies showing a strong, independent and likely causal relationship between Lp(a) and myocardial infarction, stroke, peripheral artery disease and calcific aortic valve stenosis.1,2
Despite evidence of causality and the fact that one-fifth of the general community has elevated Lp(a) levels of >50 mg/dl3, Lp(a) is not routinely measured in clinical risk assessment. This reluctance appears to be due to a lack of understanding of the pathophysiological mechanisms by which Lp(a) mediates cardiovascular disease (CVD), as well as the lack of specific therapeutic interventions to potently lower Lp(a) levels4. Consistent with known mechanisms of atherogenicity of LDL, Lp(a)’s atherogenic capacity could in part reflect the response-to-retention phenomenon of an apoB-containing particle5. The disproportionately large impact of Lp(a) on CVD risk compared with LDL, however, implies that additional pathogenic pathways need to be considered6.
Over the last decade, a large body of experimental and clinical evidence has documented that Lp(a) is the main lipoprotein carrier of phosphocholine (PC) containing oxidized phospholipids (OxPL) in plasma7–9, which has led to the hypothesis that a major component of the risk mediated by Lp(a) is through its content of OxPL10. OxPL is recognized as a danger associated molecular pattern (DAMP) by pattern recognition receptors (PRRs) on innate immune cells, leading to a wide range of pro-inflammatory and plaque destabilizing processes11–13. In epidemiological studies, the level of OxPL on apoB-containing lipoproteins (OxPL-apoB), which predominantly reflects Lp(a) associated OxPL, was found to be highly predictive of future CVD risk14,15. Most recently, a further link between Lp(a), OxPL and inflammation was discovered, showing that the risk of Lp(a) and OxPL-apoB in mediating coronary atherogenesis and cardiovascular events was conditional on an inflammatory IL-1 haplotype16.
Here, we report that subjects with elevated plasma Lp(a) levels are characterized by increased inflammatory activity of the arterial wall as well as an enhanced inflammatory response of circulating monocytes, for which OxPL carried by Lp(a) were identified as obligatory intermediates. These findings indicate a novel link between Lp(a), its associated OxPL and accelerated atherogenesis in humans.
Methods
Study subjects
This was a single-center study in subjects with elevated Lp(a) and subjects with normal Lp(a), matched for age, gender and BMI. Exclusion criteria consisted of active smoking, history of clinically-manifest cardiovascular disease or diabetes, and lipid lowering therapies including statins. Each subject provided written informed consent. The study and radiation exposure (<10mSv in total) was approved by the local institutional review board and conducted according to the principles of the International Conference on Harmonization–Good Clinical Practice guidelines.
Biochemical Measurements
EDTA plasma obtained through venous blood samples were taken after overnight fasting and stored using standardized protocols. Plasma Lp(a), total cholesterol (TChol), high density lipoprotein cholesterol (HDL-C) and triglyceride (TG) levels were analyzed using commercially available enzymatic methods. Low density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald equation and subsequently corrected for Lp(a)-cholesterol (LDLc – [Lp(a) in mmol/L*0.3])17. Oxidized phospholipids on apolipoprotein B-100 (OxPL-apoB) and on apolipoprotein(a) (OxPL-apo(a)), were measured as follows: apoB-100 and apo(a) were captured on microtiter wells using appropriate antibodies and the content of OxPL quantified by monoclonal antibody E06 and reported as nM, as previously described10.
MR imaging
MR images were obtained with a 3.0 T whole-body scanner (Ingenia, Philips Medical Systems, Best, The Netherlands), using an 8 channel dedicated bilateral carotid artery coil (Shanghai Chenguang Medical Technologies, Shanghai, China). The normalized wall index (NWI= mean wall area/outer wall area) was calculated18 by one blinded experienced reader at the core laboratory using semi-automated measurement software (VesselMass, Leiden, the Netherlands).
PET/CT imaging
PET/CT scans were performed on a dedicated scanner (Philips, Best, the Netherlands)19. All subjects fasted for at least 6 hours prior to infusion of 18F-FDG (200 MBq). After 90 min, subjects underwent PET imaging initiated with a low-dose non-contrast enhanced CT for attenuation correction and anatomic co-registration. PET/CT images were analyzed by blinded readers using OsiriX (Geneva, Switzerland; http://www.osirix-viewer.com/). The target-to-background-ratio (TBR) was calculated from the ratio of arterial SUV and venous background activity, as described previously19.
SPECT/CT imaging
SPECT/CT scans were performed at 3, 4.5 and 6 hours post infusion of 99m Tc-labeled autologous peripheral blood mononuclear cells (PBMCs) (200 MBq) on a dedicated scanner (Symbia T16, Siemens, Erlangen, Germany)20. In each subject, around 20 106 PBMCs were isolated and labeled with 99mTc-(1100 megabecquerel/2mililiter). SPECT/CT images were analyzed by blinded readers using OsiriX20. Accumulation of labeled PBMCs in the arterial wall was quantified by the ratio of the averaged maximum counts in the artery divided by the averaged mean counts in the blood, reported as the arterial-wall-to-blood-ratio (ABR)20.
Flow cytometry
Blood samples were collected into K3EDTA BD Vacutainer1 (BD Biosciences, San Jose, CA) tubes, after which 50 μL of whole blood was added to each flow cytometry tube plus the appropriate amounts of each antigen specific mAb were added, defined after titration of the dose recommended by the manufactures. See supplementary for detailed information.
Cells
Human PBMCs were isolated from blood using Ficoll-Paque Premium (d=1.077g/ml) density gradient centrifugation (GE Healthcare, UK). For in vitro experiments, human PBMCs were isolated from blood of healthy volunteers (Sanquin Bloodbank, Nijmegen, The Netherlands). CD14pos monocytes were isolated using a Ficoll-Paque plus (Pharmacia Biotech, Uppsala, Sweden) density gradient, followed by magnetic activated cell sorting (MACS) using CD14-coated MicroBeads according to manufacturer’s instructions (MACS, Miltenyi Biotec, Leiden, The Netherlands). Primary Human Arterial Endothelial Cells (HAECs) were purchased from Lonza (Baltimore, MD) and routinely cultured on fibronectin (FN; 10 μg/ml) coated culture flasks (TPP, Switzerland) or glass-slides for imaging in EGM2 medium containing SingleQuots (Lonza). Endothelial cells were cultured up to 6 passages.
Antibodies and reagents
Actin (clone AC-40) mAb was purchased from Sigma-Aldrich (Zwijndrecht, Netherlands). Filamentous actin (F-actin) was stained with Phalloidin Texas-red (Invitrogen), and the nucleus with Hoechst 33258. β-glucan (b-1,3-(D)-glucan) was kindly provided by Professor David Williams (College of Medicine, Johnson City, USA), r-apo(a) (8K-IV), r-apo(a) 17K and 17KΔLBS10 were derived as previously described9. Stimuli and inhibitors used are oxPAPC (Invivogen), LPS (Sigma-Aldrich, St. Louis, MO; from E. coli serotype 055:B5, further purified as described)21, Pam3Cys (EMC microcollections, Tübingen, Germany; L2000), anti-OxPL antibody Mouse monoclonal EO6 (Avanti Polar Lipids, Inc, Alabaster, Alabama, USA, 330001), purified mouse IgM Isotype control antibody (Biolegend, San Diego, California, USA, 401602).
