Abstract
Background
Women with high levels of mammographic density (MD) have a four- to six-fold increased risk of developing breast cancer; however, most neither have a prevalent tumor nor will they develop one. Magnetic resonance imaging (MRI) studies suggest that background parenchymal enhancement, an indicator of vascularity, is related to increased breast cancer risk. Correlations of microvessel density (MVD) in tissue, MD and biopsy diagnosis have not been defined, and we investigated these relationships among 218 women referred for biopsy.
Methods
MVD was determined by counting CD31-positive vessels in whole sections of breast biopsies in three representative areas; average MVD was transformed to approximate normality. Using digital mammograms, we quantified MD volume with single X-ray absorptiometry. We used linear regression to evaluate associations between MVD and MD adjusted for age and body mass index (BMI) overall, and stratified by biopsy diagnosis: cases (in situ or invasive cancer, n = 44) versus non-cases (non-proliferative or proliferative benign breast disease, n = 174). Logistic regression adjusted for age, BMI, and MD was used to calculate odds ratios (ORs) and 95 % confidence intervals (CIs) for associations between MVD and biopsy diagnosis. We also assessed whether the MVD-breast cancer association varied by MD.
Results
MVD and MD were not consistently associated. Higher MVD was significantly associated with higher odds of in situ/invasive disease (ORAdjusted = 1.69, 95 % CI = 1.17–2.44). MVD-breast cancer associations were strongest among women with greater non-dense volume.
Conclusions
Increased MVD in tissues is associated with breast cancer, independently of MD, consistent with MRI findings suggestive of its possible value as a radiological cancer biomarker.
Electronic supplementary material
The online version of this article (doi:10.1186/s13058-016-0746-9) contains supplementary material, which is available to authorized users.
Keywords: Mammographic density, Breast neoplasms, Angiogenesis, Pathology, Lesional density
Background
Mammographic density (MD), a radiographic reflection of the proportion of fibroglandular tissue in the breast, is one of the strongest risk factors for the development of ductal carcinoma in situ (DCIS) and invasive breast cancer [1, 2]. Nonetheless, most women with high MD have neither a prevalent tumor nor will they develop one during follow-up. Accordingly, identifying additional risk markers that are independent of MD may improve risk prediction.
Multiple breast imaging techniques [3], including breast magnetic resonance imaging (MRI), can be used to evaluate breast density, and most yield similar results with respect to breast cancer risk. In addition, recent data suggest that MRI performed with contrast provides a measure of background parenchymal enhancement (BPE), which reflects vascularity and vessel permeability [4] and has been associated with breast cancer odds in cross-sectional studies [5, 6]. Morphologically, the distribution, shape and size of radiologic patterns of dense tissue align well with areas of non-fatty tissue on gross examination of surgical pathology specimens; however, microscopically, areas of dense tissue appear highly variable, ranging from hypocellular dense collagen to hypercellular regions of invasive carcinoma. We hypothesized that vascularity is correlated with cellularity, suggesting that measurement of the former might prove useful in distinguishing dense tissues harboring neoplastic lesions from benign fibrosis. Accordingly, to assess this hypothesis, we examined relationships between microvessel density (MVD), a commonly used histologic measure of tumor angiogenesis, with MD measures and concurrent breast biopsy diagnoses.
Methods
Study population
This study included 465 women aged 40–65 years undergoing image-guided breast biopsies at the University of Vermont College of Medicine and the University of Vermont Medical Center that participated in the National Cancer Institute (NCI) Breast Radiology Evaluation and Study of Tissues (BREAST) Stamp Project from October 2007 to June 2010, as previously described [7]. Briefly, this study focused on patients referred for a diagnostic image-guided breast biopsy. At the time of mammogram, study participants completed a standard health history questionnaire which assessed known breast cancer risk factors. When a breast imaging study was considered abnormal, indicating the need for a biopsy, women were contacted by the study coordinator and screened to determine eligibility, obtain verbal consent, and administer an approximately 20-minute telephone interview to collect additional health information, including history of exogenous hormone use. Ninety percent of study participants underwent biopsy within 10 days of completing this interview. Eligible women were those without a history of breast cancer or treatment, who had not undergone breast surgery within the preceding year, did not have breast implants, were not taking breast cancer chemoprevention and were scheduled to have an image-guided breast biopsy. On the day of the breast biopsy, a research coordinator measured participants’ height and weight.
Mammographic density assessment
Mammograms were acquired on one of six full-field digital mammography systems. Raw images were encrypted and transferred to the University of California at San Francisco for quantitative volume and area density assessment. This analysis was restricted to pre-biopsy views [craniocaudal (96 %) or mediolateral oblique (4 %)] of the ipsilateral breast. For three women who underwent bilateral breast biopsies, the breast with the most severe diagnosis was selected for analysis. If more than one mammogram was available, the mammogram taken closest in time prior to the breast biopsy date was selected.
Mammographic density (global and lesional) was quantified as an absolute tissue volume (cm3) and percent tissue volume using single X-ray absorptiometry (SXA), as described previously [8, 9]. An SXA breast density phantom was affixed to the top of the compression paddle and included in the X-ray field during mammography examinations. Mammographic grayscale values were compared to the values of the SXA phantom with a known fibroglandular tissue volume (FGV) composition and thickness. In this way, volumetric measures were achieved using a planar image. Previous estimates of reproducibility for the SXA test phantoms demonstrated a repeatability standard deviation of 2 %, with a ±2 % accuracy for the entire thickness and density ranges [8]. Area-based mammographic density measures were also available as previously reported in this study population [7]. As volume and area measures were highly correlated [7], we limit our presentation of results to volumetric measures.
To compute localized density measures of the biopsied lesion, two radiologists (SDH, JMJ) recorded the biopsy location and radius of the biopsy target on the pre-biopsy standard digital mammogram (i.e., Digital Imaging and Communications in Medicine format). Absolute lesional volume (cm3) and percent lesional volume were estimated using SXA within the biopsy target, centered at the biopsy site [9]. A repeat set of 25 images was assessed for reliability. The intraclass correlation coefficients (ICCs) for percent lesional volume, absolute lesional volume, and total lesional volume were 0.99, 0.50, and 0.44 respectively, indicating fair to excellent reproducibility. Distributions of density measures were examined and images with extreme values were reviewed visually for validation. The American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS, Fourth Edition) breast density assessment (reported on the same images used for quantitative analysis) was analyzed as (I) almost entirely fat; (II) scattered fibroglandular densities; (III) heterogeneously dense; and (IV) extremely dense [10].
Pathologic diagnosis assessment
Pathology reports from the breast biopsy and surgical excision were reviewed to assign final pathologic diagnoses. We classified the following diagnoses as non-cases: non-proliferative benign breast disease, proliferative (ductal hyperplasia; sclerosing adenosis), and proliferative with atypia (atypical ductal or lobular hyperplasia) (n = 174). Cases included diagnoses of ductal or lobular carcinoma in situ (n = 36) and invasive cancer (n = 8).
