Abstract
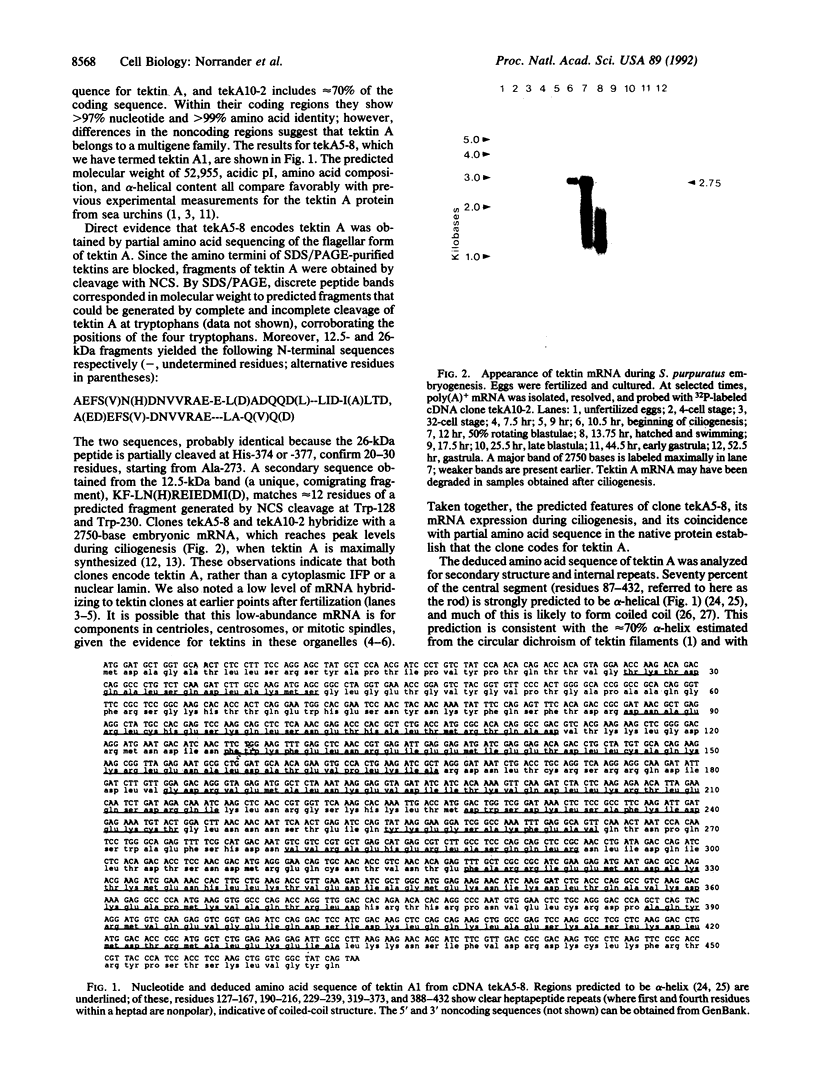
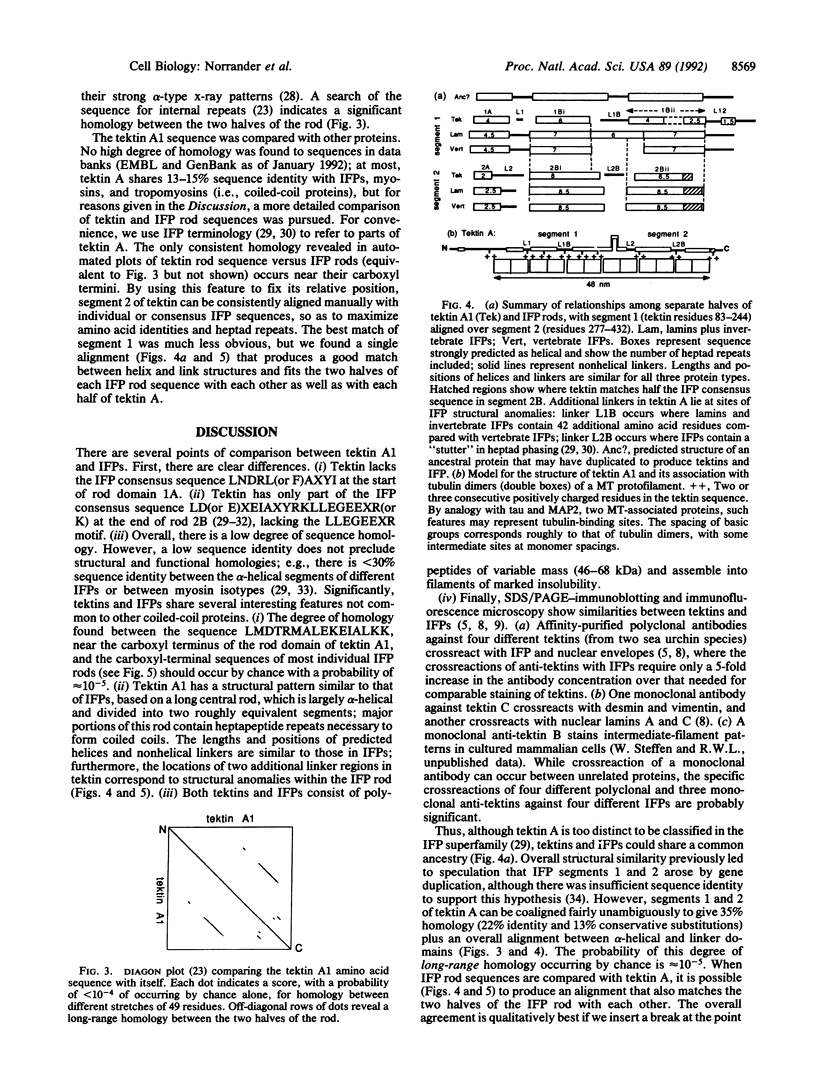
Tektins are proteins that form filamentous polymers in the walls of ciliary and flagellar microtubules and that have biochemical and immunological properties similar to those of intermediate-filament proteins. We report here the sequence of a cDNA for tektin A1, one of the main tektins from Strongylocentrotus purpuratus sea urchin embryos. By hybridization analysis, tektin A mRNA appears maximally at ciliogenesis. The predicted structure of tektin A1 (M(r) 52,955) is a series of alpha-helical rod segments separated by nonhelical linkers. The two halves of the rod appear homologous and are probably related by gene duplication. Comparison of tektin A1 with intermediate-filament proteins, including nuclear lamins, reveals a low amino acid homology but similar molecular motif, i.e., pattern of helical and nonhelical domains. This study indicates that tektins are unique proteins but may be evolutionarily related to intermediate-filament proteins, and suggests a structural basis for the interaction of tektins and tubulin in microtubules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos W. B., Amos L. A., Linck R. W. Proteins closely similar to flagellar tektins are detected in cilia but not in cytoplasmic microtubules. Cell Motil. 1985;5(3):239–249. doi: 10.1002/cm.970050306. [DOI] [PubMed] [Google Scholar]
- Amos W. B., Amos L. A., Linck R. W. Studies of tektin filaments from flagellar microtubules by immunoelectron microscopy. J Cell Sci Suppl. 1986;5:55–68. doi: 10.1242/jcs.1986.supplement_5.4. [DOI] [PubMed] [Google Scholar]
- Chang X. J., Piperno G. Cross-reactivity of antibodies specific for flagellar tektin and intermediate filament subunits. J Cell Biol. 1987 Jun;104(6):1563–1568. doi: 10.1083/jcb.104.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Goedert M., Crowther R. A., Garner C. C. Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci. 1991 May;14(5):193–199. doi: 10.1016/0166-2236(91)90105-4. [DOI] [PubMed] [Google Scholar]
