Abstract
Purpose
To determine if rectus extraocular muscle (EOM) sizes and pulley locations contribute to exotropia, we used magnetic resonance imaging (MRI) to measure these factors in normal control subjects, and subjects with concomitant and pattern exotropia.
Design
Prospective, case control study.
Participants
Nine subjects with concomitant exotropia, 6 patients with pattern exotropia, and 21 orthotropic normal controls.
Methods
High-resolution, surface coil MRI was obtained in contiguous, quasi-coronal planes. Rectus pulley locations were determined in oculocentric coordinates for central gaze, supraduction, and infraduction. Cross sections in four contiguous image planes were summed and multiplied by the 2-mm slice thickness to obtain horizontal rectus posterior partial volumes (PPVs).
Main outcome measures
Rectus pulley locations, and horizontal rectus PPVs.
Results
Rectus pulleys were differently located in subjects with A, versus V and Y, pattern exotropia. The LR pulleys were significantly displaced superiorly, the MR pulleys were displaced inferiorly, and the IR pulleys were displaced laterally in A pattern exotropia. In opposite fashion, the array of all rectus pulleys was excyclorotated in V and Y pattern exotropia. The PPV of the medial rectus muscle was statistically subnormal by about 29% in concomitant but not pattern exotropia (P<0.05). The ratio of the PPV of the LR relative to the MR in concomitant exotropia was significantly greater than in normal subjects and pattern exotropia (P<0.05).
Conclusions
Abnormalities of EOMs and pulleys contribute differently in pattern versus concomitant exotropia. Abnormal rectus pulley locations derange EOM pulling directions that contribute to pattern exotropia, but in concomitant exotropia, pulley locations are normal and relatively small medial rectus size reduces relative adducting force.
Précis
Rectus extraocular muscle pulley displacements account for pattern incomitance in A, V, and Y pattern exotropia, but rectus pulleys are normal and horizontal rectus muscles sizes imbalanced in concomitant exotropia.
At their points of transit through posterior Tenon's fascia, the rectus extraocular muscles (EOMs) are encircled by connective tissue sleeves composed of elastin, collagen, and in some cases smooth muscle.1,2 It is evident from high resolution magnetic resonance imaging (MRI) performed using surface coils that these tissue sleeves act as pulleys, greatly limiting sideslip over the globe of the posterior EOM paths during duction, and in the process influencing EOM pulling direction.3–5 However, even using high resolution MRI technique, the low intrinsic contrast of connective tissues usually precludes visualization of pulleys with detail sufficient to resolve the precise locations of the rectus pulleys. This seeming conundrum is actually not a major limitation because the EOMs themselves are readily imaged by MRI and always pass through the pulleys; it is only necessary to determine the precise paths of the EOMs to identify the possible locations of their encircling pulleys. In eccentric gazes, the pulleys prevent shift of the straight posterior paths of the EOMs, in so doing abruptly inflecting their paths so that more anteriorly the tendons follow different straight paths angulated to correspond to half the amount of globe rotation. The anatomic point of sharp inflection between the stable posterior EOM path, versus the moving anterior path, defines each pulley location functionally.6 Conceptually, EOMs direct their forces towards the pulleys as if pulleys were their mechanical origins, so the locations of the inflections in EOM paths define their pulling directions and thus their oculorotary actions.7 While precise determination of the three-dimensional locations of the rectus pulleys requires that imaging be performed in multiple gaze positions including secondary or tertiary ones, studies of pulley locations to date have made the task easier: the anteroposterior positions of rectus pulleys remain normal even if their horizontal and vertical positions are grossly abnormal. This fortunate circumstance implies that pulley coordinates in the coronal plane may be accurately approximated by the horizontal and vertical rectus EOM positions determined from quasi-coronal MRI planes located between the posterior pole and equator when obtained in central gaze.
Strabismus is described as concomitant when its angle is independent of gaze direction, yet in about 25% of cases, incomitant pattern deviations occur in which the angle changes with gaze direction.8 Heterotopic (displaced) pulleys have been proposed to be among the causes of incomitant strabismus,9,10 including congenital A-patterns with less esotropia (ET) in infraversion, and V-patterns with greater exotropia (XT) or less ET in supraversion. Pulley heterotopy can misdirect EOM force to produce over- or under-elevation in adduction that may simulate oblique EOM over- or under-contraction.9,10 Small mislocations (<2 mm) of rectus EOM pulleys can cause incomitant strabismus, which noted that abnormal superior displacement of a lateral rectus (LR) pulley can generate the clinical pattern often termed “superior oblique (SO) muscle overaction” that caused “A” pattern and an inferiorly displaced LR pulley can generate “V” pattern strabismus.9 Previous studies of EOM pulley positions in pattern strabismus measured these positions relative to the bony orbit4,9,11, but the effect could be confounded by translation of the eye itself within the orbit. Beyond static mislocations of pulleys, resultant patterns of strabismus may depend on static pulley positions, pulley instability, and coexisting globe translation that alter pulley locations relative to the globe.12
Modest pulley displacement may also result from abnormal ocular torsion,13 and modest changes in pulley positions are induced by aggressive surgical EOM transpositions performed for macular transposition.14 Recently, combined mechanical effects of medial rectus (MR), superior rectus (SR), and inferior rectus (IR) muscle pulley displacements alone were found to explain the pattern of incomitant strabismus in SO palsy, although such pulley displacements were small.15
Consensus is scarce regarding causes of concomitant strabismus. Electrophysiologic recordings of the horizontal rectus motor neurons in monkeys with experimental strabismus, found the relationship between motor neuron firing rate and eye position to be the same in strabismic and normal monkeys.16, 17 MRI in humans with concomitant ET shows that the MR is larger than normal, but the LR is not subnormal. This suggests that human concomitant ET is associated with peripheral EOM abnormality,18 yet it remains mysterious how abnormal sized EOMs could be commanded by normal motor unit behavior to execute normal eye movements, and remains uncertain if abnormal EOM size is cause or effect of strabismus. Changes in EOM length do not explain strabismus. Rabinowitz and Demer found normal horizontal rectus EOM path lengths in intermittent and alternating ET and XT.19
While pulley heterotopy and instability have been observed in pattern strabismus,9,10 it is unknown if abnormal pulley locations contribute to concomitant XT. It remains possible that the horizontal EOMs might also become larger or stronger in pattern XT. The present study sought to obtain MRI data on rectus pulley positions, as well as horizontal muscle size, asking if abnormalities are associated with encountered concomitant or pattern XT.