As part of the clinical workup, tumor blocks from in situ and invasive cancer were sectioned and stained for estrogen receptor (ER) and progesterone receptor (PR). We obtained these slides and one observer (RLC) rescored all sections microscopically. The percentage of stained cells (range: 0–100 %) and intensity (0 = negative, 1 = weak, 2 = moderate, 3 = strong) were recorded and an overall score was calculated as the product of these components (range: 0–300). We dichotomized expression as negative (<10) or positive (≥10). When information on these stains was missing, we obtained tumor blocks and performed immunohistochemistry according to routine protocols, also scored by the same microscopist (RLC).
CD31 immunohistochemical staining and MVD assessment
Paraffin-embedded whole section slides were deparaffinized and antigen retrieval performed with citrate buffer pH6 using a pressure cooker. Endogenous activity was blocked with 3 % peroxidase and primary antibody CD31 (Clone JC70A, DAKO, Glostrup, Denmark) was applied for 2 hours at room temperature. Subsequently, antibody was detected using DAKO Env + labeling system and visualized with 3,3′-diaminobenzadine (DAB) for 20 min. After rinsing, slides were counterstained with modified Harris hematoxylin, dehydrated with graded alcohol, cleared with xylene and a coverslip applied.
We performed a pilot study including 20 participants to determine the within-woman variability of MVD on whole section slides, based on counting vessels within three regions, each consisting of ten × 400 high-power fields, randomly selected from the (1) top, (2) center, and (3) bottom portions of the slide. When multiple tissue fragments existed, one randomly selected region from each of the three largest fragments was selected for MVD counting. Coefficient of variations (CVs) and ICCs were similar based on analyzing 30 or 15 fields; accordingly, in the full study, 15 MVD scores per slide were assessed by a pathologist (PL) masked to biopsy diagnosis (see Fig. 1). We analyzed the average MVD score per woman.
Fig. 1.
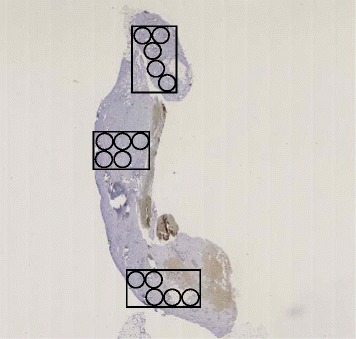
Assessment of microvessel density (MVD) in CD31-stained whole section slides. Three random regions from the top, central, and bottom portions of the whole section slide were selected. Within each region, five adjacent, non-overlapping × 400 high-power fields were assessed for the number of CD31-staining microvessels
A masked independent review of 20 randomly selected slides after study completion by two pathologists (PL, MES) demonstrated an intra-rater agreement (ICC) of 0.88 and inter-rater agreement (ICC) of 0.82.
Analytic population
Of 465 participants enrolled, 12 were not subsequently biopsied and two went straight to surgery without a biopsy and were excluded. We excluded women who underwent ultrasound-guided core (n = 225) and MR-guided vacuum-assisted biopsies (n = 1). Ultrasound-guided biopsies were generally used to sample mass lesions, providing less non-lesional tissue for MVD analysis than vacuum-assisted breast biopsies, and were therefore excluded. We further excluded participants with invalid SXA volumetric MD measurements (n = 5) and insufficient tumor tissue on slide (n = 2), resulting in a final sample of 218 women.
Statistical analysis
Relationships between baseline characteristics according to final pathologic diagnosis (in situ and invasive cancer versus benign diagnoses) were examined using chi-square or Fisher’s exact tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Spearman rank correlation coefficients were computed to estimate correlations of MVD with age at biopsy, body mass index (BMI), and MD measures overall and by final pathologic diagnosis. Based on a Box-Cox transformation analysis, we used a power of 0.2 to improve normality of the average MVD score. Age- and BMI-adjusted linear regression models were used to examine relationships between MVD with participant characteristics and MD measures overall and by case status. We tested for differences by case status by including a multiplicative term between participant characteristics and MD measures with a binary indicator for case status and evaluating the Wald test p value. Age at biopsy (39–44, 45–49, 50–54, 55–59, 60–65 years) and BMI (<25, 25– < 30, 30+ kg/m2) were coded as categorical variables. Other breast cancer risk factors were not significantly associated with MVD, and were therefore not included as potential confounders. We also examined the relationship between MVD and MD measures stratified by menopausal status based on previous findings demonstrating a decline in BPE after menopause in some women [11].
Logistic regression models were used to estimate odds ratios (ORs) and 95 % confidence intervals (CIs) for associations between MVD (the average of MVD within a woman was standardized by one standard deviation) and final pathologic diagnosis (benign versus in situ/invasive) adjusted for age at biopsy and BMI. Subsequent models were adjusted for individual MD measures. We also evaluated whether a “gradient” of MVD and lesion severity existed using polytomous logistic regression with the following outcome categories: (1) benign, carcinoma in situ (ductal or lobular), and invasive cancer and, in a second model, (2) benign, proliferative, proliferative with atypia, carcinoma in situ (ductal or lobular) and invasive cancer. We also examined the association between MVD and final pathologic diagnosis stratified by MD. In these analyses, each MD measure was dichotomized at the median based on the distribution among the non-cases. We formally tested whether the ORs in each MD stratum were significantly different by including a multiplicative term between the dichotomous MD variable and MVD and evaluating the Wald test p value. We used SAS (version 9.3, SAS Institute, Inc., Cary, NC, USA) software for all analyses and two-sided p values less than 0.05 were considered statistically significant.
Results
Participant characteristics
Among the non-cases, 50 had benign diagnoses, 99 had proliferative diagnoses, and 25 had proliferative disease with atypia (Table 1). Cases reported past menopausal hormone therapy use (40.9 % vs. 20.1 %) and current use of oral contraceptives (9.1 % vs. 1.7 %) more frequently than non-cases. Global volumetric density did not significantly differ between cases and non-cases; however, lesional non-dense volumes were significantly higher in cases compared with non-cases. In univariate analysis, MVD in cases and non-cases was not significantly different (2.5 vs. 2.2, Kruskal-Wallis p value = 0.13).
Table 1.
