Summary
Introduction.
Pneumonia remains a common reason for hospitalizing infants and the elderly worldwide, and streptococcal infection is often responsible. The aim of this study was to assess the burden of pneumonia in a large general population.
Methods.
All pneumonia-related hospitalizations from 2004 to 2013 in north-east Italy were identified from the hospital records with a first-listed diagnosis on discharge of bacterial pneumonia, or a first-listed diagnosis on discharge of meningitis, septicemia or empyema associated with a secondary diagnosis of bacterial pneumonia. We identified major comorbidities, calculated agespecific case-fatality rates (CFR), and estimated the related cost to the health care system.
Results.
Of the 125,722 hospitalizations identified, 96.9% were cases of pneumonia, 2.4% of septicemia, 0.4% of meningitis, and 0.3% of empyema; 75.3% of hospitalizations involved ≥ 65-yearolds. The overall CFR was 12.4%, and it increased with age, peaking in people over 80 (19.6%).
The mean annual pneumonia-associated hospitalization rate was 204.6 per 100,000 population, and it peaked in 0- to 4-year-old children (325.6 per 100,000 in males, 288.9 per 100,000 in females), and adults over 65 (844.9 per 100,000 in males, 605.7 per 100,000 in females).
Hospitalization rates dropped over the years for the 0-4 year-olds, and rose for people over 80. The estimated overall annual cost of these pneumonia-related hospitalizations was approximately € 41 million.
Conclusions.
This study shows that the burden on resources for pneumonia-related hospitalization is an important public health issue. Prevention remains the most valuable tool for containing pneumonia, and vaccination strategies can help in the primary prevention of infection, possibly reducing the number of cases in all age groups.
Key words: Hospitalization, Epidemiology, PCV, Pneumonia, Streptococcus Pneumoniae, Vaccination
Introduction
Pneumonia remains a common reason for the hospitalization of infants and elderly adults [1-3]. The disease can be caused by a variety of micro-organisms, but the pathogen most often responsible for pneumonia is the bacterium Streptococcus pneumoniae, especially in industrialized countries [4, 5].
Pneumonia pathogens may be reported in different ways, depending on the capabilities of in-hospital laboratories, how studies on them are designed, and the season or region in which studies are conducted [6]. They can cause a spectrum of diseases of variable severity and, when the organism invades normally sterile sites such as the bloodstream and meninges, the resulting forms are classified as invasive pneumococcal disease (IPD) [1].
Pneumococcal infections in adults can be a cause of community-acquired pneumonia (CAP), a respiratory disease with a high prevalence in the general population, a marked clinical heterogeneity, and a variable severity [2]. CAP remains a leading cause of morbidity and about 30% of CAP patients require hospitalization.
The introduction of a 7-valent pneumococcal conjugate vaccine (PCV7) that targets seven serotypes (4, 6B, 9V, 14, 18C, 19F and 23F) as part of children's national immunization programs has led to a reduction in the hospital admission rates for all-cause pneumonia, particularly among children. A herd effect has also been reported in some countries [7-10].
The arrival on the market of more valent conjugate vaccines (10-valent and 13-valent) and the availability of 13-valent pneumococcal conjugate vaccine (PCV13) for adults, offers new opportunities to prevent the diseases caused by S. pneumoniae [11].
An immunization program based on a 7-valent pneumococcal conjugate vaccine (PCV7) was first introduced in the Veneto and Friuli Venezia Giulia regions of north-east Italy in the early 2000s as an optional vaccination only for infants at risk (at 2-3 and 14 months old). The program was subsequently extended to offer vaccination to all children in the Veneto and Friuli Venezia Giulia regions in 2005 and 2009, respectively. In 2010, PCV13 was adopted in both regions instead of the PCV7 vaccine, and a catch-up program was implemented for infants up to 36 months old.
The present study investigated the pneumonia potentially preventable by vaccination, considering hospitalization for pneumonia and for meningitis, septicemia and empyema associated with pneumonia. We estimated: i) the pneumonia-related hospitalization (PRH) rate; ii) the age-specific case-fatality rate (CFR); iii) the trends of the PRH rates by PCV7 or PCV13 vaccination period for each region; and iv) the economic cost of PRH.