In the monocyte assays, Lp(a), LDL and HDL were isolated from 3.5 mL plasma from subjects with either high or normal Lp(a) levels by KBr-density gradient ultracentrifugation; plasma density was adjusted to d=1.25 g/mL with solid KBr (0.385 g/mL plasma), a discontinuous gradient was formed by carefully layering 2 mL of d=1.225 g/mL KBr solution, followed by 4 mL of d=1.100 g/mL KBr solution, and a top layer of 3 mL of d=1.006 g/mL KBr solution was added. The samples were centrifuged for 20 hours at 10°C at 29,000 rpm in a SW 41 Ti rotor (Beckman Coulter Inc., CA). The LDL, Lp(a) and HDL fractions were sliced out and dialyzed against PBS. LAL assay showed negligible endotoxin levels (<0.05 EU/mL) in the isolated fractions. For the western immunoblots, Lp(a) was purified from the lipid apheresis eluate of a single donor undergoing LDL apheresis with high Lp(a) levels (140 mg/dL) and 16 KIV and 18 KIV repeats. The apheresis eluate was subjected to ultracentrifugation in NaBr and the 1.08 > d > 1.06 g/mL fraction was collected. This fraction was applied to a SW-400 gel filtration column, which was eluted in 0.5mL fractions. Each fraction was assayed for the presence of apo(a) and apoA-I by ELISA. The fractions containing apo(a) but not apoAI were pooled, buffer exchanged into PBS and concentrated using Amicon centrifugal filter units (Millipore). Apo(a) was dissociated from Lp(a) based on the methods described previously22,23. Briefly, the solution containing Lp(a) was adjusted to a final concentration of 100 mM ACA and 2 mM dithiothrietol and incubated at room temperature for 1 h. The reaction mixture was adjusted to a density of 1.3 g/ml with NaBr and the solution was centrifuged at 50,000 rpm to separate apo(a) from the LDL released by reduction and intact Lp(a).
Western immunoblot
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of apo(a), Lp(a), and LDL (100 ng protein), in reducing (with beta-mercaptoethanol (BME)) and non-reducing conditions, was carried out using 3–8% gradient polyacrylamide gels in Tris-acetate SDS running buffer. Proteins were transferred to a PVDF membrane, which was blocked with 3% BSA in PBS-Tween. Membranes were then incubated with either biotinylated LPA4 (0.004 mg/ml), biotinylated E06 (0.004 mg/ml), or MB47 (0.006 mg/ml) probing for apo(a), OxPL, and human apoB-100 respectively. Protein detection was performed with streptavidin conjugated to horseradish peroxidase (HRP) from (R and D Systems) and visualized with ECL (Amersham).
Detection of OxPL by LC-MS/MS
To document the presence of PC-containing OxPL in Lp(a) purified by ultracentifugation, Lp(a) was isolated from 3 donors. Details of the LC-MS/MS procedures is described in the Supplement.
Transendothelial migration assay
Human aortic endothelial cells (HAECs) were cultured on a fibronectin (FN)-coated glass cover to confluency and stimulated overnight, i.e. 12 hours with TNFα (10 ng/ml)24. Monocytes (1*106 cells/ml) were added to the HAECs monolayer for 30 min in a humidified atmosphere of 5% CO2 at 37 °C and fixed with 3.7% formaldehyde (Sigma-Aldrich, Zwijndrecht, the Netherlands). After fixation, multiple images were recorded with a Zeiss Axiovert 200 microscope (Plan-apochromat 10x/0.45 M27 Zeiss-objective; Carl Zeiss Inc., Jena, Germany), and analyzed using Image-J software (http://rsb.info.nih.gov/nih-image/). After treatment and fixation in 3.7% formaldehylde in PBS (+1 mM CaCl2, 0.5 mM MgCl2), cells were permeabilized in PBS-T (PBS + 0.1% Triton-X100) for 10 min, stained for nuclei and filamentous actin for 45 min, washed and stored in PBS (+1 mM CaCl2, 0.5 mM MgCl2) until imaged using a confocal laser-scanning microscope (LSM510 META, Carl Zeiss MicroImaging, Jena, Germany) with a Zeiss Plan-Apochromat, 63x /1.40 oil objective. Monocyte motility was determined by plating freshly isolated monocytes into fibronectin (Sanquin Reagents, Netherlands) coated Lab-Tek Chambers (Thermo-Scientific, Rochester, NY) containing N-2-Hydroxyethylpiperazine-N'-2-Ethanesulfonic Acid (HEPES)-buffer + 1mM CaCl2 + 0.5% v/v human albumin and 0.1% w/v glucose. Videos were acquired using a wide-field microscope (Axio Observer Z1, Carl Zeiss MicroImaging, Jena, Germany) equipped with a humidified atmosphere climate chamber with 5 % CO2 and 37 °C, and analysed using the Tracking Tool (Gradientech, Uppsala Science Park, Uppsala, Sweden).
In vitro monocyte priming and challenge assay
Monocytes were isolated from PBMCs by adhering 5·106 PBMCs/ml to polystyrene for 1h at 37°C, 5% CO2 in 96-, or 6-well culture plates (Corning, New York, USA). Non-adherent cells were removed by washing three times with warm PBS. Monocytes were cultured in RPMI 1640 Dutch-modified culture medium (Life Technologies/Invitrogen, Breda, The Netherlands) supplemented with 10 mM glutamine (Invitrogen), 10 μg/mL gentamicin (Centraform), 10 mM pyruvate (Invitrogen) and 10% pooled human plasma. The monocytes were primed for 24 hours with either RPMI, β-glucan (5 μg/ml), isolated Lp(a) (0.5–250 μg/ml), LDL (10 μg/ml), HDL (10 μg/ml ), r-apo(a) constructs (8K-IV 0.001–0.5 μg/ml; 17K and 17KΔLBS10 0.1 μg/ml), OxPAPC (0.05–10 μg/ml) or 10% plasma from subjects with high or normal Lp(a) levels (before and after apoB precipitation of the apoB-containing lipoprotein fraction with polyethylene glycol 8000, Sigma P-213925), after which the cells were washed and incubated in supplemented culture medium for 6 days. In inhibition experiments using E06 (1 nM) or IgM control (1 nM), stimuli were pre-incubated with the inhibitors 1h prior to adding the stimuli to the cells. After 6 days of rest in supplemented RPMI medium, cells were exposed to either medium alone, 10 μg/mL Pam3Cys or 10 ng/ml lipopolysaccharide. After 24h of incubation, supernatants were collected and stored at −20°C. Production of cytokines was measured in supernatants by ELISA according to the manufacturer’s instructions (TNFα (R&D Systems, Minneapolis, USA) and IL-6 (Sanquin, Amsterdam, The Netherlands), or the human inflammatory cytokine Cytometric Bead Array according to the manufacturer’s instructions (BD Biosciences, 551811).
Statistical Analysis
Data are presented as the mean (standard deviation) or the median (min-max) for continuous variables, and as a number (percentage) for categorical variables. To examine the difference in clinical characteristics, imaging parameters and ex vivo monocyte phenotype and function between subjects with elevated or normal Lp(a) we performed student T-test or Mann-Whitney U test for normal and non-normal distributed variables, respectively. Normality was examined by means of inspection of histograms and the Shapiro-Wilk test. Variances in both groups were examined and tested for similarity using Levene's Test for Variance. To assess the differences in PBMCs accumulation as imaged with SPECT, a linear mixed model for repeated measurements was applied (fig 1D–F). In vitro monocyte experiments were performed at least 6 times, and analyzed using the Wilcoxon signed-rank test. A two-sided P-value below 0.05 was considered statistically significant. All data were analyzed using Prism version 5.0 (GraphPad software, La Jolla, California) or SPSS version 21.0 (SPSS Inc., Chicago, Illinois).