Selected characteristics of women referred to an image-guided vacuum-assisted breast biopsy in The BREAST Stamp Project by breast biopsy diagnosis (n = 218)
| Characteristics | Non-cases (n = 174) | Cases (n = 44) | p a | ||
|---|---|---|---|---|---|
| Median age at biopsy (range) | 52 (40–65) | 52 (40–64) | 0.25 | ||
| n | %b | n | %b | ||
| Age at biopsy (years) | 0.11 | ||||
| 39 to 44 | 25 | 14.4 | 6 | 13.6 | |
| 45 to 49 | 46 | 26.4 | 5 | 11.4 | |
| 50 to 54 | 51 | 29.3 | 13 | 29.5 | |
| 55 to 59 | 26 | 14.9 | 13 | 29.5 | |
| 60 to 65 | 26 | 14.9 | 7 | 15.9 | |
| Race | 0.99 | ||||
| White | 159 | 91.4 | 41 | 93.2 | |
| Non-white | 15 | 8.6 | 3 | 6.8 | |
| Education | 0.53 | ||||
| < High school or high school graduate | 26 | 15.1 | 5 | 11.4 | |
| College/graduate school degree | 146 | 84.9 | 39 | 88.6 | |
| Cigarette smoking (≥100) | 0.24 | ||||
| Never | 83 | 48.5 | 17 | 38.6 | |
| Ever | 88 | 51.5 | 27 | 61.4 | |
| BMI (kg/m2) | 0.49 | ||||
| < 25 | 92 | 52.9 | 24 | 54.5 | |
| 25–<30 | 45 | 25.9 | 8 | 18.2 | |
| ≥ 30 | 37 | 21.3 | 12 | 27.3 | |
| Age at menarche | 0.79 | ||||
| ≤ 12 | 65 | 37.8 | 18 | 40.9 | |
| 13 | 63 | 36.6 | 17 | 38.6 | |
| ≥ 14 | 44 | 25.6 | 9 | 20.5 | |
| Oral contraceptives | 0.02 | ||||
| Never | 21 | 12.2 | 8 | 18.2 | |
| Former | 148 | 86.1 | 32 | 72.7 | |
| Current | 3 | 1.7 | 4 | 9.1 | |
| Parity | 0.17 | ||||
| Nulliparous | 46 | 26.4 | 6 | 13.6 | |
| 1 | 23 | 13.2 | 10 | 22.7 | |
| 2 | 68 | 39.1 | 20 | 45.5 | |
| ≥ 3 | 37 | 21.3 | 8 | 18.2 | |
| Age at first birth (years) | 0.62 | ||||
| < 30 | 91 | 52.6 | 25 | 56.8 | |
| Nulliparous or ≥ 30 | 82 | 47.4 | 19 | 43.2 | |
| Menopausal status | 0.24 | ||||
| Premenopausal | 100 | 57.5 | 21 | 47.7 | |
| Postmenopausal | 74 | 42.5 | 23 | 52.3 | |
| Age at menopause (years) | 0.59 | ||||
| Premenopausal | 100 | 57.5 | 21 | 47.7 | |
| < 45 | 12 | 6.9 | 4 | 9.1 | |
| 45–49 | 17 | 9.8 | 3 | 6.8 | |
| ≥ 50 | 32 | 18.4 | 12 | 27.3 | |
| Postmenopausal, age unknown | 13 | 7.5 | 4 | 9.1 | |
| Menopausal hormone therapy | 0.04 | ||||
| Never | 121 | 69.5 | 23 | 52.3 | |
| Former | 35 | 20.1 | 18 | 40.9 | |
| Current | 10 | 5.7 | 2 | 4.5 | |
| Missing/unknown | 8 | 4.6 | 1 | 2.3 | |
| Family history of breast cancer | 0.83 | ||||
| No | 131 | 75.7 | 34 | 77.3 | |
| Yes | 42 | 24.3 | 10 | 22.7 | |
| Lump at the time of mammography | 0.47 | ||||
| No | 159 | 91.4 | 43 | 97.7 | |
| Yes | 8 | 4.6 | 1 | 2.3 | |
| Missing/unknown | 7 | 4.0 | 0 | 0.0 | |
| Reason for mammography | 0.91 | ||||
| Screening | 156 | 89.7 | 41 | 93.2 | |
| Short-interval follow-up | 10 | 5.7 | 2 | 4.5 | |
| Evaluation of breast problem | 8 | 4.6 | 1 | 2.3 | |
| BI-RADS breast density | 0.74 | ||||
| I (entirely fat) | 20 | 11.5 | 3 | 6.8 | |
| II (scattered densities) | 78 | 44.8 | 20 | 45.5 | |
| III (heterogeneously dense) | 51 | 29.3 | 17 | 38.6 | |
| IV (extremely dense) | 14 | 8.0 | 2 | 4.5 | |
| Missing/unknown | 11 | 6.3 | 2 | 4.5 | |
| Final BI-RADS mammography assessment | 0.03 | ||||
| 3 (probably benign finding) | 3 | 1.7 | 1 | 2.3 | |
| 4 (suspicious abnormality) | 169 | 97.1 | 39 | 88.6 | |
| 5 (highly suggestive of malignancy) | 1 | 0.6 | 3 | 6.8 | |
| Biopsy laterality | 0.64 | ||||
| Left | 82 | 47.1 | 24 | 54.5 | |
| Right | 89 | 51.1 | 20 | 45.5 | |
| Bilateral | 3 | 1.7 | 0 | 0.0 | |
| Pathologic diagnosisc | |||||
| Benign | 50 | 28.7 | -- | -- | |
| Proliferative | 99 | 56.9 | -- | -- | |
| Proliferative with atypia | 25 | 14.4 | -- | -- | |
| In situ | -- | -- | 36 | 81.8 | |
| Invasive carcinoma | -- | -- | 8 | 18.2 | |
| Lesion size | 0.24 | ||||
| < 1 cm | 93 | 54.1 | 19 | 44.2 | |
| ≥ 1 cm | 79 | 45.9 | 24 | 55.8 | |
| Volumetric mammographic density measures | Median | Range | Median | Range | |
| Global | |||||
| % density (volume) | 36.0 | 0.6–99.3 | 35.2 | 10.0–97.3 | 0.59 |
| Dense volume (cm3) | 178.6 | 6.7–497.8 | 177.0 | 80.3–683.5 | 0.70 |
| Non-dense volume (cm3) | 324.7 | 1.6–1977.0 | 433.0 | 2.2–1291.5 | 0.31 |
| Lesional | |||||
| % density (volume) | 42.4 | 0.0–100.0 | 44.5 | 6.1–100.0 | 0.86 |
| Dense volume (cm3) | 1.4 | 0.0–26.8 | 1.7 | 0.2–16.9 | 0.08 |
| Non-dense volume (cm3) | 1.4 | 0.0–26.3 | 2.8 | 0.0–37.1 | 0.03 |
| MVDd | 2.2 | 0.6–5.0 | 2.5 | 1.0–5.0 | 0.13 |
Variables with P values < 0.05 are presented in bold font
BREAST Breast Radiology Evaluation and Study of Tissues, BMI body mass index, BI-RADS Breast Imaging Reporting and Data System, MVD microvessel density
aChi-square test was used for categorical variables; where 25 % of cells had counts less than 5 we used Fisher’s exact test. Wilcoxon rank sum test was used for continuous variables
bWhen more than 3 % of data were missing for a covariate, missing values were included as a separate category. Otherwise, missing data were excluded from column percentages and chi-square or Fisher’s exact test
cBenign: normal lobules or ducts defined as sclerotic/atrophied; non-proliferative fibrocystic change; discrete entities. Proliferative: ductal hyperplasia; sclerosing adenosis. Proliferative with atypia: atypical ductal or lobular hyperplasia
dThe average of MVD within a woman was computed and standardized by one standard deviation
Relationships between MVD with participant characteristics and MD measures
Among non-cases, MVD was inversely correlated with age at biopsy (rho = -0.33, p < 0.0001) and BMI (rho = -0.28, p < 0.0002) (data not tabled). In multivariable linear regression models adjusted for age and BMI, MVD was inversely associated with age at biopsy and BMI among non-cases (Table 2). Among cases, MVD was higher among women with three or more live births (p = 0.04). We did not observe significant associations between MVD and other risk factors.