Methods
After obtaining written informed consent according to a protocol conforming to the Declaration of Helsinki and approved by the Institutional Review Board of the University of California, Los Angeles, we prospectively studied 9 subjects with constant, concomitant XT who had mean age 34±13 (standard deviation, SD) yrs, and six subjects with pattern XT of mean age 29±10 yrs, including two with A-pattern XT, two with V-pattern XT and two with Y-pattern XT. These included all subjects recruited for a prospective study of MRI in strabismus between July 2002 and January 2013 who had non-paralytic XT but had not undergoing prior strabismus surgery, in whom orbital MRI was obtained in quasi-coronal planes in central gaze, had adequate image quality for analysis. We compared these with 21 normal control subjects of mean age 31±14 yrs, who were orthotropic and had vision correctable to normal with spectacles if necessary. No strabismic subject had been included in prior studies of pulley positions.
Normal subjects were examined to verify normal ocular motility, stereopsis, and binocular alignment. All cases of XT were constant rather than intermittent XT. Exotropic subjects had similar visual acuity bilaterally. By prism and cover testing, subjects with “A” pattern exhibited XT at least 10Δ greater in infraversion than supraversion. Subjects with “V” pattern exotropia had XT at least 15Δ greater in supraversion than infraversion. “Y” pattern XT was diagnosed when XT was greatest in supraversion, but similar in central gaze and infraversion.
Subjects underwent high-resolution, T1 or T2-weighted MRI with a 1.5T scanner (Signa; General Electric, Milwaukee, WI) and surface coils (Medical Advances, Milwaukee, WI) as detailed elsewhere,3, 20 including monocular fixation of a centered afocal target by the eye scanned to eliminate eye position as a potential confounder.9, 21, 22 Axial images were obtained to localize placement of subsequent higher resolution quasicoronal images perpendicular to the orbital axis. Digital MRI images were quantified with the computer program ImageJ64 (Rasband, W. S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2015). Horizontal rectus EOMs were manually outlined in coronal plane images.23, 24 Rectus EOM locations were determined by their area centroids6 that were transformed into a standard coordinate system originating at center of the spherical globe6 as determined in 3-D from multiple cross-sectional images.4,25 Data were rotated about globe center using cranial landmarks into a standard anatomical coordinate system whose positive coordinates were anterior, superior, and lateral.6 In most cases, imaging was performed in central gaze, supraduction, and infraduction, enabling determination of the anteroposterior locations of the horizontal rectus pulleys from the inflections in EOM paths in the eccentric gazes. While imaging was also performed in horizontal eccentric gazes, it was not possible to image the paths of the vertical rectus EOMs sufficiently anteriorly without injected contrast to identify path inflections that would have defined the anteroposterior locations of the pulleys. These were identified quantitatively by piecewise linear regression.6 Therefore, published anteroposterior pulley coordinates were assumed to determine anteroposterior coordinates of the vertical rectus pulleys.