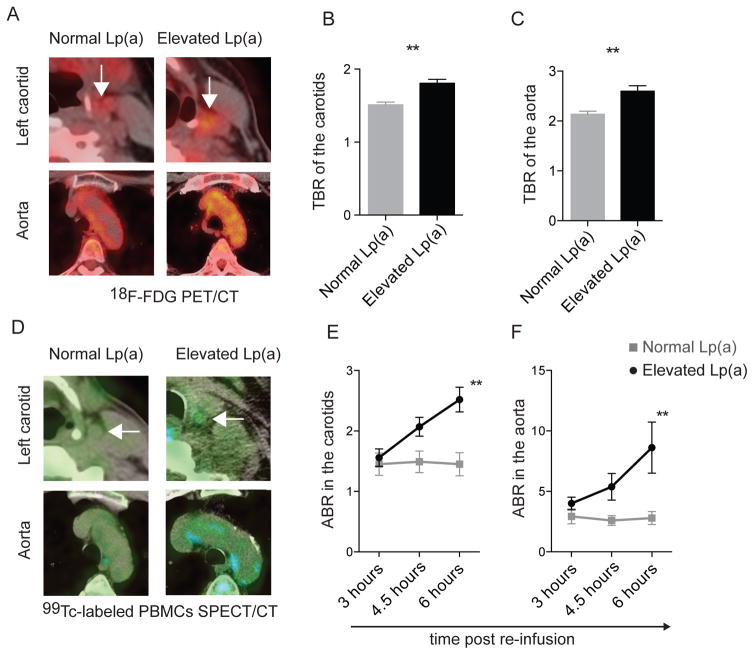
Figure 1. Increased arterial wall inflammation in subjects with elevated Lp(a).
(A) Cross-sectional 18F-FDG PET/CT images demonstrating an increased 18F-FDG uptake (yellow) in the left carotid (top, indicated by white arrow) and aorta (bottom) in a subject with normal Lp(a) (left) and a subject with elevated Lp(a) (right), quantified as the maximum target to background ratio (TBR) in the (B) carotid arteries, and (C) ascending aorta in subjects with elevated Lp(a) (n=30) and normal Lp(a) (n=30), (D) cross-sectional SPECT/CT images demonstrating increased autologous 99mTc-labeled PBMCs accumulation (blue; at T=6 hours post-infusion), depicted as the arterial wall to blood pool ratio (ABR) at the level of (E) the carotids and (F) ascending aorta in subjects with elevated Lp(a) (n=15) and normal Lp(a) (n=15). **=p<0.01.
Results
Increased arterial wall inflammation in subjects with elevated Lp(a)
To determine if Lp(a) influences arterial wall inflammation, we included subjects with elevated Lp(a) levels (mean of 108 mg/dL [range 50–195]; n=30) or normal Lp(a) levels (mean of 7 mg/dL [range 2–28]; n=30) matched for age, gender and body mass index (Table 1). None of the subjects were currently smoking, had a history of clinically-manifest cardiovascular disease or diabetes, or used lipid-lowering therapies such as statins. Aside from Lp(a) levels, subjects did not differ in blood pressure, non-Lp(a) lipoprotein profile or circulating leukocytes (Table 1). As expected, subjects with elevated Lp(a) exhibited significantly higher levels of OxPL-apoB and specifically, OxPL on apo(a) lipoproteins (OxPL-apo(a)) (Table 1). The normalized wall index (NWI) of the carotids, as assessed with magnetic resonance imaging (MRI)18, was not significantly different between groups (Table 1, Figure S2A).
Table 1.
Clinical characteristics of included subjects
| Characteristic | Subjects with normal Lp(a) (n=30) | Subjects with elevated Lp(a) (n=30) | P value |
|---|---|---|---|
| Age, y | 53 ± 12 | 52 ± 11 | 0.858 |
| Gender, %male (n) | 45 (9) | 43 (15) | 0.820 |
| BMI, kg/m2 | 24 ± 4 | 24 ± 3 | 0.914 |
| DBP, mmHg | 79 ± 7 | 81 ± 8 | 0.399 |
| SBP, mmHg | 131 ± 8 | 134 ± 12 | 0.144 |
| Smoking, %active | 0 (0) | 0 (0) | - |
| NWI | 0.42 ± 0.06 | 0.39 ± 0.04 | 0.161 |
| Lp(a), mg/dL | 7 [2–28] | 108 [50–195] | <0.001 |
| OxPL-apo(a), nM | 3.0 [0.5–26.0] | 69.1 [40.9–92.5] | <0.001 |
| OxPL-apoB, nM | 4.1 [2.3–6.3] | 15.8 [5.9–31.1] | <0.001 |
| TChol, mmol/L | 5.21 ± 0.83 | 5.79 ± 1.44 | 0.127 |
| LDLc*, mmol/L | 2.91 ± 0.80 | 2.80 ± 1.16 | 0.621 |
| HDLc, mmol/L | 1.68 ± 0.42 | 1.60 ± 0.40 | 0.481 |
| TG, mmol/L | 0.80 [0.24–2.18] | 0.82 [0.39–2.16] | 0.684 |
| CRP, mg/L | 2.30 [0.40–4.40] | 1.13 [0.30–1.90] | 0.180 |
| HbA1c, mmol/mol | 36 [34–39] | 35 [33–38] | 0.100 |
| Creatinine, umol/L | 76 [66–80] | 70 [55–87] | 0.304 |
| ALT, U/L | 27 ± 10 | 27 ± 6 | 0.766 |
| AST, U/L | 25 ± 11 | 27 ± 8 | 0.427 |
| WBC, 109/L | 5.4 ± 1.3 | 5.7 ± 1.3 | 0.522 |
| Monocytes, 109/L | 0.43 ± 0.15 | 0.43 ± 0.12 | 0.996 |
| Neutrophils, 109/L | 3.1 ± 1.4 | 3.3 ± 1.09 | 0.710 |
| Lymphocytes, 109/L | 2.4 ± 2.6 | 2.2 ± 1.7 | 0.666 |
| Eosinophils, 109/L | 0.16 ± 0.13 | 0.15 ± 0.16 | 0.891 |
| Basophils, 109/L | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.809 |
Data are presented as mean ± SD, n (%) or median [min-max]. ALT indicates alanine transaminase; apo(a), apolipoprotein(a); apoB, apolipoprotein B-100; AST, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein, CVD, cardiovascular disease (including myocardial infarction, stroke or peripheral artery disease); HbA1c, hemoglobin A1c; HDLc, high density lipoprotein cholesterol; DBP, diastolic blood pressure; LDLc, low density lipoprotein cholesterol; Lp(a), lipoprotein(a); NWI, normalized wall index; OxPL, oxidized phospholipids; SBP, systolic blood pressure; Tchol, total cholesterol; TG, triglycerides; WBC, white blood cell count, *LDLc corrected for Lp(a)-cholesterol.
Positron emission tomography with computed tomography (PET/CT) imaging was performed to image arterial wall 18F-fluorodeoxyglucose uptake (18F-FDG), as a marker of arterial wall inflammation26. In subjects with elevated Lp(a), arterial wall 18F-FDG uptake, quantified as the target to background ratio (TBR), was significantly higher compared with subjects with normal Lp(a) (Figure 1A); both in the carotids (Figure 1B) as well as in the aorta (Figure 1C). Of note, the inflammatory activity in subjects with elevated Lp(a) did not correlate to arterial wall dimensions (NWI, normalized wall index) as assessed with MRI (Figure S2B).