Table 2.
Age- and body mass index (BMI)-adjusted linear regression results for the association between participant characteristics and microvessel density (MVD), overall and by case status
| All women | Non-cases | Cases | |||||
|---|---|---|---|---|---|---|---|
| N = 218 | n = 174 | n = 44 | |||||
| Characteristics | βa | p | βa | p | βa | p | P heterogeneityb |
| Age (years) | 0.009 | 0.01 | 0.46 | 0.63 | |||
| 45 to 49 | -0.03 | -0.03 | 0.01 | ||||
| 50 to 54 | -0.07 | -0.07 | -0.07 | ||||
| 55 to 59 | -0.08 | -0.11 | -0.03 | ||||
| 60 to 65 | -0.10 | -0.09 | -0.12 | ||||
| BMI (kg/m2) | 0.09 | 0.02 | 0.87 | 0.24 | |||
| 25– < 30 | -0.009 | -0.01 | 0.02 | ||||
| ≥ 30 | -0.05 | -0.07 | 0.03 | ||||
| Age at menarche (years) | 0.53 | 0.77 | 0.49 | 0.68 | |||
| 13 | 0.02 | 0.01 | 0.05 | ||||
| ≥ 14 | 0.02 | 0.01 | 0.07 | ||||
| Oral contraceptive use | 0.81 | 0.68 | 0.62 | 0.44 | |||
| Ever | -0.01 | -0.01 | 0.03 | ||||
| Parity | 0.52 | 0.89 | 0.04 | 0.02 | |||
| 1 | -0.01 | -0.02 | 0.03 | ||||
| 2 | -0.01 | -0.01 | -0.01 | ||||
| ≥ 3 | 0.02 | -0.01 | 0.16 | ||||
| Age at first birth (years) | 0.33 | 0.29 | 0.98 | 0.83 | |||
| Nulliparous or ≥ 30 | 0.02 | 0.02 | 0.00 | ||||
| Menopausal status | 0.14 | 0.43 | 0.25 | 0.99 | |||
| Postmenopausal | -0.04 | -0.02 | -0.09 | ||||
| Age at menopause (years) | 0.08 | 0.11 | 0.68 | 0.92 | |||
| < 45 | -0.10 | -0.09 | -0.02 | ||||
| 45–49 | -0.003 | 0.02 | -0.11 | ||||
| ≥ 50 | -0.06 | -0.05 | 0.08 | ||||
| Menopausal hormone therapy | 0.73 | 0.66 | 0.52 | 0.75 | |||
| Ever | 0.01 | -0.01 | 0.03 | ||||
| Family history of breast cancer | 0.76 | 0.87 | 0.76 | 0.92 | |||
| Yes | -0.01 | -0.003 | -0.02 | ||||
Non-cases: non-proliferative benign breast disease, proliferative (ductal hyperplasia; sclerosing adenosis), proliferative with atypia (atypical ductal or lobular hyperplasia). Cases: ductal or lobular carcinoma in situ and invasive cancer.
aBased on linear regression with the Box-Cox-transformed average MVD (within a woman) as the outcomeand adjusted for age at biopsy (39–44, 45–49, 50–54, 55–59, 60–65 years) and BMI (<25, 25– < 30, 30+ kg/m2)
b P heterogeneity based on a Wald test in the regression model corresponding to an interaction term between case status and the corresponding variable
Age- and BMI-adjusted linear regression models were used to evaluate associations between MVD and the MD measures (Table 3). MVD was not significantly associated with global or lesional percent dense volume measures. Among all women and the non-cases, MVD tended to be inversely associated with global measures of both absolute dense and non-dense volume. In particular, among all women, we observed statistically significant inverse associations between MVD and non-dense volume (β = -0.00007, p = 0.04). Among non-cases, MVD was inversely related to absolute dense volume (β = -0.0003, p = 0.008) and non-dense volume (β = -0.00008, p = 0.01). We observed positive associations of MVD with non-dense lesional volumes among all women and among cases (p ≤ 0.04). Finally, when the association between MVD and MD measures was stratified by menopausal status, we did not observe significant deviations from these patterns (data not tabled).
Table 3.
Age- and body mass index (BMI)-adjusted linear regression results for the association between microvessel density (MVD) and mammographic density (MD) measures, overall and by case status
| All women | Non-cases | Cases | |||||
|---|---|---|---|---|---|---|---|
| N = 218 | n = 174 | n = 44 | |||||
| MD measure | βa | p | βa | p | βa | p | P heterogeneityb |
| Global | |||||||
| % density (volume) | 0.0004 | 0.35 | 0.0003 | 0.49 | 0.0005 | 0.70 | 0.67 |
| Dense volume (cm3) | -0.0001 | 0.11 | -0.0003 | 0.008 | -0.00004 | 0.84 | 0.43 |
| Non-dense volume (cm3) | -0.00007 | 0.04 | -0.00008 | 0.01 | 0.00001 | 0.89 | 0.31 |
| Lesional | |||||||
| % dense volume (cm3) | 0.0005 | 0.16 | 0.0006 | 0.16 | 0.0001 | 0.93 | 0.83 |
| Dense volume (cm3) | 0.004 | 0.08 | 0.002 | 0.37 | 0.01 | 0.15 | 0.22 |
| Non-dense volume (cm3) | 0.004 | 0.03 | 0.0003 | 0.90 | 0.006 | 0.04 | 0.09 |
Beta coefficients with P values < 0.05 are presented in bold font. Non-cases: non-proliferative benign breast disease, proliferative (ductal hyperplasia; sclerosing adenosis), proliferative with atypia (atypical ductal or lobular hyperplasia). Cases: ductal or lobular carcinoma in situ and invasive cancer
aBased on linear regression with the Box-Cox-transformed, standardized average MVD (within a woman) as the outcome and adjusted for age at biopsy (39–44, 45–49, 50–54, 55–59, 60–65 years) and BMI (<25, 25– < 30, 30+ kg/m2)
b P heterogeneity based on a Wald test in the regression model corresponding to an interaction term between case status and the corresponding MD measure
Associations between MVD and breast cancer odds
In logistic regression models adjusted for age and BMI, we observed that a one standard deviation increase in MVD was associated with a significant increased OR of carcinoma in situ/invasive disease compared with non-cases (OR = 1.69, 95 % CI = 1.17–2.44) (Table 4). Compared with non-cases, MVD was positively associated with carcinoma in situ (OR = 1.36, 95 % CI = 0.91–2.04) and invasive cancer (OR = 4.86, 95 % CI = 1.91–12.38) (Table 4). In models that further parsed the biopsy diagnoses as benign, proliferative, proliferative with atypia, carcinoma in situ, and invasive cancer, we observed a significant increase in the proportional odds of more invasive disease associated with higher MVD (OR = 1.66, 95 % CI = 1.27 - 2.16) (Table 4). These relationships persisted after further adjustment for MD measures.