The quasi-coronal image plane closest to the junction of the globe and optic nerve was defined to be image plane zero, with more anterior image planes designated positive, and posterior planes designated negative. Cross sections in image planes -4, -5, -6, -7 (8–14 mm posterior to the globe-optic nerve junction) were summed and multiplied by 2-mm to form posterior partial volumes (PPVs).26 Results were analyzed using analysis of variance (ANOVA, GraphPad Prism version 6, Graphpad Software, La Jolla, CA). Differences were considered significant at P<0.05.
Results
Rectus Pulley Locations In the Coronal Plane
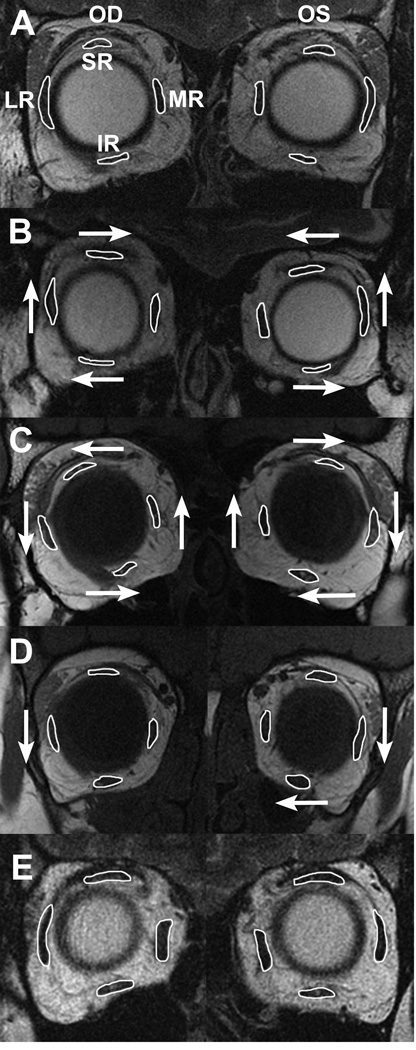
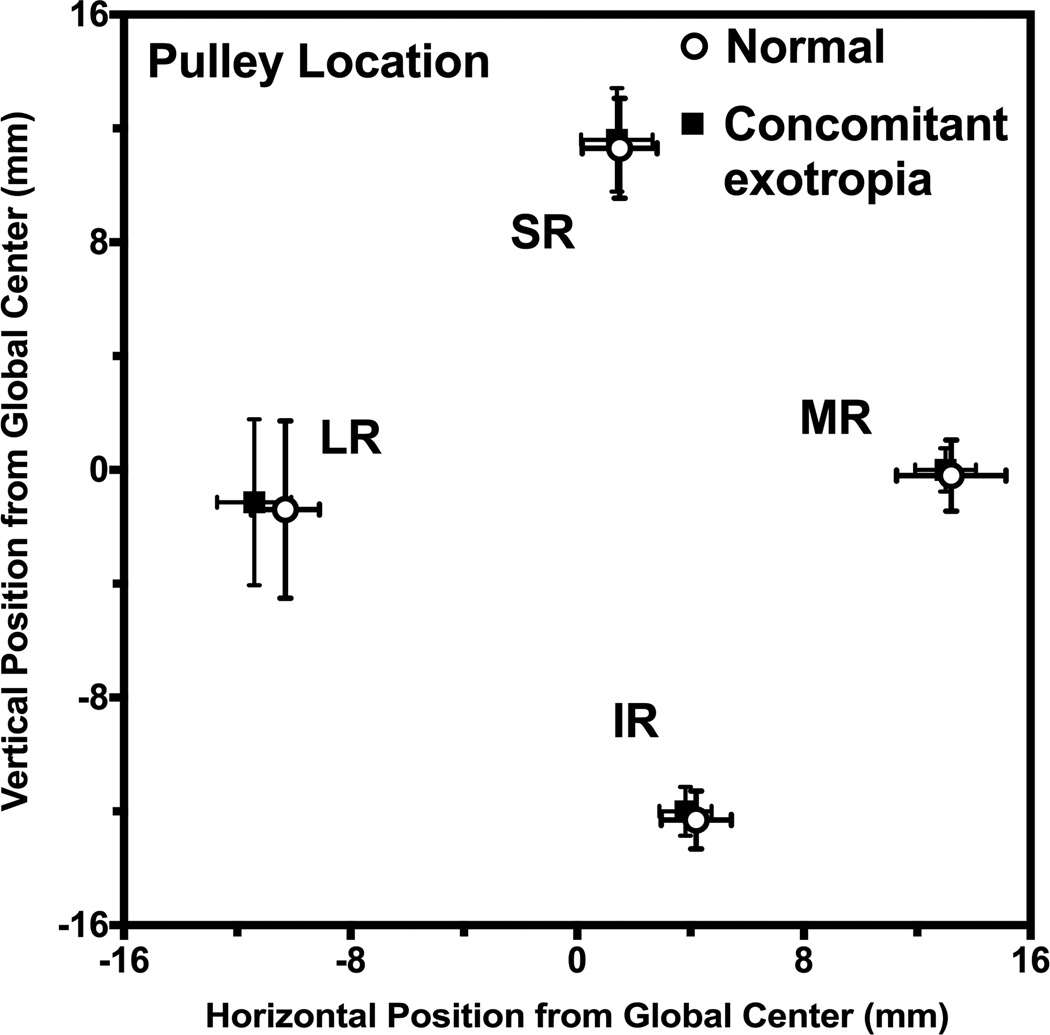
Cross sections of rectus EOMs pulleys were readily identifiable in quasi-coronal MRI. Figure 1A illustrates binocular MRI at the level of the rectus EOM pulleys in a representative normal subject. It is evident that rectus cross sections are bilaterally symmetrical, with similar vertical positions of all four horizontal rectus EOMs, and with the SR located slightly lateral to the IR. This arrangement was also typical of concomitant XT (Fig. 1E). A quantitative analysis is illustrated in Fig. 2, which superimposes mean coordinates of the four rectus pulleys in both normal subjects, and those with concomitant XT. The pulley locations of normal control subjects are consistent with a previous study,6 and were statistically indistinguishable from those of subjects with concomitant XT (P>0.05).
Fig. 1.
MRI in representative subject groups. (A) Normal. (B) A pattern exotropia - lateral rectus (LR) muscles are displaced superiorly, inferior rectus (IR) muscles laterally, and superior rectus (SR) muscles medially. (C) V pattern exotropia - array of all rectus muscles is excyclorotated bilaterally. (D) Y pattern exotropia - LR muscles in both eyes are displaced inferiorly, and IR in the left eye is displaced nasally. (E) concomitant exotropia (A, B and E are T2-weighted, C and D are T1-weighted.) MR - medial rectus muscle.
Fig. 2.
Rectus pulley locations of normal subjects and subjects with concomitant exotropia. No pulley positions are abnormal in concomitant exotropia. Error bands ±2 SD. LR - lateral rectus, IR - inferior rectus, MR - medial rectus muscle, SR - superior rectus.
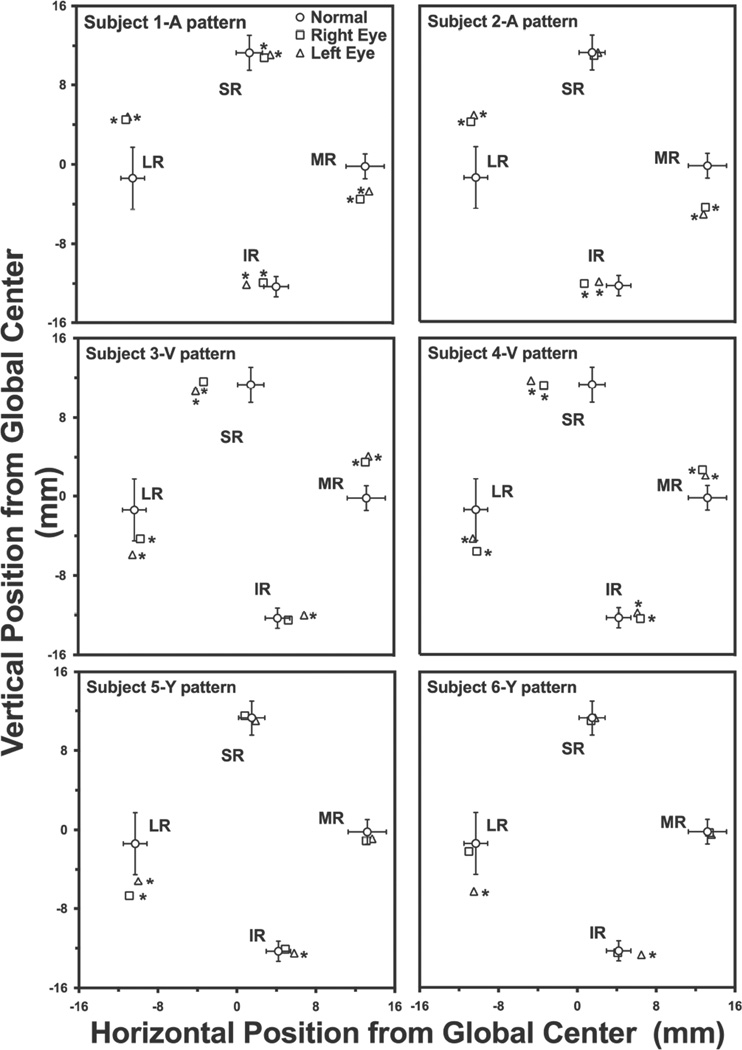
Unlike subjects with concomitant strabismus, all six subjects who had XT exhibiting Vor Y- or A-pattern incomitance had at least one heterotopic rectus pulley significantly outside the 95% confidence interval of normal. Fig. 1 B–D demonstrates MRI examples of the consistent finding of abnormal rectus pulley locations in pattern exotropia. In A-pattern XT, the array of rectus pulleys appears bilaterally incyclorotated (Fig. 1B), with the LR superior to the MR, and the SR nasal to the IR. The opposite finding was typical of V-pattern exotropia (Fig. 1C), where the array of rectus pulleys appears bilaterally excyclorotated, with the LR inferior to the MR, and SR markedly temporal to the IR. The foregoing effect was less extreme in Y-pattern exotropia (Fig. 1D). Quantitative analyses of pulley positions are illustrated in Fig. 3, with examples of two subjects each with A-, V-, and Y-pattern exotropia.