In view of the intricate relation between resident arterial wall macrophages and circulating monocytes27, we subsequently investigated the impact of elevated Lp(a) on circulating immune cells in vivo. We used an imaging approach to monitor the trafficking of technetium-99m (99mTc)-labeled autologous PBMCs to the arterial wall, using single photon emission computed tomography with computed tomography (SPECT/CT)20. PBMCs were found to accumulate at a substantially higher rate in the arterial wall within 3–6 hours after re-infusion in subjects with elevated Lp(a) compared with those with normal Lp(a) (Figure 1D). The arterial wall-to-blood-pool ratio (ABR)20 values were significantly higher in subjects with elevated Lp(a) in both the carotids (Figure 1E) and aorta (Figure 1F). Collectively, these in vivo imaging studies indicate that subjects with elevated Lp(a) levels have increased arterial inflammatory activity, which coincides with more autologous immune cell accumulation in their arterial wall. Further, there is a strong correlation between the TBR indices from PET/CT imaging and the ABR values from PMBCs imaging and respective Lp(a) plasma levels (Table 2).
Table 2.
Correlations between inflammatory metrics and Lp(a) levels
| Method | Parameter | Spearman correlation with Lp(a) level |
|---|---|---|
| PET imaging | TBR, carotid | 0.31^ |
| TBR, aorta | 0.45** | |
| SPECT imaging | ABR, carotid | 0.72* |
| ABR, aorta | 0.65* | |
| Whole plasma level | CRP, mg/L | 0.24 |
| Flow cytometric assay | CD11b, ΔMFI | 0.27 |
| CD11c, ΔMFI | 0.42^ | |
| CD29, ΔMFI | 0.50* | |
| SRA, ΔMFI | 0.42^ | |
| CD36, ΔMFI | 0.48* | |
| Ex vivo monocyte | TNFα, pg/mL | 0.27 |
| cytokine production | IL-6, pg/mL | 0.18 |
Correlation coefficients of the Spearman's rho test are shown, correlations are flagged with
p<0.06,
p<0.05,
p<0.01,
p<0.001.
TBR and ABR are imaging metrics, CRP was measured in whole plasma, TNFα and IL-6 were measured as products of monocytes after TLR ligand challenge, and CD11b, CD11c, CD29 and CD36 expression on monocytes were measured by flow cytometry as described in Methods.
Monocytes increasingly migrate in subjects with elevated Lp(a)
We next questioned whether the increased arterial wall immune cell accumulation was preceded by an intrinsic cellular activation leading to enhanced migration. To this end, we analyzed adhesion and migration markers of blood monocytes in subjects with elevated versus normal Lp(a) using flow cytometry (gating strategy in Figure S3A)28. Monocytes from subjects with elevated Lp(a) expressed more C-C chemokine receptor type 7 (CCR7), L-selectin (also described as the cluster of differentiation 62 ligand; CD62L) and integrin alpha and beta chains; CD11b, CD11c and CD2929 (Figure 2A), for which the latter two showed a (borderline) significant correlation with the Lp(a) plasma levels (Table 2).
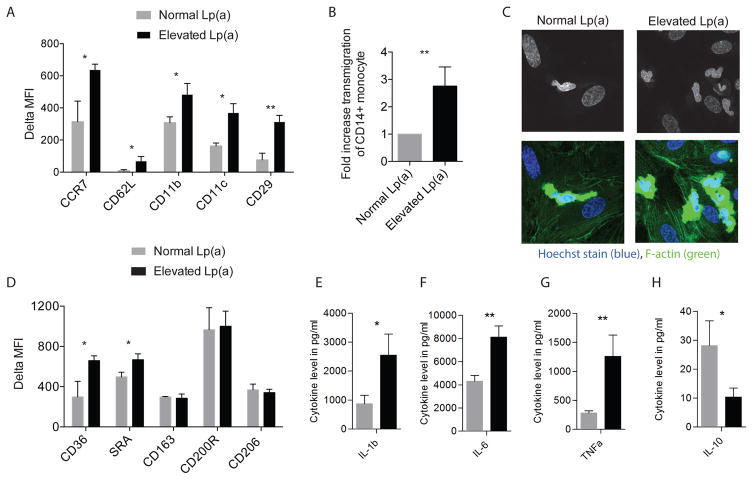
Figure 2. Monocytes have an activated and inflammatory phenotype in Lp(a) subjects.
(A) Bar graphs display the expression (quantified as delta MFI) of chemokine, adhesion and transmigration markers on monocytes as assessed with flow cytometry in subjects with elevated Lp(a) (n=15, black bars) compared with normal Lp(a) (n=15, grey bars), (B) bar graph and (C) microscope images demonstrating increased endothelial transmigration of monocytes (calculated per at least 4 fields of view) isolated from subjects with elevated Lp(a) (n=15, black bar) compared with normal Lp(a) (n=15, grey bar), coinciding with augmented spreading and adhesion as illustrated nucleus (Hoechst, blue) and F-actin (green) stain, (D) bar graphs showing expression (quantified as delta MFI) of scavenger and other receptors on monocytes in subjects with elevated Lp(a) (n=15, black bars) compared with subjects with normal Lp(a) (n=15, grey bars), (E-H) in response to an overnight challenge to Pam3Cys (10 μg/ml), monocytes isolated from subjects with elevated Lp(a) (n=15, black bars) produced higher levels of IL-1β (E), IL-6 (F) and TNFα (G) and lower levels of IL-10 (H), compared with monocytes of subjects with normal Lp(a) (n=15, grey bars). ^=p<0.06, *=p<0.05, **=p<0.01, ***=p<0.001.
To evaluate the functional relevance of increased expression of adhesion and migration markers, we performed transendothelial migration (TEM) experiments24 with monocytes isolated from subjects with and without elevated Lp(a). Twelve hours prior to performing the migration assay, human arterial endothelial cells (HAECs) were stimulated with TNFα to mimic dysfunctional endothelium. Freshly isolated monocytes of subjects with elevated Lp(a) showed a 3-fold higher TEM rate compared with monocytes isolated from subjects with normal Lp(a) (Figure 2B).
Staining of the nucleus and F-actin illustrated that monocytes derived from subjects with elevated Lp(a) have augmented spreading and adhesion compared with monocytes of subjects with normal Lp(a) (Figure 2C). In addition, monocytes of subjects with elevated Lp(a) also showed higher motility when plated on fibronectin-coated surfaces (movie S1), lending further support to an increased transmigratory tendency.
Thus, in subjects with elevated Lp(a), circulating monocytes exhibit an enhanced capacity to adhere and transmigrate the endothelium. Whereas generalized markers of inflammation, such as plasma levels of CRP, do not relate with Lp(a) levels, a number of inflammatory markers measured on the monocytes of the subjects are proportional to their Lp(a) levels (Table 2).
Atherogenic monocyte response in subjects with elevated Lp(a)
In addition to migration markers, monocytes of subjects with elevated Lp(a) expressed increased levels of the scavenger receptors CD36 and SRA (Figure 2D), also correlating to Lp(a) plasma levels (Table 2), whereas the expression of other receptors such as CD163, CD200R and CD206 was not different (Figure 2D). To assess whether this activated phenotype also translated to an enhanced inflammatory response, we performed a challenge assay using Toll-like receptor (TLR) ligands. After an overnight stimulation with a TLR2 ligand (Pam3Cys), monocytes isolated from subjects with elevated Lp(a) produced higher levels of tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β) and IL-6 (Figure 2E–G). Conversely, the production of IL-10 was lower (Figure 2H). Similar results were observed upon stimulation with the TLR4 ligand lipopolysaccharide (LPS) (Figure S4A–D). In aggregate, we observed an activated state of monocytes, potentially resulting from their priming by Lp(a), or some associated component found in the plasma of subject with elevated Lp(a).