Table 4.
Logistic regression estimates for the association between microvessel density (MVD) and breast biopsy diagnosis
| Breast biopsy diagnosis | N | OR (95 % CI)a | P |
|---|---|---|---|
| Non-case | 174 | 1.00 | 0.005 |
| Case | 44 | 1.69 (1.17–2.44) | |
| Non-case | 174 | 1.00 | 0.002 |
| In situ carcinoma | 36 | 1.36 (0.91–2.04) | |
| Invasive | 8 | 4.86 (1.91–12.38) | |
| Benign | 50 | 1.00 | 0.002 |
| Proliferative | 99 | 1.56 (1.04–2.33) | |
| Proliferative with atypia | 25 | 1.47 (0.84–2.58) | |
| In situ carcinoma | 36 | 1.89 (1.13–3.15) | |
| Invasive | 8 | 6.81 (2.52–18.42) | |
| Ordinal odds ratio | 1.66 (1.27–2.16) | 0.002 |
The average of MVD within a woman was computed and standardized by one standard deviation. Non-cases: non-proliferative benign breast disease, proliferative (ductal hyperplasia; sclerosing adenosis), proliferative with atypia (atypical ductal or lobular hyperplasia). Cases: ductal or lobular carcinoma in situ and invasive cancer
aAdjusted for age at biopsy (39–44, 45–49, 50–54, 55–59, 60–65 years) and BMI (<25, 25– < 30, 30+ kg/m2)
To test our primary hypothesis of an interaction between MVD and MD, Table 5 presents associations between MVD and breast cancer odds stratified by MD measures. We observed increased breast cancer odds with increasing MVD among women classified as low (i.e., below the median) percent density, which was consistent across MD measures. For example, among women with low global or low lesional percent dense volume, higher MVD was associated with higher breast cancer odds (OR = 2.35, 95 % CI = 1.33–4.14) and (OR = 3.03, 95 % CI = 1.61–5.70), respectively. The effect estimates in the low percent density stratum significantly differed from the effects observed in the high (i.e., above the median) percent density stratum for lesional density (p heterogeneity = 0.03) but not global density (p heterogeneity = 0.11).
Table 5.
Logistic regression estimates for the association between microvessel density (MVD) and breast biopsy diagnosis stratified by mammographic density (MD) measures
| Density measure | Below median of mammographic density measure | Above median of mammographic density measure | |||||
|---|---|---|---|---|---|---|---|
| Cases | Non-cases | OR (95 % CI)a | Cases | Non-cases | OR (95 % CI)a | P heterogeneityb | |
| Volume | Effect of microvessel density (MVD)c on breast cancer risk | ||||||
| Global | |||||||
| % density (volume) | 23 | 87 | 2.35 (1.33–4.14) | 21 | 87 | 1.23 (0.73–2.07) | 0.11 |
| Dense volume (cm3) | 22 | 87 | 1.60 (0.84–3.05) | 22 | 87 | 1.78 (1.07–2.98) | 0.23 |
| Non-dense volume (cm3) | 21 | 87 | 1.14 (0.67–1.95) | 23 | 87 | 2.51 (1.41–4.46) | 0.08 |
| Lesional | |||||||
| % dense volume (cm3) | 21 | 88 | 3.03 (1.61–5.70) | 23 | 86 | 1.10 (0.69–1.79) | 0.03 |
| Dense volume (cm3) | 19 | 88 | 1.25 (0.67–2.32) | 25 | 86 | 1.90 (1.15–3.13) | 0.61 |
| Non-dense volume (cm3) | 15 | 87 | 0.97 (0.50–1.90) | 29 | 87 | 2.13 (1.30–3.48) | 0.06 |
Each MD measure was dichotomized at the median based on the distribution among non-cases. ORs with P values < 0.05 are presented in bold font. Non-cases: non-proliferative benign breast disease, proliferative (ductal hyperplasia; sclerosing adenosis), proliferative with atypia (atypical ductal or lobular hyperplasia). Cases: ductal or lobular carcinoma in situ and invasive cancer
aAdjusted for age at biopsy (39–44, 45–49, 50–54, 55–59, 60–65 years) and BMI (<25, 25– < 30, 30+ kg/m2)
b P heterogeneity based on a Wald test in the regression model corresponding to an interaction term between the dichotomous MD measure and MVD
cThe average of MVD within a woman was computed and standardized by one standard deviation
Among women categorized as high (i.e., above the median) absolute dense volume, higher MVD was significantly associated with increased breast cancer odds. For example, among women with high global absolute dense volume, higher MVD was associated with a 1.78-fold increase in breast cancer odds (95 % CI = 1.07–2.98); however, this was not significantly different than the odds observed among women in the low global absolute dense volume category (OR = 1.60, 95 % CI = 0.84–3.05, p heterogeneity = 0.23). A similar pattern was noted for lesional absolute dense volume.
Regarding the non-dense volume measures, the association between MVD and breast cancer differed between women with lower versus higher amounts of non-dense volumes, such that higher breast cancer odds were observed among women with greater amounts of non-dense tissues. For example, higher MVD was associated with a 2.51 (95 % CI = 1.41–4.46) times higher odds of breast cancer among women in the high absolute non-dense volume stratum compared with a 1.14 (95 % CI = 0.67–1.95) times higher breast cancer odds among women in the low absolute non-dense volume stratum (p heterogeneity = 0.08). A similar pattern was noted for lesional non-dense volume.
Lastly, relationships between tumor characteristics and MVD were evaluated among the 44 cases (Additional file 1: Table S1). We did not observe significant differences by grade, histology, or hormone receptor positivity; however, this exploratory analysis was based on small numbers.