Fig. 3.
Locations of rectus EOM pulleys in normal subjects and subjects with pattern exotropia relative to orbital center. Left orbits have been reflected to the configuration of right orbits. Error bands ±2 SD. *- significantly abnormal.
Anteroposterior Pulley Locations
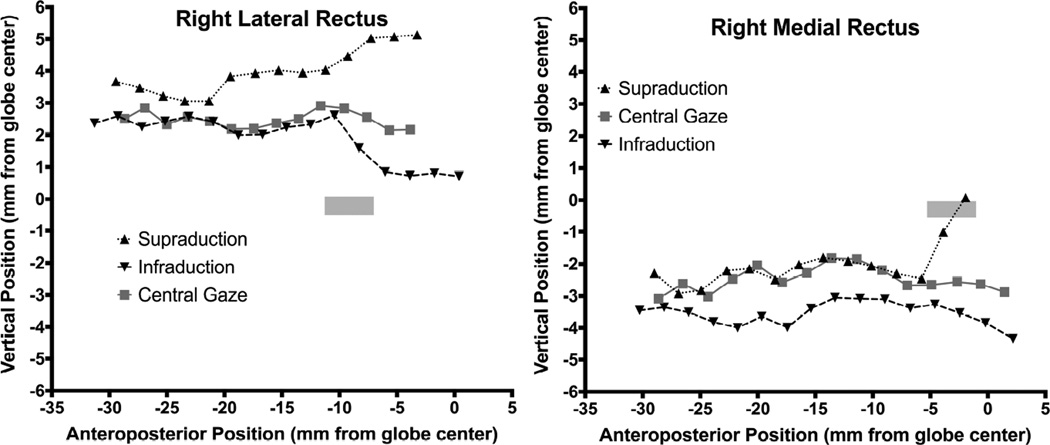
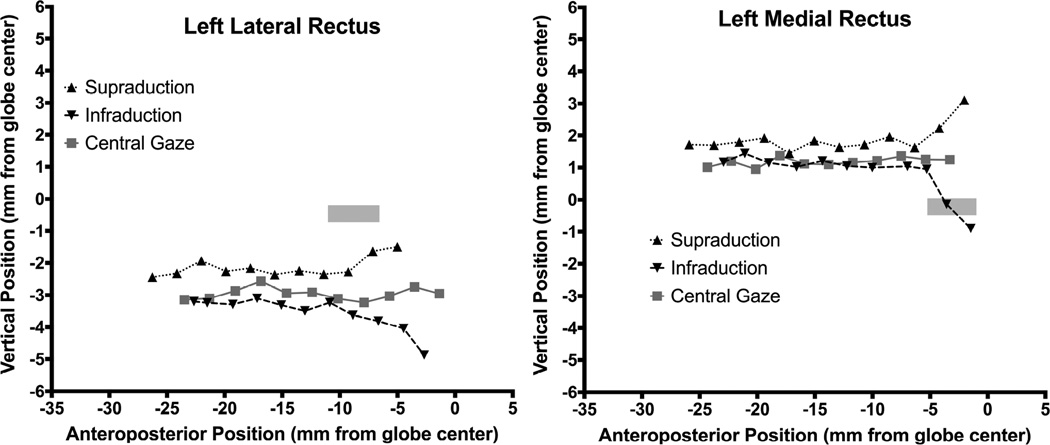
In order to determine anteroposterior pulley locations, MRI was repeated in multiple vertical gaze positions only in subjects with pattern XT. Figure 4 shows vertical projections of the anteroposterior paths of the LR and MR muscles for Apattern XT in central gaze, supraduction, and infraduction. There was roughly 3 mm superior displacement from normal of the posterior LR path, and roughly 2.5 mm inferior shift of the posterior MR path; these posterior paths were similar in all vertical gaze positions. However, about 10 mm posterior to globe center, LR path was sharply inflected inferiorly in infraduction, and superiorly in supraduction, as the anterior LR tendon traveled anteriorly from its pulley to the correspondingly rotated scleral insertion (Fig. 4 left). The anteroposterior location of this inflection point in the LR path was similar for both infraduction and supraduction, and corresponds to the normal anteroposterior range of pulley locations denoted by the rectangle in Fig. 4. While anteroposterior location of the LR pulley for the case of A-pattern XT in Fig. 4 may thus be seen to be normal, inflections of LR path during vertical gaze shift were 2 – 4 mm superior to the vertical extent of the normal pulley location denoted by the gray rectangle in Fig. 4. Medial rectus paths for the same case of A-pattern XT are illustrated in the right panel of Fig. 4. Inverse to the vertical path of the LR, posterior MR path may be seen to be shifted about 3 mm inferiorly in all vertical gaze positions, but exhibits vertical inflection 5–6 mm posterior to globe center at the posterior end of the normal range denoted by the gray rectangle (Fig. 4 right). This implies that the MR pulley is displaced significantly inferiorly, but remains in a normal anteroposterior location.