Methods
SETTING AND DATA SOURCE
We conducted this retrospective study in the Veneto and Friuli Venezia Giulia regions, which have populations of 4.9 and 1.2 million, respectively. We analyzed the hospital discharge records (HDRs) of public and accredited private hospitals in the two regions from 1 January 2004 to 31 December 2013. HDRs include the following data: details of the hospital; patient's name, date of birth, gender, place of residence, and date of admission; surgical and other procedures; and date and type of discharge. Each HDR contained one primary diagnosis (or first-listed diagnosis) and up to five secondary diagnoses based on the diagnostic codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Generally speaking, the first-listed diagnosis concerns the main condition identified during the patient's hospital stay, while other diagnoses indicate associated or contributing conditions (comorbidities and/or complications).
INCLUSION AND EXCLUSION CRITERIA
The PRH rate was calculated considering all hospitalizations identified from the HDRs as having a first-listed diagnosis on discharge of bacterial pneumonia potentially preventable by vaccination, or a first-listed diagnosis on discharge of meningitis, septicemia, or empyema associated with a secondary diagnosis of bacterial pneumonia potentially preventable by vaccination. Table I shows the diagnostic codes used as inclusion criteria. Only HDRs concerning residents of the Veneto or Friuli Venezia Giulia regions were considered. Day hospital admissions were disregarded for the purpose of this analysis.
Tab. I.
Diagnostic codes used for hospital discharge record selection.
| Pneumonia-related hospitalization Discharge Diagnoses Group |
International Classification of Diseases 9 – Clinical Modification Codes | |
|---|---|---|
| First-listed discharge diagnosis | Another discharge diagnosis |
|
| Pneumococcal pneumonia [S. pneumoniae pneumonia] |
481 | |
| Unspecified pneumonia [pneumonia without a causative organism identified] |
485-487; 482.9 | |
| Meningitis | 321, 013.0, 003.21, 036.0, 036.1, 047, 047.0, 047.1, 047.8, 047.9, 049.1, 053.0, 054.72, 072.1, 091.81, 094.2, 098.82, 100.81, 112.83, 114.2, 115.01, 115.11, 115.91, 130.0, 320, 320.0, 320.1, 320.2, 320.3, 320.7, 320.81, 320.82, 320.89, 320.8, 320.9, 322, 322.0, 322.9 |
plus 481; 485-487; 482.9 |
| Septicemia | 038.1, 038.4, 003.1, 020.2, 022.3, 031.2, 036.2, 038, 038.0, 038.2, 038.3, 038.8, 038.9, 054.5, 790.7 |
plus 481; 485-487; 482.9 |
| Empyema | 510 |
plus 481; 485-487; 482.9 |
COMORBIDITY
We obtained information on comorbid conditions from all hospital diagnoses mentioned in the HDRs. The ICD- 9-CM diagnostic codes were selected on the basis of the current recommendations for pneumococcal vaccination in the Italian vaccination program [12] (see Table II). The presence of at least one of these comorbidity codes was used to classify patients by specific comorbidities.
Tab. II.
Diagnostic codes considered to identify comorbidities.
| Code description | International Classification of Diseases 9 – Clinical Modification Codes |
|---|---|
| Asplenia or severe dysfunction of the spleen | 282.5-282.6, 289.4-289.5; 759.0 |
| Chronic renal disease or nephrotic syndrome | 581, 582, 584, 585; |
| Immunodeficiency or immunosuppression | 0420-0449, 279 |
| Chronic heart disease | 393-398, 414, 416 |
| Chronic lung disease | 490-496 |
| Chronic liver disease | 571-573 |
| Diabetes mellitus | 250 |
| Malignant neoplasms | 140-209 |
PNEUMONIA-RELATED HOSPITALIZATION RATE
Based on the total number of hospital admissions concerning Veneto and Friuli Venezia Giulia residents in each year considered, annual hospitalization rates were calculated by dividing the annual number of hospitalizations by the population in the year considered, according to the Veneto Regional Authority's statistical office, and expressing the rates as hospitalizations per 100,000 population. For patients readmitted within 30 days, only the first hospital stay was considered when calculating the hospitalization rate. The length of hospital stays was calculated as the days elapsing between the dates of admission and discharge, and the mean hospital stay was calculated. The case-fatality rate (CFR) was calculated by dividing the number of in-hospital deaths by the number of patients hospitalized with a diagnosis of pneumonia-related diseases, expressed as a percentage. Only first hospital stays were considered.