Discussion
Our results demonstrate that women with diagnostic vacuum-assisted breast biopsies and a pathology finding of in situ or invasive breast cancer are more likely than women with benign lesions to have higher tissue MVD. MVD was not associated with MD, although the relationship of higher MVD and in situ/invasive breast cancer was higher among women with increased total breast adipose content. These findings are consistent with data suggesting that women whose MRIs show higher BPE are more likely to have a prevalent breast cancer, and support further research on parenchymal patterns in breast imaging as markers of breast cancer risk.
MRI-assessed BPE is directly related to vascular supply and vessel permeability [4], which enables the detection of breast cancer secondary to increased blood flow and leaking of contrast from abnormally permeable cancer-associated vessels. Our findings are consistent with two retrospective breast MRI studies, in which higher levels of MRI-assessed BPE were associated with breast cancer independent of MRI-assessed fibroglandular tissue (i.e., density). In the study by King and colleagues [5], among 1275 women who underwent breast MRI screening, 39 women had a prevalent breast cancer. Increased BPE was strongly associated with elevated breast cancer odds after adjustment for fibroglandular tissue [5]. In the second MRI study to evaluate BPE level and breast cancer odds, 23 women with a breast cancer diagnosed a median of 2 years after the index MRI and 23 age- and BRCA1/2 mutation status-matched women who did not develop breast cancer were included. Compared with minimal BPE, breast cancer risk was nine times higher (95 % CI = 1.1–71.0) among those with mild, moderate, or marked BPE [6]. This latter study suggests that MRI-assessed BPE can be used to predict future breast cancer risk, broadening the clinical utility of this marker.
When we launched the BREAST Stamp Project in 2007, there was not sufficient scientific evidence to recommend supplemental MRI screening for women with dense breasts [12]. In our study, only two of 218 women (0.9 %) were recommended to have a supplemental MRI. As a result, we are unable to assess the relationship between MRI-assessed BPE and MVD in this study population. Although we did not capture MRI as part of the study protocol, increasingly, MRI is being considered as a supplemental screening tool among women with dense breasts [13], and our work may therefore be important for future studies relating breast tissue vascularity to radiologic measures of breast density.
In a cohort of approximately 2000 women who underwent a biopsy for a benign breast lesion, 24 women subsequently developed breast cancer (median time to diagnosis was not provided) [14]. For each case, one control subject who did not develop breast cancer in follow-up was randomly selected and matched by age at biopsy, year of biopsy, and follow-up time. Tissue from the initial biopsy lesion was assessed for MVD, and the authors reported a seven times higher breast cancer risk (95 % CI = 0.9–52.2) for women in the highest category of MVD compared with the lowest [14]. Other studies have evaluated MVD levels across a spectrum of breast histology in convenience samples, including benign tissue from patients undergoing reduction surgery, hyperplasia, in situ, and invasive breast cancers [15–18]. These studies have demonstrated a gradient of higher MVD with increasing lesion severity, which was also evident in our multivariable polytomous logistic regression analyses that separately considered the non-benign diagnoses.
We initially hypothesized that the combination of high MD and high MVD would be significantly associated with breast cancer compared with other combinations of these two features (e.g., high MD/low MVD). The underlying rationale for this hypothesis was two-fold: first, while higher mammographic breast density is strongly associated with breast cancer risk, many women with dense breasts do not subsequently develop breast cancer. Therefore, other biological mechanisms must work in concert with elevated density to increase breast cancer risk. Second, higher levels of BPE, a measure of vascular supply, are positively associated with breast cancer independent of fibroglandular content [5, 6]. We posited that dense breast tissue characterized by high vascularity would be metabolically “active” with a higher potential for malignant transformation compared with dense, avascular breast tissue. We indeed observed a higher odds of breast cancer associated with higher MVD within strata of high absolute density measures; however, these associations were not statistically different than the effects observed within strata of low absolute density measures.
We observed that the MVD-breast cancer association was stronger among women with greater amounts of non-dense tissue, as revealed in the analyses stratified by percent density and absolute non-dense volume. Regardless of the density measure (global or lesional), these analyses consistently demonstrated a significant positive OR for the relationship between MVD and breast cancer odds within strata of low percent density and within strata of high absolute non-dense volume. Reasons for this association are unclear. Non-dense breast tissue is primarily composed of adipose tissue, which is radiolucent and appears dark on a mammogram. In a recent meta-analysis of area density measure studies, absolute non-dense area was inversely associated with breast cancer risk among pre- and postmenopausal women, with slight attenuation after adjustment for absolute dense area, particularly among premenopausal women [19]. Conversely, some have suggested that higher amounts of breast adipose tissue are related to increased breast cancer risk [20, 21]. Adipose tissue secretes a number of factors including adipokines, pro-inflammatory molecules, chemokines, hormones, and growth factors [22] – when dysregulated, these factors have been shown to contribute to the development and progression of breast cancers [23–25]. We hypothesize that in a microenvironment characterized by dysregulated pathways, in this case angiogenesis, adipose tissue may facilitate the carcinogenic process. Although additional studies are needed, our results would suggest that MVD is a potentially important biomarker in identifying a high-risk segment of women that are traditionally perceived as having lower breast cancer risk based on their MD.
We did not detect clear relationships between MVD and the quantitative assessments of volumetric MD (global or lesional) in the overall study population or stratified by menopausal status. In accordance with our findings, several MRI reports also failed to observe an association between BPE and MD [26–28]. Our menopausal status-stratified analyses conflict with a previous study of 28 women with paired breast MRIs – one available while the woman was pre-menopausal and one following menopause – that demonstrated a decline in BPE after menopause among 61 % (17/28) of women [11]. The lack of association between MVD and MD measures in our study, coupled with the observation that the MVD-breast cancer association was not substantially attenuated when MD was included in the models, suggests that MVD and MD affect breast cancer through independent biological mechanisms. Further, apart from lesional non-dense volume, we did not observe significant differences in MD measures by pathologic diagnosis, which has been previously reported in this study population [7]. Although percent MD is a strong and established risk factor for breast cancer development, higher MD may not necessarily predict breast cancer risk among women referred for biopsy [29].
We are unaware of any study relating breast cancer risk factors to histologic markers of angiogenesis among women with benign breast disease. Our analysis revealed few associations: MVD was inversely associated with age and BMI among women without breast cancer. Interestingly, BPE appears to be hormonally regulated: premenopausal women have been reported to have higher BPE levels compared with postmenopausal women [11] whereas use of tamoxifen [30] or aromatase inhibitors [31] has been associated with declines in BPE levels in the contralateral unaffected breast. Although we did not observe a statistically significant association between MVD and menopausal status, the age range of our study population was limited, which may have affected our ability to detect an association. Furthermore, we did not observe significant relationships between MVD and other tumor characteristics, probably due to low numbers of cases.