Fig. 4.
Vertical area centroid positions of the right lateral rectus (LR) muscle and right medial rectus (MR) muscle in a subject with A pattern exotropia along the anteroposterior orbit axis, referenced to globe center. The LR path in central gaze shows marked superior displacement of the LR pulley, well superior to the 95% confidence region for the normal LR and MR pulley6 (gray rectangle).
Figure 5 shows vertical projections of the anteroposterior paths of the LR and MR paths in V-pattern XT in central gaze, supraduction, and infraduction. There was roughly 2.5 mm inferior displacement from normal of the posterior LR path, and roughly 2 mm superior shift of the posterior MR path; these posterior paths were similar in all vertical gaze positions. However, about 10 mm posterior to globe center, LR path was sharply inflected inferiorly in infraduction, and superiorly in supraduction, as the anterior LR tendon traveled anteriorly from its pulley to the correspondingly rotated scleral insertion (Fig. 5 left). The anteroposterior location of this inflection point in the LR path was similar for both infraduction and supraduction, and corresponds to the normal anteroposterior range of pulley locations denoted by the rectangle in Fig. 5. While anteroposterior location of the LR pulley for the case of V-pattern XT in Fig. 5 may thus be seen to be normal, inflections of LR path during vertical gaze shift were 1 – 3 mm inferior to the vertical extent of the normal pulley location denoted by the gray rectangle in Fig. 5. Medial rectus paths for the same case of V-pattern XT are illustrated in the right panel of Fig. 5. Inverse to the vertical path of the LR, posterior MR path may be seen to be shifted about 2 mm superiorly in all vertical gaze positions, but exhibits vertical inflection about 5 mm posterior to globe center at the posterior and of the normal range denoted by the gray rectangle (Fig. 5 right). This implies that the MR pulley is displaced significantly superiorly, but remains in a normal anteroposterior location in this subject.
Fig. 5.
Vertical area centroid positions of the left lateral rectus (LR) muscle and left medial rectus (MR) muscle in V pattern exotropia along the anteroposterior orbit axis, referenced to globe center. The LR path in central gaze was consistent with a marked inferior displacement of the LR and MR pulley, well inferior to the 95% confidence region for the normal LR pulley6 (gray rectangle).
Computational Simulation