TEMPORAL TRENDS OF THE PRH RATE BY VACCINATION PERIOD
In the Veneto, the vaccination coverage for 24-monthold infants was low (10-40%) up until 2005, then rapidly increased from 2006 to 2010 (70-80%), and has exceeded 80% since 2011. In Friuli Venezia Giulia, the coverage rate was low up until 2009, then reached 70-80% in 2009, and has exceeded 80% since 2010.
In classifying vaccination coverage at 24 months, we distinguished between: a "PCV7 period", when the coverage rate was < 40%; a "late PCV7 period" when it was 70-80%; and a "PCV13 period", when it exceeded 80% (Tab. III).
Tab. III.
Study period and vaccination coverage in children by region.
| Vaccine | Period | Vaccination coverage in children (24 months of age) |
Type of offer |
|
|---|---|---|---|---|
| Veneto | Friuli Venezia Giulia |
|||
| PCV7 | 2004-2005 | 2004-2008 | < 40% | limited to groups at risk |
| 2006-2010 | 2009-2010 | 70-80% | extended to all children |
|
| PCV13 | 2011-2013 | 2011-2013 | > 80% | |
Tab. IV.
Hospital admissions for pneumonia by patients' characteristics and age group (2004-2013).
| Variable | Total | Age groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0-4 | 5-14 | 15-64 | 65-79 | 80 + | ||||||||
| Number of subjects | 125,722 | 8,510 | 3,924 | 18,573 | 32,926 | 61,789 | ||||||
| Gender [n (%)] | ||||||||||||
| Males | 66,131 | (52.6) | 4,625 | (54.3) | 2,167 | (55.2) | 11,450 | (61.6) | 20,646 | (62.7) | 27,243 | (44.1) |
| Females | 59,591 | (47.4) | 3,885 | (45.7) | 1,757 | (44.8) | 7,123 | (38.4) | 12,280 | (37.3) | 34,546 | (55.9) |
| First-listed diagnosis [n (%)] | ||||||||||||
| Pneumonia | 121,823 | (96.9) | 8,319 | (97.8) | 3,838 | (97.8) | 17,600 | (94.8) | 31,677 | (96.2) | 60,389 | (97.7) |
| S. pneumoniae | 4,002 | (3.2) | 659 | (7.7) | 371 | (9.5) | 967 | (5.2) | 930 | (2.8) | 1075 | (1.7) |
| without a causative organism identified |
117,821 | (93.7) | 7,660 | (90.0) | 3,467 | (88.4) | 16,633 | (89.6) | 30,747 | (93.4) | 59,314 | (96.0) |
| Septicemia | 3,025 | (2.4) | 88 | (1.0) | 28 | (0.7) | 582 | (3.1) | 1015 | (3.1) | 1,312 | (2.1) |
| Meningitis | 539 | (0.4) | 50 | (0.6) | 16 | (0.4) | 248 | (1.3) | 177 | (0.5) | 48 | (0.1) |
| Empyema | 335 | (0.3) | 53 | (0.6) | 42 | (1.1) | 143 | (0.8) | 57 | (0.2) | 40 | (0.1) |
| Comorbidity [n (%)] | ||||||||||||
| Chronic lung disease | 13,965 | (11.1) | 307 | (3.6) | 115 | (2.9) | 1,255 | (6.8) | 4,994 | (15.2) | 7,294 | (11.8) |
| Diabetes mellitus | 13,574 | (10.8) | 1 | (0.0) | 7 | (0.2) | 1,494 | (8.0) | 5,186 | (15.8) | 6,886 | (11.1) |
| Chronic heart diseases | 10,990 | (8.7) | 10 | (0.1) | 1 | (0.0) | 454 | (2.4) | 3,319 | (10.1) | 7,206 | (11.7) |
| Malignant neoplasm | 7,380 | (5.9) | 19 | (0.2) | 26 | (0.7) | 1,088 | (5.9) | 3,154 | (9.6) | 3,093 | (5.0) |
| Chronic liver disease | 2,882 | (2.3) | 9 | (0.1) | 6 | (0.2) | 1,000 | (5.4) | 1,161 | (3.5) | 706 | (1.1) |
| Immunodeficiency | 135 | (0.1) | 16 | (0.2) | 10 | (0.3) | 62 | (0.3) | 36 | (0.1) | 11 | (0.0) |
| Asplenia | 93 | (0.1) | 33 | (0.4) | 31 | (0.8) | 17 | (0.1) | 6 | (0.0) | 6 | (0.0) |
| Chronic renal disease | 110 | (0.1) | 1 | (0.0) | 5 | (0.1) | 27 | (0.1) | 39 | (0.1) | 38 | (0.1) |
| Length of hospital stay [days (DS)] | 11.9 | (10.1) | 4.7 | (3.9) | 4.9 | (3.7) | 10.9 | (10.3) | 13.,0 | (10.2) | 12.9 | (10.2) |
Throughout the study period, the 23-valent pneumococcal polysaccharide vaccine (PPV23) was optionally offered to 65-year-olds, achieving a low coverage (< 50%) in both regions.