Strengths of our study include the quantitative, reliable global density measures that have been validated with respect to breast cancer risk factors [7] and risk [32, 33] and analysis of biopsies prior to surgical intervention, which can induce granulation tissue and neovascularity. Limitations of our study include the potential for misclassification of MVD. Although robust methods for MVD assessment in breast tumors exist [34], methods for benign breast tissues have not been well validated. Therefore, we undertook a rigorous and agnostic approach to score MVD in all tissue sections, masked to diagnosis. Importantly, we did not observe large differences in MVD between randomly selected regions on the whole section slides within a woman/biopsy, nor did we note differences when the number of high-power fields reviewed per tissue section was reduced. Moreover, the high intra-rater and inter-rater agreement we observed suggests our MVD assessment method was reproducible. Like many studies of MD, our study population consists of mostly white and well-educated women, and the extent to which these results apply to the general population is unknown. Further, our study population consists of women referred for an image-guided breast biopsy – identifying biomarkers among women referred to biopsy is important and future studies in larger, more diverse populations may be warranted to determine the utility of this biomarker.
In summary, our histopathologic analysis suggests that tissue vascularity, as reflected by MVD, is associated with breast cancer independently of MD, and the effect may be stronger among women with lower breast density. Although women with low MD tend to have lower breast cancer risk, these women account for a high percentage of breast cancers overall. Our results add to prior findings suggesting that the features of benign parenchyma, assessed radiologically or histologically, may be related to breast cancer. Specifically, prior analyses have found that women with benign breast disease who have more associated terminal duct lobular units (TDLUs) experience increased breast cancer risk [35, 36], independent of MD [37], although the two are correlated [9, 38]. In future studies, it may be possible to integrate MD, MVD (assessed histologically or radiologically) with histologic assessment of TDLU involution to assess future breast cancer risk, particularly following a biopsy diagnosis of benign breast disease.
Conclusions
Microvessel density was positively associated with breast cancer odds in our study, independent of mammographic density, an established breast cancer risk factor. Further, the association between microvessel density and higher breast cancer odds was stronger among women with lower mammographic breast density, which account for a high percentage of breast cancers overall. These results need to be replicated in larger studies, particularly in prospective studies with mammograms and breast biopsy material available years prior to the cancer diagnosis. This would provide evidence that increased vascularity is related to cancer development, rather than a finding that manifests after cancer occurs.
Acknowledgements
The authors are indebted to the participants in the BREAST Stamp Project for their outstanding cooperation and to the physicians, pathologists, nurses, technologists, and interviewers for their efforts in the field. The authors thank Clair Bove, Patricia Lutton, Ellen Young, Aileen Burke, Laura Linville, and Daphne Papathomas for research assistance. We also thank Janet Lawler-Heaver and Kerry Grace Morrissey from Westat for study management support and Jane Demuth at Information Management Services for data support and analysis.
Authors’ contributions
BG, PMV, DLW, REC, MES, LAB, and GLG conceived and designed the study and collected participant data. JS, APM, JW, BF, SM, SDH, and JMJ contributed to the mammographic density measures. JM and SMH conducted immunohistochemical analysis of the tissue samples. PL, RLC, and MES interpreted immunohistochemical stains. ASF, RMP, DAP, and GLG performed statistical analysis and all co-authors interpreted the data. ASF, PL, RMP, MES, and GLG drafted the manuscript. All authors contributed with critical revision, editing of the final version of the manuscript, approved the final version for publication, and agree to be accountable for the accuracy and integrity of the work.
Competing interests
Sally Herschorn is a stockholder in Hologic, which may represent a potential competing interest. There are no other competing interests.
Ethics approval and consent to participate
All participants provided written informed consent, and the study was approved by Institutional Review Boards at the University of Vermont and the NCI.
Abbreviations
- BI-RADS
Breast Imaging Reporting and Data System
- BMI
Body Mass Index
- BPE
Background Parenchymal Enhancement
- BREAST
Breast Radiology Evaluation and Study of Tissues
- CI
Confidence Interval
- CV
Coefficient of Variation
- DAB
Diaminobenzadine
- ER
Estrogen Receptor
- ICC
Intraclass Correlation Coefficients
- MD
Mammographic Density
- MRI
Magnetic Resonance Imaging
- MVD
Microvessel Density
- NCI
National Cancer Institute
- OR
Odds Ratio
- PR
Progesterone Receptor
- SXA
Single X-ray Absorptiometry
- TDLU
Terminal Duct Lobular Unit
Additional file
Association between microvessel density (MVD) and tumor characteristics among breast cancer cases (n = 44). (DOC 54 kb)
Contributor Information
Ashley S. Felix, Phone: 614-688-1477, Email: Felix.20@osu.edu
Petra Lenz, Email: plenz@mail.nih.gov.
Ruth M. Pfeiffer, Email: pfeiffer@mail.nih.gov
Stephen M. Hewitt, Email: hewitts@mail.nih.gov
Jennifer Morris, Email: R_JMorris712@aol.com.
Deesha A. Patel, Email: Deesha84@gmail.com
Berta Geller, Email: berta.geller@uvm.edu.
Pamela M. Vacek, Email: pvacek@uvm.edu
Donald L. Weaver, Email: donald.weaver@uvm.edu
Rachael E. Chicoine, Email: rchicoin@uvm.edu
John Shepherd, Email: John.Shepherd@ucsf.edu.
Amir Pasha Mahmoudzadeh, Email: AmirPasha.Mahmoudzadeh@ucsf.edu.
Jeff Wang, Email: jeff.wing.wang.walla.walla@gmail.com.
Bo Fan, Email: bo.fan@ucsf.edu.
Serghei Malkov, Email: Serghei.Malkov@ucsf.edu.