Computational simulations of binocular alignment were performed using measured rectus coronal plane pulley locations in subjects with A-, V- and Y-pattern XT using the Orbit 1.8 model (see Supplemental Fig. S1, available at aaojournal.org). Implementing only these abnormalities that were measured in the same oculocentric coordinates employed by Orbit 1.8, the computer simulations replicated the three patterns of incomitance observed for these subjects in infraversion and supraversion. These simulations suggest that the pulley heterotopies might cause the incomitant XT.
Horizontal Rectus EOM Volumes
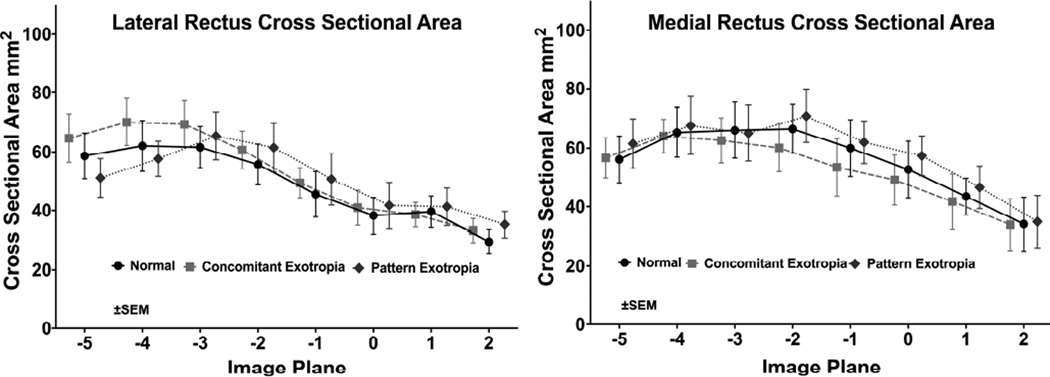
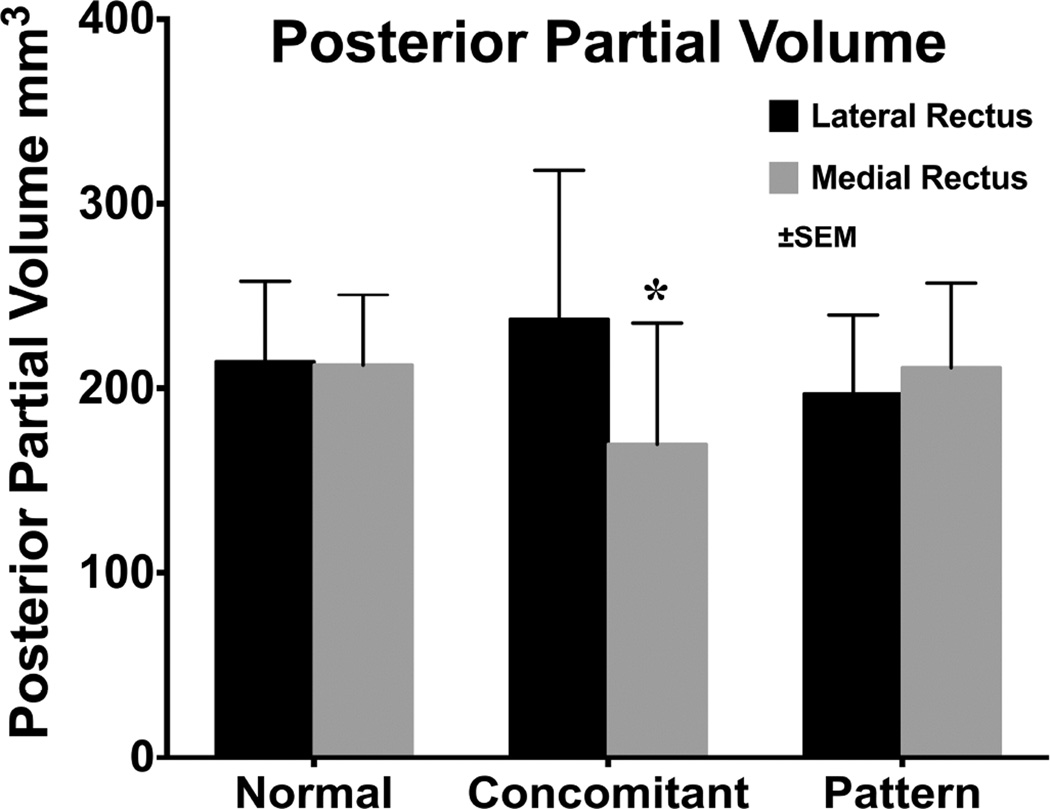
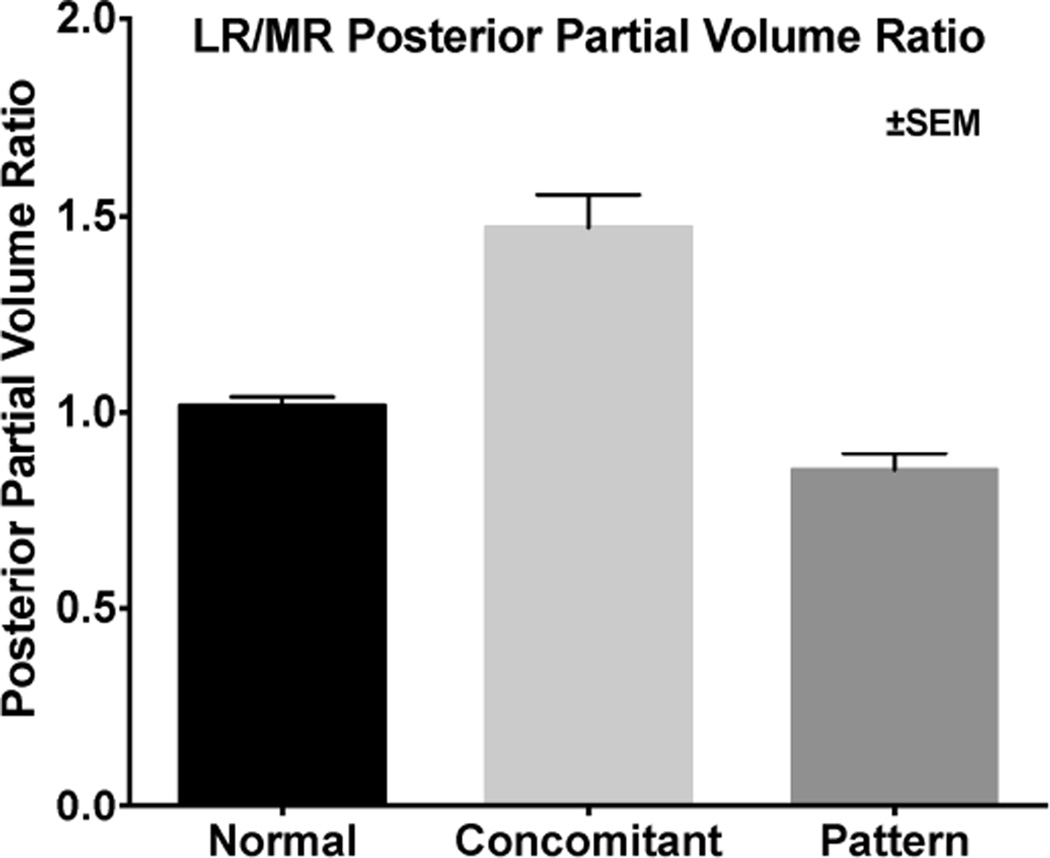
We measured cross sectional area distributions in each image plane of the horizontal rectus EOMs in central gaze and plotted these against anteroposterior position in the orbit (Fig. 6) to determine if EOM size might vary with type of XT. There were no significant differences for LR and MR in normal subjects, and subjects with concomitant and pattern XT. But Figure 6 demonstrated greater difference between LR and MR in subjects with concomitant XT. From the summed cross sections, we computed the PPVs of the LR and MR (Fig. 7). In concomitant XT, the PPV of the MR was 28.6% smaller than normal (P=0.01). The ratio of PPV of the LR to MR was 1.01±0.15 for normal subjects, and 0.86±0.16 for subjects with pattern XT, (Fig. 8, P=0.05), but it was significantly greater than both at 1.47±0.37 in concomitant XT (P=0.006).
Fig. 6.
Mean cross-area (mm2) of the lateral rectus (LR) and medial rectus (MR) muscles in 2 mm thick quasi-coronal planes from posterior at left to anterior at right, for normal subjects, and concomitant and pattern exotropia. Differences were not significant (P=0.67 for LR, P=0.43 for MR, using ANOVA).
Fig. 7.
Posterior partial volumes of the lateral and medial rectus muscles in normal subjects and concomitant and pattern exotropia. * - statistically subnormal (P<0.05).
Fig. 8.
Ratio of lateral rectus to medial rectus PPV in normal subjects, and concomitant and pattern exotropia. The ratio was significantly greater in concomitant exotropia than in the other groups (P<0.05).
Superior Oblique Size
Mean PPV of the SO was 116±7 mm3 in normal subjects, 117±5 mm3 in concomitant XT, 123±4 mm3 in A-pattern XT, and 112±3 mm3 in V- and Y-pattern XT, not varying significantly among groups. While PPV of the SO did not differ significantly from normal in any of the exotropic subject groups, it was about 9% smaller in V-Y pattern XT that in A pattern XT (P=0.03). Inferior oblique muscle size could not be measured from the image planes acquired.