Lp(a) and its associated OxPL induce prolonged responsiveness in monocytes
Lp(a) was isolated by ultracentrifugation, the lipid soluble material was extracted, and LC-MS/MS was performed on the extracted material an shown to have the following OxPLs: POVPC, PGPC, PONPC, PAzPC and KDdiA-PC, with PONPC being the most abundant (Figure S5A–B).
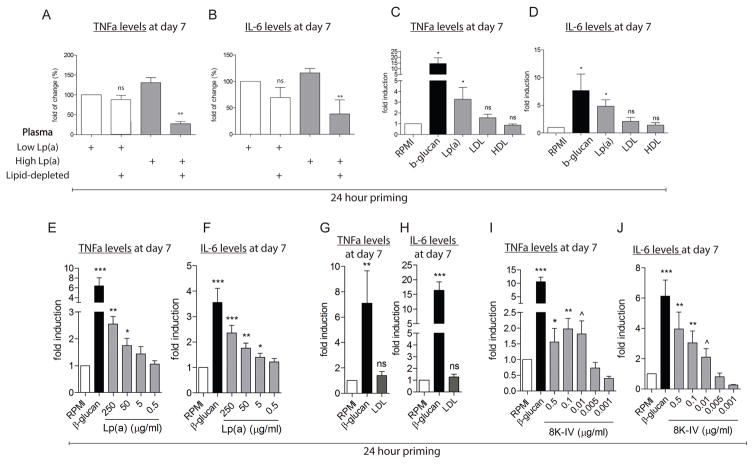
To address priming by Lp(a), or some associated component found in the plasma of subject with elevated Lp(a), we performed a series of in vitro studies, in which we analyzed the ability of various plasma components to activate monocytes. As a positive control for monocyte activation we used β-glucan, a cell wall component of C. Albicans, since we have extensively demonstrated that β-glucan induces a long-lasting pro-inflammatory phenotype in monocytes30. First, priming of healthy monocytes with plasma from subjects with high Lp(a) for 24 hours, with a subsequent washout and resting period, increased the production of TNFα and IL-6 after an overnight challenge with the TLR2 and TLR4 ligands Pam3Cys or LPS, respectively (Figure 3A,B and Figure S6A,B), whereas normal Lp(a) plasma did not activate monocytes. Lipid depletion of the above plasma profoundly decreased the cytokine production of the in-origin high Lp(a) plasma, indicating that a lipophilic particle might be responsible for the observed effect (Figure 3A,B and Figure S6A,B).
Figure 3. Isolated Lp(a) primes monocytes towards a responsive state.
(A,B) priming of healthy monocytes with plasma of a subject with high Lp(a) (1st grey bar) for 24h resulted in an increased production of TNFα and IL-6 upon re-stimulation with Pam3Cys (10 μg/mL) on day 6, compared with plasma of normal Lp(a) (1st white bar). In addition, after lipid-depletion (2nd white and grey bar) this effect of high Lp(a) plasma was profoundly reduced compared to the undepleted control (n=6), (C,D) priming of healthy monocytes with β-glucan (5 μg/mL, positive control, black bar) or Lp(a) (grey bar, 250 μg/mL) for 24h, induced an increased production of TNFα and IL-6 upon re-stimulation with Pam3Cys (10 μg/mL) on day 6, compared with priming with RPMI (negative control, white bar). LDL (grey bar, 10 μg/mL) or HDL (grey bar, 10 μg/mL) did not induce this increase (n=6) (E,F) in addition, various concentrations of Lp(a) (grey bars) for 24h, resulted in a dose-dependent increased production of TNFα and IL-6 upon re-stimulation with Pam3Cys (10 μg/mL) on day 6 (n=6) compared to priming with RPMI (negative control, white bar) (G,H) priming of healthy monocytes with LDL (10 μg/mL) (grey bar) did not result in higher cytokine levels after Pam3Cys (10 μg/mL) compared to the negative control RPMI (white bar), in contrast to β-glucan (5 μg/mL, positive control, black bar) (n=6), whereas (I,J) priming of healthy monocytes with β-glucan (5 μg/mL, positive control, black bar) or the r-apo(a) construct 8K-IV (grey bars) induced increased cytokine levels after Pam3Cys (10 μg/mL) (n=6), compared with RPMI (negative control, white bar). *=p<0.05, **=p<0.01, ***=p<0.001.
In line with the difference in Lp(a) levels between both plasma, priming of healthy monocytes with purified Lp(a) for 24 hours, induced an increased production of TNFα and IL-6 at day 7, after an overnight TLR challenge (Figure 3C–F and Figure S6C–F). Please note that priming with the isolated lipoprotein fractions LDL and HDL did not induce monocyte activation (Figure 3C,D and Figure S6C,D).
Next, we assessed the effects of Lp(a)’s main components separately; using isolated LDL, apo(a) separated from Lp(a), and recombinant apo(a) [r-apo(a)] constructs containing various numbers of kringles9. Whereas priming with LDL had no prolonged effects on monocytes (Figure 3G,H and Figure S6G,H), priming with an 8 kringle IV (8K-IV) r-apo(a) construct elicited an enhanced cytokine response in healthy donor monocytes at day 7 after overnight stimulation with TLR ligands (Figure 3I,J and Figure S6I,J).
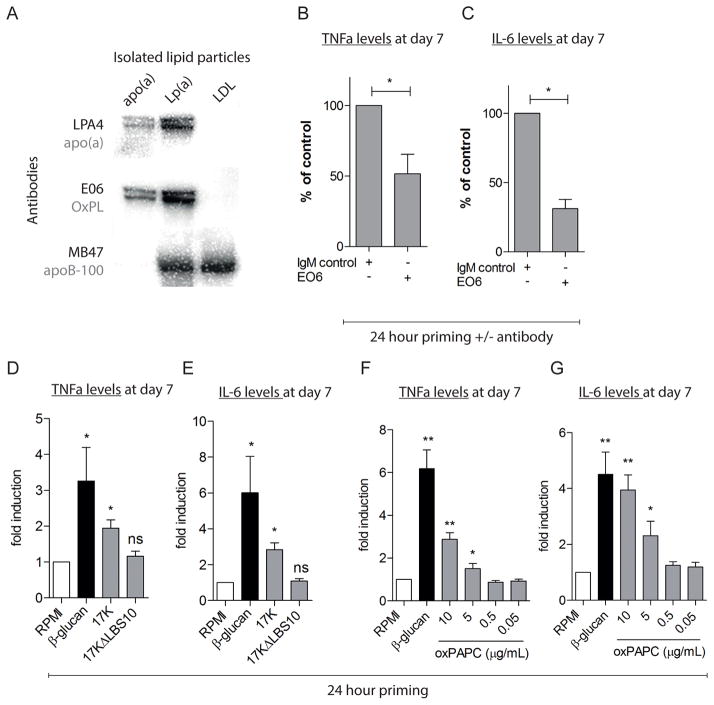
In view of the role of Lp(a) as carrier of OxPL7–9, we hypothesized an important role for OxPL in monocyte activation. In support of this concept, we show via immunoblotting that both Lp(a) and apo(a) contain OxPL, whereas LDL isolated from the same plasma as Lp(a) does not (Figure 4A). To further demonstrate the role of OxPL in mediating the activation of monocytes, we utilized monoclonal antibody E06, which binds to the PC moiety of OxPL and potently inhibits their pro-inflammatory properties, but does not bind to native, non-oxidized phospholipids31–33. Whereas the 8K-IV apo(a) construct, containing covalently bound OxPL9, potently activated monocytes (Figure 3I,J and Figure S6I,J), pre-incubation of the 8K-IV construct with E06 inhibited its priming effects (Figure 4B,C and Figure S6K,L).
Figure 4. OxPL induce the enhanced monocyte response.