For each region (considering only first hospital admissions), the trends of the PRH rates were calculated for the three vaccination periods and correlated with the pneumococcal vaccination coverage in children.
ESTIMATED COST
The annual cost of the PCV13 vaccination campaign was calculated using the acquisition cost of the vaccine (€ 42.5 per dose; three doses for children and one dose for a one cohort strategy (in the elderly), the time per injection for immunization activities (physician, health visitor or nurse, records: 8.6 minutes [13]). To convert this time into a cost, we considered the hourly cost of each actor (€ 71/hour for a physician, € 18 for a health visitor). The vaccination coverage rate considered to estimate the overall cost was 90% of children and 50% of elderly people. The population considered was the resident population in 2014 (newborn and 65 year-olds).
The estimated cost to the health care system of PRHs was calculated using the diagnosis-related groups (DRGs) of hospitalized patients. The DRGs depend on the patients' ICD classification at the time of their discharge from hospital, their age and gender, and the consumption of resources during their hospital stay. According to the DRG-based reimbursement system, every hospitalized patient belongs to a group of diagnostically homogeneous cases. Patients within each category are therefore similar in clinical terms, and are expected to require the same level of hospital resources. As a result, patients in the same DRG are assigned the same reimbursement charges. All hospital stays were considered for patients readmitted within 30 days.
STATISTICAL ANALYSIS
The data were analyzed using the chi-square test, Student's t-test for unpaired data, and Pearson's test, and 95% confidence intervals were calculated, as appropriate. A p value < 0.05 was considered significant. Analyses were performed using the Statistical Package for the Social Sciences (SPSS 16.0; SPSS Inc., Chicago, IL, USA). Significant trends over the years considered were assessed as average annual percent changes (AAPC), a summary measure of the trend over a given fixed interval that is computed as a weighted average of the annual percent change (APC) emerging from the joinpoint model, using weights equating to the length of the APC interval. If an AAPC lies entirely within a single joinpoint segment, the AAPC is the same as the APC for that segment [14].
ETHICAL PRINCIPLES
The study was conducted on data routinely collected by the health services and linked to anonymized records that make it impossible to identify the individuals concerned. The data analysis was performed on anonymized aggregated data in the Local Health Authority registries that are recorded with the patient's consent and can be used in aggregate form for scientific studies without further authorization [15]. This study complies with the Declaration of Helsinki and with Italian privacy law (Decree n. 196/2003 on the protection of personal data).
Results
PNEUMONIA-RELATED HOSPITALIZATIONS
Overall, we identified 133,936 PRHs and 8,214 (6.1%) of them were disregarded because they concerned readmissions within 30 days. Of the 125,722 hospitalizations thus included in the analysis, 6.8% (8,510 hospitalizations) concerned children under five years of age, and 75.3% (94,715 hospitalizations) concerned adults over 65. The first diagnosis was pneumonia in 121,823 (96.9%) of cases, while it was pneumonia-related septicemia in 3,025 (2.4%), meningitis in 539 (0.4%) and empyema in 335 (0.3%).