- Holberton D., Baker D. A., Marshall J. Segmented alpha-helical coiled-coil structure of the protein giardin from the Giardia cytoskeleton. J Mol Biol. 1988 Dec 5;204(3):789–795. doi: 10.1016/0022-2836(88)90370-1. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Ivanov I. E., Lee G. H., Cowan N. J. Organization of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989 Nov 30;342(6249):498–505. doi: 10.1038/342498a0. [DOI] [PubMed] [Google Scholar]
- Linck R. W., Amos L. A., Amos W. B. Localization of tektin filaments in microtubules of sea urchin sperm flagella by immunoelectron microscopy. J Cell Biol. 1985 Jan;100(1):126–135. doi: 10.1083/jcb.100.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck R. W., Goggin M. J., Norrander J. M., Steffen W. Characterization of antibodies as probes for structural and biochemical studies of tektins from ciliary and flagellar microtubules. J Cell Sci. 1987 Nov;88(Pt 4):453–466. doi: 10.1242/jcs.88.4.453. [DOI] [PubMed] [Google Scholar]
- Linck R. W., Langevin G. L. Structure and chemical composition of insoluble filamentous components of sperm flagellar microtubules. J Cell Sci. 1982 Dec;58:1–22. doi: 10.1242/jcs.58.1.1. [DOI] [PubMed] [Google Scholar]
- Linck R. W., Stephens R. E. Biochemical characterization of tektins from sperm flagellar doublet microtubules. J Cell Biol. 1987 Apr;104(4):1069–1075. doi: 10.1083/jcb.104.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Periodic charge distribution in the intermediate filament proteins desmin and vimentin. J Mol Biol. 1982 Dec 15;162(3):693–698. doi: 10.1016/0022-2836(82)90396-5. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax-Jeuken Y. E., Quax W. J., Bloemendal H. Primary and secondary structure of hamster vimentin predicted from the nucleotide sequence. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3548–3552. doi: 10.1073/pnas.80.12.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Schneider A., Hemphill A., Wyler T., Seebeck T. Large microtubule-associated protein of T. brucei has tandemly repeated, near-identical sequences. Science. 1988 Jul 22;241(4864):459–462. doi: 10.1126/science.3393912. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Patchornik A., Burstein Y. Selective chemical cleavage of tryptophanyl peptide bonds by oxidative chlorination with N-chlorosuccinimide. Biochemistry. 1976 Nov 16;15(23):5071–5075. doi: 10.1021/bi00668a019. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Methods to define and locate patterns of motifs in sequences. Comput Appl Biosci. 1988 Mar;4(1):53–60. doi: 10.1093/bioinformatics/4.1.53. [DOI] [PubMed] [Google Scholar]