(A) Immunoblotting of purified apo(a), Lp(a) and LDL; OxPL, apo(a), and apoB-100 resolved on SDS-PAGE in reducing and non-reducing conditions, were detected using the monoclonal antibodies E06, LPA4 and MB47 respectively. E06 immunostained apo(a) and Lp(a) but not LDL isolated from the same subject, (B,C) pre-treatment with the E06 antibody (1 nM) against OxPL inhibited the increased monocyte response after priming with 8K-IV (0.1 μg/mL, grey bars) and subsequent challenge with Pam3cys, shown as % of the initial priming with 8K-IV, corrected for IgM control antibody (n=6), (D,E) priming with β-glucan (5 μg/mL, positive control, black bar) and the r-apo(a) construct 17K (0.1 μg/mL, grey bar) induced increased cytokine production after Pam3Cys (10 μg/mL), whereas 17KΔLBS lacking OxPL (0.1 μg/mL, grey bar) did not (n=6), (F,G) priming with β-glucan (5 μg/mL positive control, black bar) and oxPAPC (grey bars) increased the monocyte responsiveness compared with RPMI (negative control, white bar) (n=6). ^=p<0.06, *=p<0.05, **=p<0.01, ***=p<0.001.
The role of OxPL was further validated using 2 additional constructs consisting of 17 kringles (17K) but varying in the functionality of the lysine binding site (LBS) on kringle IV type 10 (KIV10), which in turn is related to the ability of the apo(a) constructs to have covalently bound OxPL 9,34. Thus, 17K is a r-apo(a) construct with an intact LBS that has covalently bound OxPL, whereas 17KΔLBS10 has a mutated LBS and lacks immunologically detected OxPL9,34. Consistent with our previous findings with Lp(a), priming with 17K induced a state of increased responsiveness in monocytes, whereas such effects were absent with the mutant 17KΔLBS10 (Figure 4D,E and Figure S6M,N). Finally, we show that short-term priming with purified oxidized-1-palmitoyl-2-arachidonoyl-sn-3-glycero-phosphocholine (OxPAPC), a mixture of OxPL, also results in a dose-dependent enhanced cytokine production at day 7 in response to an overnight challenge with TLR ligands (Figure 4F,G and Figure S6O,P).
Collectively, these data show that subjects with elevated Lp(a) exhibit increased arterial wall inflammation coinciding with an increased responsiveness of monocytes, in which OxPL carried by Lp(a) are obligatory mediators.
Discussion
In this translational work, we provide evidence in support of the concept that pro-inflammatory OxPL are major contributors to the atherogenicity of Lp(a) by showing that (i) subjects with elevated Lp(a) have increased arterial inflammation in vivo, (ii) monocytes isolated from subjects with elevated Lp(a) remain in a long-lasting activated state ex vivo, and (iii) Lp(a) elicits the pro-inflammatory response in healthy monocytes in vitro, an effect markedly attenuated by removing or inactivating OxPL present on Lp(a) and specifically on apo(a).
We demonstrate an increased inflammatory activity in the arterial wall in subjects with elevated Lp(a), who present with enhanced 18F-FDG uptake in the arterial wall proportional to their Lp(a) levels. In analogy to the enhanced glucose consumption in inflamed tissue, arterial wall 18F-FDG uptake has been proposed as a marker of atherogenic inflammation, supported by previous studies documenting 18F-FDG uptake primarily in macrophage-rich areas in atherosclerotic plaques26. In addition, we report that this increased local inflammatory activity coincides with a higher accumulation rate of autologous PBMCs in the arterial wall as visualized by SPECT/CT imaging. Whereas we previously demonstrated augmented influx of PBMCs in advanced atherosclerotic plaques20, the present finding emphasizes that in subjects at increased risk for atherosclerotic disease due to Lp(a) elevation, PBMC accumulation in the arterial wall is significantly enhanced also at sites without plaques. The increased arterial inflammatory signal in our subjects with elevated Lp(a) cannot be attributed to differences in atherosclerotic burden for two reasons: first, the normalized wall index of the carotids on MRI did not differ from controls, and second, inflammatory measures of the arterial wall did not correlate with structural arterial wall metrics. The absence of a consistent relation between Lp(a) elevation and established surrogate markers of atherosclerotic burden observed in this as well as in previous studies35,36 implies that Lp(a) may increase CVD risk by enhancing plaque vulnerability rather than accelerating plaque burden.
To address whether intrinsic cellular activation precedes enhanced PBMC migration in Lp(a) subjects, we subsequently analyzed isolated circulating monocytes, which are key contributors to the technetium uptake by PBMCs20. Using ex vivo assays, we were able to recapitulate our in vivo findings. First, we found that monocytes isolated from subjects with elevated Lp(a) displayed an increased capacity to transmigrate the endothelium. Second, we observed that monocytes isolated from subjects with elevated Lp(a) have enhanced capacity to produce pro-inflammatory cytokines upon TLR stimulation. Previous experimental studies already showed that the apo(a) component of Lp(a) was able to also activate endothelial cells37, whereas apo(a) also co-localized with the adhesion molecule Mac-1 in the arterial wall38. When integrating these data, subjects with elevated Lp(a) might be burdened by a double hit comprising activated monocytes as well as endothelial cells, both predisposing for increased arterial wall inflammation.
In support of a causal role for Lp(a) in driving monocyte activation, a series of in vitro experiments substantiate that (i) high Lp(a) plasma induce monocyte activation in vitro, which is abolished after lipid-depletion, (ii) among the isolated lipoprotein fractions, only Lp(a) and apo(a) (particles both carrying OxPL) have the capacity to induce long-term monocyte activation. In humans, more than 85% of plasma lipoprotein-associated OxPL are present on Lp(a). On Lp(a), OxPL is present in the lipid phase (i.e. removable from Lp(a) by delipidation with organic solvents) as well as covalently attached to apo(a)8,9. Here we found five oxPL species on Lp(a), comprising POVPC, PGPC, PONPC, PAzPC and KDdiA-PC, with PONPC being the most abundant. Previously, PONPC was shown to be the most abundant OxPL in the debris of distal protection devices from coronary bypass graft, carotid and renal interventions.39 Recombinant 17K apo(a) containing covalently bound OxPL but not 17KΔLBS r-apo(a), which lacks E06 detectable OxPL9, reproduced Lp(a) priming of monocyte activation. The key epitope that E06 recognizes is the phosphocholine (PC) headgroup when presented in the proper conformation on OxPL. Sequencing the VH and VL chains of E06 revealed that these were identical to those regions of the T15 IgA natural antibody, which has been crystallized with its antigen, the PC moeity40. Further detailed lipidomic study of a large variety of lipids, oxidized lipids and oxidized-lipid adducts revealed that like T15, the IgM E06 only bound to oxidized phosphocholine containing phospholipids (e.g. PC - and only when the sn2 side chain was oxidized and or adducted to a protein), but not to native PAPC suggesting that oxidation products of the sn2 PUFA generated from oxidized PAPC appeared to generate the correct conformational epitope of the PC to allow recognition by E0632,40.
Here, we demonstrate that the proinflammatory effects of apo(a) can be blocked by E06. Further we show that a recombinant apo(a) that contains bound OxPL is capable of activating monocytes, whereas the nearly identical, but mutated recombinant apo(a) that has lost the ability to bind OxPL does not have monocyte activation properties, indicating the necessity for OxPL to mediate monocyte activation. In aggregate, these data indicate that the OxPL carried by Lp(a) are obligatory danger signals in eliciting the prolonged potentiation of the monocyte response in vitro.