Discussion
Pulleys in Concomitant and Pattern XT
High resolution MRI here demonstrated that rectus EOMs pulleys are normally located in concomitant XT, thus excluding coronal plane pulley heterotopy as a causal or associated factor in this form of strabismus. In contrast, all six subjects who had XT with V- or Y- or A-pattern incomitance exhibited at least one heterotopic rectus pulley significantly outside the 95% confidence region for normal oculocentric coordinates in the coronal plane. This finding confirms and extends the observations of a previous study that examined the normal rectus pulley positions relative to the orbit (orbitocentric coordinates).6 The current findings support the conclusion that displacement of rectus pulleys perpendicular to their planes of action by only a few millimeters may increase the risk of strabismus by altering EOM pulling directions sufficiently to exceed compensatory capabilities of fusional vergence.27
For the both subjects with A pattern XT, the LR pulley was shifted superiorly and the IR laterally. In subjects with V pattern XT, the LR pulley was displaced inferiorly, the IR medially, MR superiorly, and SR laterally in both eyes (Fig. 2C), amounting to bilateral excyclorotation of the entire rectus pulley array. This phenomenon was incomplete in subject 4, who exhibited abnormal positions only of the left MR and right IR pulleys. The subject with Y-pattern XT had pulley locations similar to those in V pattern XT, with the LR bilaterally shifted inferiorly, and the left IR pulley shifted nasally.
Previous studies demonstrated the effects of insertional transposition,11,20 and showed conventional recession and resection of horizontal rectus EOMs did not result in horizontal or vertical displacement of their pulleys.28 Sekeroglu et al. found that half tendon width transposition of horizontal muscles significantly decreased the amount of V pattern.29 Children with craniosynostosis often have V-pattern horizontal strabismus.30,31 One explanation for pattern strabismus in craniosynostosis is excyclotorsion of the orbital contents, resulting in oblique pulling directions for the nominally rectus EOMs.31–33 Weiss et al. have provided evidence that the pattern and dissociated vertical strabismus associated with Crouzon syndrome can be caused by extorsion of rectus pulley array.34 The present cases illustrate that abnormalities of locations of horizontal rectus pulleys occur in pattern XT, consistent with a prior report in two subjects.4 There are several reasons why the present findings suggest that rectus pulley heterotopy is probably the cause, rather than the effect, of pattern strabismus. First, SO palsy, a wellrecognized cause of paralytic pattern strabismus, produces small pulley displacements of MR, SR and IR, but not LR, which suggest that these pulley displacements are not secondary to excyclotorsion caused by SO palsy.15 Second, even a 16 mm inferior oblique muscle advancement performed in one patient that corrected 25° incyclotropia induced by macular translocation did not shift the MR pulleys as much as observed here in V- and Y-pattern XT.15 Third, computational simulation using the Orbit 1.8 model predicted the alignment effects of the pulley heterotopies in all cases of pattern XT. Abnormal rectus pulley locations may explain why some cases of pattern strabismus respond poorly to oblique EOM surgery. This suggests that orbital imaging might be clinically valuable in surgical planning for patients with pattern XT.
Multipositional MRI sufficient to locate anteroposterior pulley locations by EOM path inflections was performed only in some cases of pattern XT. Anteroposterior locations of the LR and MR pulleys were normal in the cases of A- and V-pattern XT that were examined, supporting the conventional assumption that anteroposterior pulley position remains normal even in the setting of considerable rectus pulley heterotopy in the coronal plane. Anteroposterior pulley location was therefore presumed normal for the other cases of XT.
Posterior Partial Volumes of Extraocular Muscles
Subjects with concomitant XT had significantly subnormal PPV for the MR, and a trend towards supranormal PPV of the LR muscles, such that the ratio of LR to MR PPV significantly exceeded normal. It seems logical to presume that larger EOMs would be stronger than smaller ones, since changes in EOM size closely correlate with contractility.24 In context of the normal rectus pulley locations observed here in concomitant XT, larger LR than MR PPV seems to offer a satisfactory mechanism for understanding the XT on the basis of abducting LR strength exceeding adducting MR strength. Differential strength in the LR-MR antagonist pair may be related to the tendency for recurrence of XT after surgical treatment. It may be that the MR in concomitant XT is weaker than in normal subjects and subjects with pattern XT, where the pattern of the strabismus is presumably the result of normal EOM force exerted in abnormal directions due to heterotopic pulleys.
Volume of the SO is reflective of its strength, and did not significantly differ from normal in concomitant or wither form of pattern XT. While PPV of the SO in V and Y pattern XT was about 9% less than in A pattern XT, the significance of this small difference is uncertain. For comparison, the ratio of LR to MR PPV in concomitant XT was about 47% greater than normal (Fig. 8). A larger sample of subjects would be required to determine if the SO plays a significant role in pathophysiology of pattern XT. The present investigation did not examine the inferior oblique muscle (IO) for a possible relationship to XT. While highly significant in all groups with pattern XT, pulley heterotopy by itself would not in any obvious way determine whether associated strabismus would be ET vs. XT; other factors, such as central innervational commands, must be presumed decisive in this regard.
In summary, the current study confirms and extends previous reports of pulley positions in pattern XT,4,5,7,9 and suggests that PPV changes in horizontal rectus EOMs may be related to concomitant XT. This study is preliminary in that it included few subjects who had concomitant XT and pattern XT, did not evaluate the IO muscle, and only determined anteroposterior pulley locations in some cases of pattern XT. However, these data indicate that highly significant and widespread coronal plane pulley positions are probably primary abnormalities in pattern XT.
Supplementary Material
Simulated binocular alignment based upon measured pulley positions for every subject with A-, V- and Y-pattern exotropia.
Acknowledgments
Funding/Support: This study was supported by grants to Joseph L. Demer from the U.S. Public Health Service, National Institutes of Health, grants EY008313 and EY000331, and an unrestricted grant from Research to Prevent Blindness. The funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: Accepted for presentation at American Association for Pediatric Ophthalmology and Strabismus (AAPOS) Annual Meeting, Vancouver, BC, April 9, 2016.
Conflict of Interest: No conflicting relationship exists for any author.