The patients were male in 52.6% of cases (66,131 patients); the mean hospital stay was 11.9 ± 10.1 days, and did not vary between genders. The mean hospital stay increased significantly with age (p < 0.001), reaching about 13 days for patients aged 65 or more.
The characteristics of the sample are shown in Table I.
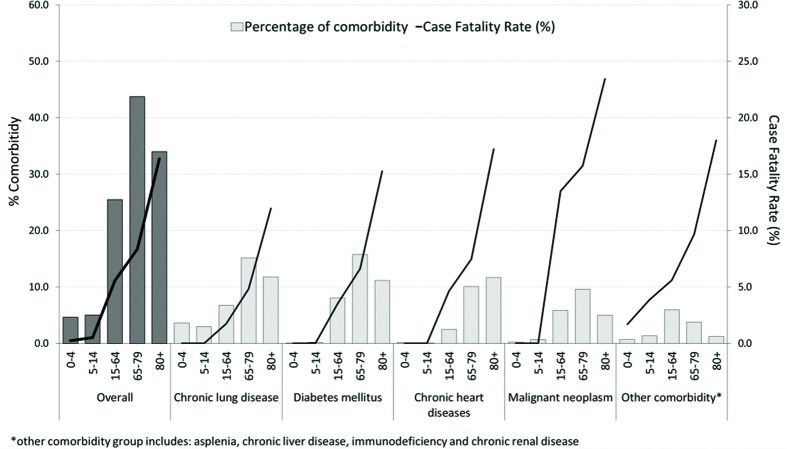
In the sample as a whole, 32.4% of patients had at least one chronic comorbidity and the number of comorbidities tended to increase with age (p < 0.001); their frequencies by principal type are shown in Figure 1. As for outcome, the overall CFR was 12.4% and remained stable during the study period. This rate increased with age, peaking in people over 80 years old (19.6%).
Fig. 1.
Percentage of comorbidity and case fatality rate by type of comorbidity and age group (2004-2013).
The overall annual PRH rate during the study period was 204.6 per 100,000 population (221.2 per 100,000 males and 188.8 per 100,000 females). Both genders showed two age-related peaks, one in children aged 0-4 years (325.6 per 100,000 in males, 288.9 per 100,000 in females), the other in adults over 65 years old (844.9 per 100,000 in males, 605.7 per 100,000 in females). The hospitalization rates for pneumonia were highest for the 0-4 and 80+ age groups, irrespective of the different types of vaccine used or their coverage. The rates for children up to 4 years of age were similar to those for 65- to 79-year-olds, at about 330 hospitalizations per 100,000 population a year. The PRH rate was stable during the study period, ranging from 200.9 per 100,000 in 2004 to 198.7 per 100,000 in 2013 [AAPC: 0.3%; (95% CI: -1.0; 1.6)]. The rate dropped significantly among the 0- to 4-year-olds, from 379.7 per 100,000 in 2004 to 211.9 per 100,000 in 2013 [AAPC: -4.6%; (95% CI: -6.5; -2.7)], while it rose significantly for adults aged 80 or more, from 1,370.3 per 100,000 in 2004 to 1,658.4 per 100,000 in 2013 [AAPC: 2.7%; (95% CI: 1.2; 4.3)] (Fig. 2).
Fig. 2.
Pneumonia-related hospitalization rate (per 100,000 population) by age group (2004-2013).
Figure 3 shows that the PRH rate was consistent with the timing of the vaccination programs by region. The PRH rate was 204.9 per 100,000 in the Veneto, and 203.3 per 100,000 in Friuli Venetia Giulia. In the Veneto, the PRH rate dropped for the 0- to 4-year-olds from the early PCV7 period (2004-2005) to the late PCV7 period (2006-2010), and to the PCV13 period (2011-2013): -9.3% and -29.0% respectively; p < 0.01. A significant inverse trend was seen for people over 80 years old, whose PRH rates rose by 9.9% and 25.2%, respectively, over the same periods. In Friuli Venezia Giulia, the figure was much the same for the 0- 4-year-olds, with reductions of 21% and 31.7% from the early to the late PCV7 period, to the PCV13 period (p < 0.01); while the PRH rate rose by 8.4% (p < 0.01) from the first to the last period among individuals aged 80 years or more (Fig. 3).
Fig. 3.