These findings are also complementary to prior reports demonstrating that OxPL are danger associated molecular patterns (DAMPs). The PC headgroup on OxPL is recognized by multiple innate PRRs12; for example, both CD36 and SR-B1 bind the PC on OxPL31,41 and consequently, E06 blocked the uptake of OxLDL by these macrophage scavengers42. In addition, both acute and chronic lung injury in mice have been shown to lead to the generation of OxPL in the brochiolavage fluid, which in turn caused cytokine production by macrophages that was dependent on TLR4, and E06 was capable of blocking the pro-inflammatory properties of the OxPL43. Similarly, the OxPL content of apoptotic cells was shown to induce endothelial expression of IL-8, and more recently, the OxPL on apo(a) was shown to be necessary for apo(a) mediated upregulation of IL-8 by macrophages33,34. Hence, the mechanisms by which OxPL accelerates atherosclerosis have been attributed both to mediating uptake of OxLDL into macrophages to generate foam cells, as well as to a variety of pro-inflammatory and plaque destabilizing processes11–13. In addition, recent studies suggest that such DAMP/PAMPs induce long-term potentiation of their inflammatory response in vitro via epigenetic alterations30,44,45. Future studies will need to focus on elucidating how and for how long OxPL-Lp(a) induce adaptive responses of monocytes.
Whereas the present study cannot substantiate that OxPL also is a prerequisite for monocyte activation in humans in vivo, it is interesting to note that our subjects with elevated Lp(a) exhibited 4-fold higher OxPL-apoB levels (OxPL associated with all apoB lipoproteins) and even 20-fold higher OxPL-apo(a) levels (OxPL associated with Lp(a) lipoproteins) compared with subjects with normal Lp(a). This increased circulating OxPL content in subjects with elevated Lp(a) suggests that a comparable impact of OxPL may hold true in vivo. In support, we have previously shown that OxPL-apoB levels were potent predictors of progressive atherosclerosis as well as the risk of cardiovascular disease and death in patients14–16,46.
It remains to be established whether lowering of Lp(a) and OxPL will also lead to a reduction in arterial wall inflammation and eventually CVD risk. Recently, an antisense-based approach was reported that specifically lowered Lp(a) levels by ~80%, and to a similar degree their associated OxPL-apoB and OxPL-apo(a) levels47,48. With the development of such therapies to effectively lower Lp(a)49, we should be able to design appropriate clinical trials to aid in dissecting the (causal) relation between Lp(a) and cellular as well as arterial wall inflammation. In addition to Lp(a)-lowering therapies, the present findings may also provide novel targets to modulate the atherogenic impact of Lp(a). Because the OxPL content of Lp(a) appears to mediate the pro-inflammatory effect on monocytes, oxidation-specific epitope targeted therapy using specific antibodies may also bear clinical potential50. At present these approaches are being evaluated in experimental settings only51,52.
In summary, our findings strengthen the case for the inflammatory hypothesis of Lp(a) by addressing the increased inflammatory activity in the arterial wall, corresponding to an enhanced accumulation of activated immune cells, in which Lp(a)’s associated OxPL are obligatory intermediates.
Supplementary Material
Clinical Perspective.
What is new?
We provide evidence in support of the concept that pro-inflammatory oxidized phospholipids are major contributors to the atherogenicity of Lp(a) by showing that subjects with elevated Lp(a) have increased arterial wall inflammation in vivo, that monocytes from subjects with elevated Lp(a) remain in a long-lasting activated state ex vivo and that Lp(a) elicits a pro-inflammatory response in healthy monocytes in vitro, an effect markedly attenuated by removing or inactivating oxPL on Lp(A) and specifically on apo(a)
What are the clinical implications?
The present findings of oxPL induced pro-inflammatory effects of Lp(a) may provide novel targets to modulate the atherogenic impact of Lp(a). Because the oxPL content of Lp(a) appears to mediate the pro-inflammatory effect on monocytes, oxidation-specific epitope targeted therapy using specific antibodies or single chain antibodies may bear clinical potential. At present, these approaches are being evaluated in experimental settings.
Acknowledgments
We thank M.F. Lam and M.E. Hemayat for assistance in the nuclear imaging scans, and Andrea Edel for her help with the LC-MS/MS experiments.
Funding Sources: This work was partly supported by a European Framework Program 7 grant (ESS: FP7-Health 309820: Nano-Athero), a European Horizon2020 grant (ESS, NPR: H2020-PHC-2015-667873-2), and by the Dutch CardioVascular Research Initiative (CVON 2011/ B019 GENIUS). NPR is financially supported by a grant from the Netherlands Heart Foundation (2012T051). Drs Tsimikas and Witztum are supported by NIH R01 grants HL119828, P01-HL088093, P01 HL055798, R01-HL106579, R01-HL078610, and R01-HL124174.
Footnotes
Disclosures: ESS has received lecturing fees from Merck, Novartis, Ionis, Amgen - none of which are related to the contents of this manuscript. NPR has served on a scientific advisory board of AstraZeneca, unrelated to the context of this study. ST and JLW are co-inventors of and receive royalties from patents or patent applications owned by the University of California San Diego on oxidation-specific and other antibodies and apoC-III-lipoprotein assays. ST has a dual appointment at UCSD and Ionis Pharmaceuticals, Inc. JLW has received honoraria for consulting for Ionis, CymaBay, Intercept and Prometheus Pharmaceuticals.
All other authors declare that they have no conflict of interest and no relationships with industry relevant to this study.
References
- 1.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273:6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 2.Thanassoulis G. Lipoprotein(a) in calcific aortic valve disease: from genomics to novel drug target for aortic stenosis. J Lipid Res [Internet] 2015 doi: 10.1194/jlr.R051870. Available from: http://www.jlr.org/content/early/2015/12/18/jlr.R051870.abstract. [DOI] [PMC free article] [PubMed]
- 3.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen M-R, Tokgözoglu L, Tybjærg-Hansen A. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsimikas S, Hall JL. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: a rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 6.Dubé JB, Boffa MB, Hegele Ra, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr Opin Lipidol. 2012;23:133–140. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 7.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 8.Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin M-J, Binder CJ, Hörkkö S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, Yang X, Dennis E, Witztum JL, Koschinsky ML, Tsimikas S. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54:2815–30. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsimikas S, Witztum JL. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol. 2008;19:369–377. doi: 10.1097/MOL.0b013e328308b622. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner Ja. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller YI, Choi S-H, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leibundgut G, Witztum JL, Tsimikas S. Oxidation-specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol. 2013;13:168–179. doi: 10.1016/j.coph.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL, Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 16.Tsimikas S, Duff GW, Berger PB, Rogus J, Huttner K, Clopton P, Brilakis E, Kornman KS, Witztum JL. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a) J Am Coll Cardiol. 2014;63:1724–1734. doi: 10.1016/j.jacc.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seman LJ, Jenner JL, McNamara JR, JSE Quantification of Lipoprotein ( a ) Plasma Fraction in Plasma and Cholesterol in Lectin-Bound. Clin Chem. 1994;40:400–403. [PubMed] [Google Scholar]