This article contains additional online-only material. Figure S1 should appear online-only.
References
- 1.Koornneef L. New insights in the human orbital connective tissue: Results of a new anatomical approach. Arch Ophthalmol. 1977;95:1269–1273. doi: 10.1001/archopht.1977.04450070167018. [DOI] [PubMed] [Google Scholar]
- 2.Demer JL, Miller JM, Poukens V, et al. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 3.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 4.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys: muscle paths as a function of gaze. Invest Ophthalmol Vis Sci. 1997;38:227–240. [PubMed] [Google Scholar]
- 5.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- 6.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 7.Demer JL, Miller JM, Poukens V. Surgical implications of the rectus extraocular muscle pulleys. J Pediatr Ophthalmol Strabismus. 1996;33:208–218. doi: 10.3928/0191-3913-19960701-03. [DOI] [PubMed] [Google Scholar]
- 8.Costenbader FD. Symposium: The ‘A’ and ‘V’ patterns in strabismus. Trans Am Acad Ophthalmol Otolaryngol. 1964;68:385–386. [PubMed] [Google Scholar]
- 9.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic rectus muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 10.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Buren, The Netherlands: Aeolus Press; 1999. pp. 91–94. [Google Scholar]
- 11.Clark RA, Rosenbaum AL, Demer JL. Magnetic resonance imaging after surgical transposition defines the anteroposterior location of the rectus muscle pulleys. J AAPOS. 1999;3:9–14. doi: 10.1016/s1091-8531(99)70088-1. [DOI] [PubMed] [Google Scholar]
- 12.Oh SY, Clark RA, Velez F, et al. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–2178. [PubMed] [Google Scholar]
- 13.Guyton DL, Weingarten PE. Sensory torsion as the cause of primary oblique muscle overaction/underaction and A- and V-pattern strabismus. Binocular Vision & Eye Muscle Surgery Quarterly. 1994;9:209–236. [Google Scholar]
- 14.Kono R, Ohtsuki H, Okanobu H, Kinugasa K. Displacement of rectus muscle pulleys by torsional muscle surgery for treatment of full macular translocation-induced incyclotropia. Am J Ophthalmol. 2005;140:144–146. doi: 10.1016/j.ajo.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Suh SY, Le A, Clark RA, Demer JL. Rectus pulley displacements without abnormal oblique contractility explain strabismus in superior oblique palsy. Ophthalmology. doi: 10.1016/j.ophtha.2016.02.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das V. Investigating mechanisms of strabismus in non-human primates. J AAPOS. 2008;12:324–325. doi: 10.1016/j.jaapos.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi AC, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2011;52:6697–6705. doi: 10.1167/iovs.11-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoeff K, Chaudhuri Z, Demer JL. Functional magnetic resonance imaging of horizontal rectus muscles in esotropia. J AAPOS. 2013;17:16–21. doi: 10.1016/j.jaapos.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinowitz R, Demer JL. Muscle path length in horizontal strabismus. J AAPOS. 2014;18:4–9. doi: 10.1016/j.jaapos.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JM, Demer JL, Rosenbaum AL. Effect of transposition surgery on rectus muscle paths by magnetic resonance imaging. Ophthalmology. 1993;100:475–487. doi: 10.1016/s0161-6420(93)31618-0. [DOI] [PubMed] [Google Scholar]
- 21.Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of extraocular muscles: A new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–188. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 22.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 23.Clark RA, Demer JL. Functional morphometry demonstrates extraocular muscle compartmental contraction during vertical gaze changes. J Neurophysiol. 2016;115:370–378. doi: 10.1152/jn.00825.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark RA, Demer JL. Functional morphometry of horizontal rectus extraocular muscles during ocular duction. Invest Ophthalmol Vis Sci. 2012;53:7375–7379. doi: 10.1167/iovs.12-9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason TE, Hazard CT. Brief Analytic Geometry. Boston: Ginn & Company; 1935. p. 72. [Google Scholar]
- 26.Demer JL, Clark RA. Magnetic resonance imaging of differential compartmental function of horizontal rectus extraocular muscles during conjugate and converged ocular adduction. J Neurophysiol. 2014;112:845–855. doi: 10.1152/jn.00649.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews DE, Farewell VT. Using and Understanding Medical Statistics. New York: Karger; 1988. pp. 177–178. [Google Scholar]
- 28.Clark RA, Demer JL. Magnetic resonance imaging of the effects of horizontal rectus extraocular muscle surgery on pulley and globe positions and stability. Invest Ophthalmol Vis Sci. 2006;47:188–194. doi: 10.1167/iovs.05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekeroglu HT, Turan KE, Uzun S, et al. Horizontal muscle transposition or oblique muscle weakening for the correction of V pattern? Eye (Lond) 2014;28:553–556. doi: 10.1038/eye.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SH, Nischal KK, Dean F, et al. Visual outcomes and amblyogenic risk factors in craniosynostotic syndromes: A review of 141 cases. Br J Ophthalmol. 2003;87:999–1003. doi: 10.1136/bjo.87.8.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morax S. Oculo-motor disorders in craniofacial malformations. J Maxillofac Surg. 1984;12:1–10. doi: 10.1016/s0301-0503(84)80201-5. [DOI] [PubMed] [Google Scholar]
- 32.Cheng H, Burdon MA, Shun-Shin GA, Czypionka S. Dissociated eye movements in craniosynostosis: A hypothesis revived. Br J Ophthalmol. 1993;77:563–568. doi: 10.1136/bjo.77.9.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan KP, Sargent MA, Poskitt KJ, Lyons CJ. Ocular overelevation in adduction in craniosynostosis: Is it the result of excyclorotation of the extraocular muscles? J AAPOS. 2005;9:550–557. doi: 10.1016/j.jaapos.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Weiss AH, Phillips J, Kelly JP. Crouzon syndrome: relationship of rectus muscle pulley location to pattern strabismus. Invest Ophthalmol Vis Sci. 2014;55:310–317. doi: 10.1167/iovs.13-13069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Simulated binocular alignment based upon measured pulley positions for every subject with A-, V- and Y-pattern exotropia.