Average annual pneumonia-related hospitalization rates for children by vaccination coverage (CVC), age group and region
The overall estimated costs of the vaccination campaign for new-born and 65-year-olds was € 8.5 million (€ 6.4 million in children and € 2.1 million in the elderly). The purchase of PCV13 vaccine cost € 7.6 million (€ 5.9 for children and € 1.7 for the elderly).
The estimated overall annual cost of the PRHs was approximately € 41 million, with an estimated cost per patient of € 3,059. People over 65 accounted for 74.9% of the estimated annual overall pneumonia-related cost (€ 30.6 million in all, corresponding to € 22.9 per person over 65 in the general population).
Discussion
This retrospective observational study assessed the trends of pneumonia-related hospitalization in a population of 6.2 million, confirming that the burden on hospital resources is an important public health issue.
The PRH rate was stable on the whole, but dropped significantly for infants (0-4 years old), and this trend may be thanks to a greater vaccination coverage in this age group since 2008. Hospital admissions for pneumonia involving children aged 0-4 years dropped once the PCV7 coverage rate improved, and even more after the PCV13 vaccine was introduced. The same situation was confirmed in both regions, but Friuli Venezia Giulia had a lower hospitalization rate than the Veneto, probably due to differences in the two regions' admission modalities for this age group. Much the same picture has emerged in other areas [9, 10, 16].
The reduction in PRHs for infants seen in our sample was initially lower than the figures reported in previous observational studies [8, 17]. This may be due to a different diffusion of pneumococcal serotypes in our region, leading to a better coverage after the addition of more serotypes in the 13-valent conjugate vaccine (especially 19A, 3 and 1) [18].
The risk of pneumonia increases considerably with age and our analysis revealed a rising trend in the hospitalization rate for the very elderly (80 + years old). In contrast with our data, a similar retrospective study conducted in the US on hospital discharge records for the years 1997 to 2009 showed a substantial reduction in adult hospital admissions for pneumonia [7]. This difference could have several explanations. The magnitude of the indirect effects of vaccine-derived immunity depends on multiple influences relating to the transmissibility of the infection, the nature of the vaccine-induced immunity, the serotypes circulating, the patterns of mixing and transmitting infections in a given population, the type and schedule of vaccine used, the coverage achieved and, more importantly, the population's immunity. The heterogeneity of immunity and the complexity of populations make it difficult to draw comparisons [19, 20]. Active surveillance systems for monitoring invasive bacterial diseases show that serotypes not covered by the 7-valent pneumococcal conjugate vaccine (such as 19A) play a significant part in the burden of pneumonia-related disease [18], suggesting the likelihood of a reversal of the herd effect [21].
Our analysis confirms the mortality rate reported in other studies, with a CFR of 12%, and even higher in highrisk patients [22].
The introduction of PCV13 for infants in north-east Italy in 2010, and its subsequent use in people over 65 years old, can be expected to produce some benefits in years to come. A limitation of this retrospective study lies in that it is impossible to rule out external factors that might confound the apparent effect of vaccination on the agespecific rates of hospitalization for pneumonia, such as changes in patient management or the diagnostic coding of pneumonia. Any systematic shift in the management of pneumonia is unlikely to have coincided with the introduction of the pneumococcal vaccine, however [8].
A better understanding of the effects of higher-valence vaccines could improve the sensitivity of surveillance systems and add to our understanding of the potential invasiveness of serotypes not included in PCVs (the prevalence of their carriage could be compared with their prevalence in invasive pneumococcal disease). This is an aspect of surveillance that has hitherto been largely overlooked.
A large trial to ascertain the efficacy of PCV-13 in adults > 65 years of age was begun in the Netherlands in 2008. The main objective of this trial was to establish the efficacy of PCV-13 in the prevention of a first episode of vaccine-serotype specific CAP (vaccine efficacy 45.6%). The study also tested the efficacy of PCV-13 against first episodes of non-bacteremic vaccine-serotype-specific CAP and vaccine-serotype-specific invasive pneumococcal disease (IPD). The results indicated that PCV-13 was effective in preventing vaccine-serotype pneumococcal, bacteremic, and non-bacteremic CAP, and vaccine- serotype IPD, but not all-cause CAP [23].