- 18.Duivenvoorden R, de Groot E, Elsen BM, Laméris JS, van der Geest RJ, Stroes ES, Kastelein JJP, Nederveen aJ. In vivo quantification of carotid artery wall dimensions: 3.0-Tesla MRI versus B-mode ultrasound imaging. Circ Cardiovasc Imaging. 2009;2:235–242. doi: 10.1161/CIRCIMAGING.108.788059. [DOI] [PubMed] [Google Scholar]
- 19.Rudd JHF, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 20.van der Valk FM, Kroon J, Potters WV, Thurlings RM, Bennink RJ, Verberne HJ, Nederveen AJ, Nieuwdorp M, Mulder WJM, Fayad Za, van Buul JD, Stroes ESG. In vivo imaging of enhanced leukocyte accumulation in atherosclerotic lesions in humans. J Am Coll Cardiol. 2014;64:1019–1029. doi: 10.1016/j.jacc.2014.06.1171. [DOI] [PubMed] [Google Scholar]
- 21.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting Edge: Repurification of Lipopolysaccharide Eliminates Signaling Through Both Human and Murine Toll-Like Receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 22.Cain WJ, Millar JS, Himebauch AS, Tietge UJF, Maugeais C, Usher D, Rader DJ. Lipoprotein [a] is cleared from the plasma primarily by the liver in a process mediated by apolipoprotein [a] J Lipid Res. 2005;46:2681–2691. doi: 10.1194/jlr.M500249-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Edelstein C, Mandala M, Pfaffinger D, Scanu AM. Determinants of Lipoprotein(a) Assembly: A Study of Wild-Type and Mutant Apolipoprotein(a) Phenotypes Isolated from Human and Rhesus Monkey Lipoprotein(a) under Mild Reductive Conditions. Biochemistry. 1995;34:16483–16492. doi: 10.1021/bi00050a032. [DOI] [PubMed] [Google Scholar]
- 24.van Rijssel J, Kroon J, Hoogenboezem M, van Alphen FPJ, de Jong RJ, Kostadinova E, Geerts D, Hordijk PL, van Buul JD. The Rho-guanine nucleotide exchange factor Trio controls leukocyte transendothelial migration by promoting docking structure formation. Mol Biol Cell. 2012;23:2831–2844. doi: 10.1091/mbc.E11-11-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franssen R, Schimmel AWM, van Leuven SI, Wolfkamp SCS, Stroes ESG, Dallinga-Thie GM. In Vivo Inflammation Does Not Impair ABCA1-Mediated Cholesterol Efflux Capacity of HDL. Cholesterol. 2012;2012:610741. doi: 10.1155/2012/610741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarkin JM, Joshi FR, Rudd JHF. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014;11:443–457. doi: 10.1038/nrcardio.2014.80. [DOI] [PubMed] [Google Scholar]
- 27.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GKM, Xystrakis E, Khamri W, Shawcross DL, Ma Y, Wendon Ja, Vergani D. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi) /CD16(neg) monocytes: Expansion of CD14(hi) /CD16(pos) and contraction of CD14(lo) /CD16(pos) monocytes in a. Cytometry A. 2012;81:823–834. doi: 10.1002/cyto.a.22104. [DOI] [PubMed] [Google Scholar]
- 29.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 30.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJW, van der Meer BMJW, Deen PMT, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JWM, Ng A, Joosten LAB, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1 -mediated aerobic glycolysis as metabolic basis for trained immunity. Science (80- ) 2014;345:1250684–1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 33.Chang M-K, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scipione CA, Sayegh SE, Romagnuolo R, Tsimikas S, Marcovina SM, Boffa MB, Koschinsky ML. Mechanistic insights into lipoprotein(a)-induced interleukin-8 expression: a role for oxidized phospholipid modification of apolipoprotein(a) J Lipid Res. 2015;56:2273–2285. doi: 10.1194/jlr.M060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein JH, Hegele RA, Hackam DG, Koschinsky ML, Huff MW, Spence JD. Lipoprotein(a) is associated differentially with carotid stenosis, occlusion, and total plaque area. Arterioscler Thromb Vasc Biol. 2008;28:1851–1856. doi: 10.1161/ATVBAHA.108.169292. [DOI] [PubMed] [Google Scholar]
- 36.Guerra R, Yu Z, Marcovina S, Peshock R, Cohen JC, Hobbs HH. Lipoprotein(a) and apolipoprotein(a) isoforms: no association with coronary artery calcification in the Dallas Heart Study. Circulation. 2005;111:1471–1479. doi: 10.1161/01.CIR.0000159263.50305.BD. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrino M, Furmaniak-Kazmierczak E, LeBlanc JC, Cho T, Cao K, Marcovina SM, Boffa MB, Côté GP, Koschinsky ML. The apolipoprotein(a) component of lipoprotein(a) stimulates actin stress fiber formation and loss of cell-cell contact in cultured endothelial cells. J Biol Chem. 2004;279:6526–6533. doi: 10.1074/jbc.M309705200. [DOI] [PubMed] [Google Scholar]
- 38.Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Economopoulou M, Isermann B, Bdeir K, Nawroth PP, Preissner KT, Gahmberg CG, Koschinsky ML, Chavakis T. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J. 2006;20:559–561. doi: 10.1096/fj.05-4857fje. [DOI] [PubMed] [Google Scholar]
- 39.Ravandi A, Leibundgut G, Hung MY, Patel M, Hutchins PM, Murphy RC, Prasad A, Mahmud E, Miller YI, Dennis EA, Witztum JL, Tsimikas S. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J Am Coll Cardiol. 2014;63:1961–1971. doi: 10.1016/j.jacc.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 42.Bird DA, Gillotte KL, Hörkkö S, Friedman P, Dennis EA, Witztum JL, Steinberg D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc Natl Acad Sci U S A. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YHC, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JSM, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, Cheng S-C, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JWM, Joosten LAB, Wijmenga C, Martens JHA, Xavier RJ, Logie C, Netea MG, Stunnenberg HG. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science (80- ) 2014;345:1251086–1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bekkering S, Quintin J, Joosten LaB, van der Meer JWM, Netea MG, Riksen NP. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arterioscler Thromb Vasc Biol [Internet] 2014;34:1731–8. doi: 10.1161/ATVBAHA.114.303887. Available from: http://atvb.ahajournals.org/cgi/doi/10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 46.Byun YS, Lee J-H, Arsenault BJ, Yang X, Bao W, DeMicco D, Laskey R, Witztum JL, Tsimikas S. Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: the TNT trial. J Am Coll Cardiol. 2015;65:1286–1295. doi: 10.1016/j.jacc.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsimikas S, Viney N, Hughes S, Singleton W, Graham M, Baker B, Burkey J, Yang Q, Marcovina S, Geary R, Crooke R, Witztum J. Antisense Therapy Targeting Apolipoprotein (a) Reduces Plasma Lipoprotein(a) Levels. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 48.Stroes ES, van der Valk FM. A sense of excitement for a specific Lp(a)-lowering therapy. Lancet. 2015;6736:23–24. doi: 10.1016/S0140-6736(15)60638-9. [DOI] [PubMed] [Google Scholar]
- 49.Kolski B, Tsimikas S. Emerging therapeutic agents to lower lipoprotein (a) levels. Curr Opin Lipidol. 2012;23:560–568. doi: 10.1097/MOL.0b013e3283598d81. [DOI] [PubMed] [Google Scholar]
- 50.Miller YI, Tsimikas S. Oxidation-specific epitopes as targets for biotheranostic applications in humans: biomarkers, molecular imaging and therapeutics. Curr Opin Lipidol. 2013;24:426–37. doi: 10.1097/MOL.0b013e328364e85a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsimikas S, Miyanohara A, Hartvigsen K, Merki E, Shaw PX, Chou M-Y, Pattison J, Torzewski M, Sollors J, Friedmann T, Lai NC, Hammond HK, Getz GS, Reardon Ca, Li AC, Banka CL, Witztum JL. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol. 2011;58:1715–1727. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonen A, Hansen LF, Turner WW, Montano EN, Que X, Rafia A, Chou M-Y, Wiesner P, Tsiantoulas D, Corr M, VanNieuwenhze MS, Tsimikas S, Binder CJ, Witztum JL, Hartvigsen K. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. J Lipid Res. 2014;55:2137–2155. doi: 10.1194/jlr.M053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.