- Steffen W., Linck R. W. Evidence for a non-tubulin spindle matrix and for spindle components immunologically related to tektin filaments. J Cell Sci. 1992 Apr;101(Pt 4):809–822. doi: 10.1242/jcs.101.4.809. [DOI] [PubMed] [Google Scholar]
- Steffen W., Linck R. W. Evidence for tektins in centrioles and axonemal microtubules. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2643–2647. doi: 10.1073/pnas.85.8.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen W., Linck R. W. Relationship between tektins and intermediate filament proteins: an immunological study. Cell Motil Cytoskeleton. 1989;14(3):359–371. doi: 10.1002/cm.970140306. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Differential protein synthesis and utilization during cilia formation in sea urchin embryos. Dev Biol. 1977 Dec;61(2):311–329. doi: 10.1016/0012-1606(77)90301-3. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Oleszko-Szuts S., Linck R. W. Retention of ciliary ninefold structure after removal of microtubules. J Cell Sci. 1989 Mar;92(Pt 3):391–402. doi: 10.1242/jcs.92.3.391. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Quantal tektin synthesis and ciliary length in sea-urchin embryos. J Cell Sci. 1989 Mar;92(Pt 3):403–413. doi: 10.1242/jcs.92.3.403. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Goldstein L. S. One motor, many tails: an expanding repertoire of force-generating enzymes. Cell. 1990 Mar 23;60(6):883–885. doi: 10.1016/0092-8674(90)90334-b. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wais-Steider C., White N. S., Gilbert D. S., Eagles P. A. X-ray diffraction patterns from microtubules and neurofilaments in axoplasm. J Mol Biol. 1987 Sep 20;197(2):205–218. doi: 10.1016/0022-2836(87)90119-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Dodemont H., Kossmagk-Stephan K. Amino acid sequences and homopolymer-forming ability of the intermediate filament proteins from an invertebrate epithelium. EMBO J. 1988 Oct;7(10):2995–3001. doi: 10.1002/j.1460-2075.1988.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J. 1989 Nov;8(11):3221–3227. doi: 10.1002/j.1460-2075.1989.tb08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]