Sally D. Herschorn, Email: sally.herschorn@uvmhealth.org
Jason M. Johnson, Email: jjohnson12@mdanderson.org
Renata L. Cora, Email: RLCora@optonline.net
Louise A. Brinton, Email: brintonl@exchange.nih.gov
Mark E. Sherman, Email: shermanm@exchange.nih.gov
Gretchen L. Gierach, Email: gierachg@mail.nih.gov
References
- 1.McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 2.Reinier KS, Vacek PM, Geller BM. Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and post-menopausal women. Breast Cancer Res Treat. 2007;103(3):343–8. doi: 10.1007/s10549-006-9375-9. [DOI] [PubMed] [Google Scholar]
- 3.Winkler NS, Raza S, Mackesy M, Birdwell RL. Breast density: clinical implications and assessment methods. Radiographics. 2015;35(2):316–24. doi: 10.1148/rg.352140134. [DOI] [PubMed] [Google Scholar]
- 4.Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol. 2011;196(1):218–24. doi: 10.2214/AJR.10.4550. [DOI] [PubMed] [Google Scholar]
- 5.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011;260(1):50–60. doi: 10.1148/radiol.11102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dontchos BN, Rahbar H, Partridge SC, Korde LA, Lam DL, Scheel JR, Peacock S, Lehman CD. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology. 2015;276(2):371–80. doi: 10.1148/radiol.2015142304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gierach GL, Geller BM, Shepherd JA, Patel DA, Vacek PM, Weaver DL, Chicoine RE, Pfeiffer RM, Fan B, Mahmoudzadeh AP, et al. Comparison of mammographic density assessed as volumes and areas among women undergoing diagnostic image-guided breast biopsy. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2338–48. doi: 10.1158/1055-9965.EPI-14-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malkov S, Wang J, Kerlikowske K, Cummings SR, Shepherd JA. Single x-ray absorptiometry method for the quantitative mammographic measure of fibroglandular tissue volume. Med Phys. 2009;36(12):5525–36. doi: 10.1118/1.3253972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gierach GL, Patel DA, Pfeiffer RM, Figueroa JD, Linville L, Papathomas D, Johnson JM, Chicoine RE, Herschorn SD, Shepherd JA, et al. Relationship of terminal duct lobular unit involution of the breast with area and volume mammographic densities. Cancer Prev Res. 2016;9(2):149–58. doi: 10.1158/1940-6207.CAPR-15-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Radiology (ACR) Breast imaging reporting and data system (BI-RADS) 4. Reston, VA: American College of Radiology; 2003. [DOI] [PubMed] [Google Scholar]
- 11.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol. 2012;22(12):2641–7. doi: 10.1007/s00330-012-2553-8. [DOI] [PubMed] [Google Scholar]
- 12.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 13.Berg WA. Current status of supplemental screening in dense breasts. J Clin Oncol. 2016. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 14.Guinebretiere JM, Le Monique G, Gavoille A, Bahi J, Contesso G. Angiogenesis and risk of breast cancer in women with fibrocystic disease. J Natl Cancer Inst. 1994;86(8):635–6. doi: 10.1093/jnci/86.8.635. [DOI] [PubMed] [Google Scholar]
- 15.Bluff JE, Menakuru SR, Cross SS, Higham SE, Balasubramanian SP, Brown NJ, Reed MW, Staton CA. Angiogenesis is associated with the onset of hyperplasia in human ductal breast disease. Br J Cancer. 2009;101(4):666–72. doi: 10.1038/sj.bjc.6605196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter PM, Chen WP, Mendez A, McLaren CE, Su MY. Angiogenesis in the progression of breast ductal proliferations. Int J Surg Pathol. 2011;19(3):335–41. doi: 10.1177/1066896909333511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlakis K, Messini I, Vrekoussis T, Yiannou P, Keramopoullos D, Louvrou N, Liakakos T, Stathopoulos EN. The assessment of angiogenesis and fibroblastic stromagenesis in hyperplastic and pre-invasive breast lesions. BMC Cancer. 2008;8:88. doi: 10.1186/1471-2407-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viacava P, Naccarato AG, Bocci G, Fanelli G, Aretini P, Lonobile A, Evangelista G, Montruccoli G, Bevilacqua G. Angiogenesis and VEGF expression in pre-invasive lesions of the human breast. J Pathol. 2004;204(2):140–6. doi: 10.1002/path.1626. [DOI] [PubMed] [Google Scholar]
- 19.Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, Vachon C, Bakker MF, Giles GG, Chia KS, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106(5). doi:10.1093/jnci/dju078. [DOI] [PMC free article] [PubMed]
- 20.Beer AE, Billingham RE. Adipose tissue, a neglected factor in aetiology of breast cancer? Lancet. 1978;2(8084):296. doi: 10.1016/S0140-6736(78)91694-X. [DOI] [PubMed] [Google Scholar]
- 21.Lokate M, Peeters PH, Peelen LM, Haars G, Veldhuis WB, van Gils CH. Mammographic density and breast cancer risk: the role of the fat surrounding the fibroglandular tissue. Breast Cancer Res. 2011;13(5):R103. doi: 10.1186/bcr3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol. 2011;55(7-9):851–9. doi: 10.1387/ijdb.113365jt. [DOI] [PubMed] [Google Scholar]
- 24.Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324(2):142–51. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev. 2010;19:45–52. doi: 10.1159/000316896. [DOI] [PubMed] [Google Scholar]
- 26.Cubuk R, Tasali N, Narin B, Keskiner F, Celik L, Guney S. Correlation between breast density in mammography and background enhancement in MR mammography. Radiol Med. 2010;115(3):434–41. doi: 10.1007/s11547-010-0513-4. [DOI] [PubMed] [Google Scholar]
- 27.Ko ES, Lee BH, Choi HY, Kim RB, Noh WC. Background enhancement in breast MR: correlation with breast density in mammography and background echotexture in ultrasound. Eur J Radiol. 2011;80(3):719–23. doi: 10.1016/j.ejrad.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Hansen NL, Kuhl CK, Barabasch A, Strobel K, Schrading S. Does MRI breast “density” (degree of background enhancement) correlate with mammographic breast density? J Magn Reson Imaging. 2014;40(2):483–9. doi: 10.1002/jmri.24495. [DOI] [PubMed] [Google Scholar]
- 29.Weaver DL, Vacek PM, Skelly JM, Geller BM. Predicting biopsy outcome after mammography: what is the likelihood the patient has invasive or in situ breast cancer? Ann Surg Oncol. 2005;12(8):660–73. doi: 10.1245/ASO.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 30.King V, Kaplan J, Pike MC, Liberman L, David Dershaw D, Lee CH, Brooks JD, Morris EA. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J. 2012;18(6):527–34. doi: 10.1111/tbj.12002. [DOI] [PubMed] [Google Scholar]
- 31.King V, Goldfarb SB, Brooks JD, Sung JS, Nulsen BF, Jozefara JE, Pike MC, Dickler MN, Morris EA. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology. 2012;264(3):670–8. doi: 10.1148/radiol.12112669. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, Malkov S, Vittinghoff E, Cummings SR. Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1473–82. doi: 10.1158/1055-9965.EPI-10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eng A, Gallant Z, Shepherd J, McCormack V, Li J, Dowsett M, Vinnicombe S, Allen S, dos-Santos-Silva I. Digital mammographic density and breast cancer risk: a case-control study of six alternative density assessment methods. Breast Cancer Res. 2014;16(5):439. doi: 10.1186/s13058-014-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 35.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98(22):1600–7. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 36.Baer HJ, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Tamimi RM. Lobule type and subsequent breast cancer risk: results from the Nurses’ Health Studies. Cancer. 2009;115(7):1404–11. doi: 10.1002/cncr.24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh K, Vachon CM, Pankratz VS, Vierkant RA, Anderson SS, Brandt KR, Visscher DW, Reynolds C, Frost MH, Hartmann LC. Independent association of lobular involution and mammographic breast density with breast cancer risk. J Natl Cancer Inst. 2010;102(22):1716–23. doi: 10.1093/jnci/djq414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS, et al. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28(13):2207–12. doi: 10.1200/JCO.2009.23.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]