It is not easy to estimate the morbidity of S. pneumoniae diseases due to variable levels of accuracy in reporting the cases diagnosed, and to differences in surveillance methods and microbiological methods, but HDRs can be useful for assessing the cases severe enough to warrant hospitalization [14].
In our sample, 96.9% of the patients with pneumonia were first-listed diagnoses, so their disease was probably community-acquired (since hospital-acquired infections are not normally recorded as a first diagnosis). Vaccination can only have an impact on CAP, not on hospitalacquired pneumonia, because of the different types of agent involved. S. pneumoniae is considered the most common pathogen responsible for pneumonia, accounting for about 24% of all cases [6, 24].
One of the difficulties in this setting concerns the availability of microbiological data and the dubious accuracy of the total number of hospital admissions identified as being related to S. pneumoniae (SP) due to coding errors in the hospital records. In our dataset, the number of SP-specific diagnoses was very low and varied by age group. The use of different diagnostic testing procedures may have influenced the pattern emerging between the diagnostic subgroups. This is the impression that emerges on comparing the percentage of pneumonia cases of unspecified etiology in our study (93.7%) with the 55% found in a recent study conducted in Australia, where cases hospitalized for CAP underwent a standardized, detailed assessment for bacterial and viral pathogens [8]. A review on the burden of CAP among adults in Europe showed that Italy had the lowest rate of identification of pneumococcal disease and recommended improvements in the diagnostic assays used to detect pneumococcal pneumonia with a view to enhancing the detection rate and generating a more accurate epidemiological picture [25].
It is important to ensure a good vaccination coverage among older people for both influenza and pneumococcal disease. In the latter period of our study, the annual influenza vaccination coverage in our elderly population dropped from about 75% in 2004 to about 60% in 2013, meaning that other measures are needed to prevent pneumonia in seniors [26].
Continued monitoring and longitudinal analyses are warranted to assess the longer-term effects of various contributing factors such as serotype replacement, and vaccination coverage, as well as catch-up programs. This will be particularly important as new pneumococcal conjugate vaccines offering additional serotype protection are licensed and included in immunization programs.
From the economic point of view, the implementation of PCV13 vaccination in the elderly is justified by the cost-effectiveness of this preventive measure. Our estimated costs are similar to those indicated in a model used in another study conducted in the same geographical area [27], presumably indicating that the benefits of vaccination could be extended to our context.
Conclusions
Prevention remains the most valuable tool to help reduce the burden of pneumonia, and vaccination strategies can be used for the primary prevention of infection and possibly to contain this disease in all age groups. The availability of new-generation pneumococcal conjugate vaccines with a broader antigenic spectrum and suitable for people of all ages suggests interesting new opportunities for improving the control of pneumococcal disease in the population as a whole.
Acknowledgements
The study was partially supported by a grant from University of Padua.
The authors have no competing interests to disclose.
References
- 1. World Health Organization. Immunization surveillance, assessment and monitoring. [Update 2014 Nov; cited 2015 Jul 28]. Available at: http://www.who.int/immunization/monitoring_surveillance/en/ [Accessed 30/09/2015].
- 2.Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet. 2006;368:1048–1050. doi: 10.1016/S0140-6736(06)69334-3. [DOI] [PubMed] [Google Scholar]
- 3.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–437. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 4.Bohte R, Furth R, Broek PJ. Aetiology of community- acquired pneumonia: a prospective study among adults requiring admission to hospital. Thorax. 1995;50:543–547. doi: 10.1136/thx.50.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Kleemola M, Koskela M, Leinonen M, Rönnberg PR, Saikku P, Stén M, et al. Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin Infect Dis. 2001;32:1141–1154. doi: 10.1086/319746. [DOI] [PubMed] [Google Scholar]
- 6.Woo JH, Kang JM, Kim YS, Shin WS, Ryu JH, Choi JH, Kim YR, Cheong HJ, Uh ST, Park CS, et al. A prospective multicenter study of communityacquired pneumonia in adults with emphasis on bacterial etiology. Korean J Infect Dis. 2001;33:1–7. [Google Scholar]
- 7.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–163. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J. 2010;29:607–612. doi: 10.1097/inf.0b013e3181d7d09c. [DOI] [PubMed] [Google Scholar]
- 9.Durando P, Crovari P, Ansaldi F, Sticchi L, Sticchi C, Turello V, Marensi L, Giacchino R, Timitilli A, Carloni R, et al. Collaborative Group for Pneumococcal Vaccination in Liguria, author. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine. 2009;27:3459–3462. doi: 10.1016/j.vaccine.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Baldo V, Cocchio S, Baldovin T, Buja A, Furlan P, Bertoncello C, Russo F, Saia M. A population-based study on the impact of hospitalization for pneumonia in different age groups. BMC Infectious Diseases. 2014;14:485–485. doi: 10.1186/1471-2334-14-485. doi:10.1186/1471- 2334-14-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres A, Bonanni P, Hryniewicz W, Moutschen M, Reinert RR, Welte T. Pneumococcal vaccination: what have we learnt so far and what can we expect in the future? Eur J Clin Microbiol Infect Dis. 2015;34:19–31. doi: 10.1007/s10096-014-2208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conferenza permanente per i rapporti tra lo stato le regioni e le province autonome di Trento e Bolzano. Piano Nazionale Prevenzione Vaccinale 2012-2014. Gazzetta Ufficiale della Repubblica Italiana, numero 60 del 12 marzo 2012 (supplemento ordinario n. 47).
- 13.Judith E. Glazner, Brenda Beaty, Stephen Berman. Cost of vaccine administration among pediatric practices. Pediatrics. 2009;124(Suppl 5):S492–S498. doi: 10.1542/peds.2009-1542H. [DOI] [PubMed] [Google Scholar]
- 14.Boehmer TK, Patnaik JL, Burnite SJ, Ghosh TS, Gershman K, Vogt RL. Use of hospital discharge data to evaluate notifiable disease reporting to Colorado's electronic disease reporting system. Public Health Rep. 2011;126:100–106. doi: 10.1177/003335491112600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garante per la protezione dei dati personali: Autorizzazione generale al trattamento dei dati personali effettuato per scopi di ricerca scientifica - 1° marzo 2012. Gazzetta Ufficiale della Repubblica Italiana numero 72 del 26 marzo 2012. [Google Scholar]
- 16.Martinelli D, Pedalino B, Cappelli MG, Caputi G, Sallustio A, Fortunato F, Tafuri S, Cozza V, Germinario C, Chironna M, et al. Apulian Group for the surveillance of pediatric IPD, author. Towards the 13-valent pneumococcal conjugate universal vaccination: Effectiveness in the transition era between PCV7 and PCV13 in Italy, 2010-2013. Hum Vaccin Immunother. 2014;10:33–39. doi: 10.4161/hv.26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grijalva CG, Nuorti JP, Arboqast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–1186. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 18.Russo F, Pozza F, Napoletano G, Zanella F, Baldovin T, Lazzari R, Cocchio S, Baldo V. Experience of vaccination against invasive bacterial diseases in Veneto Region (north-east Italy) J Prev Med Hyg. 2012;53:113–115. [PubMed] [Google Scholar]
- 19.Longini IM, Halloran ME, Nizam A. Model-based estimation of vaccine effects from community vaccine trials. Stat Med. 2002;21:481–495. doi: 10.1002/sim.994. [DOI] [PubMed] [Google Scholar]
- 20.Fine P, Eames K, Heymann DL. "Herd immunity": a rough guide. Clin Infect Dis. 2011;52:911–916. doi: 10.1093/cid/cir007. [DOI] [PubMed] [Google Scholar]
- 21.Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand J Infect Dis. 2011;43:683–689. doi: 10.3109/00365548.2011.582247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupka JE, Mortensen EM, Anzueto A, Restrepo MI. Community- acquired pneumonia in elderly patients. Aging Health. 2009;5:763–774. doi: 10.2217/ahe.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, Werkhoven CH, Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 24.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–141. [PubMed] [Google Scholar]
- 25.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2010;67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 26. Ministry of Health: Italian Vaccine Coverage. In Sintesi Anni 1999–2015. Available at: http://www.salute.gov.it/imgs/C_17_pagineAree_679_listaFile_itemName_6_file.pdf. [Accessed 15/8/2015].
- 27.Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother. 2013;9:699–706. doi: 10.4161/hv.23268. [DOI] [PMC free article] [PubMed] [Google Scholar]