Abstract
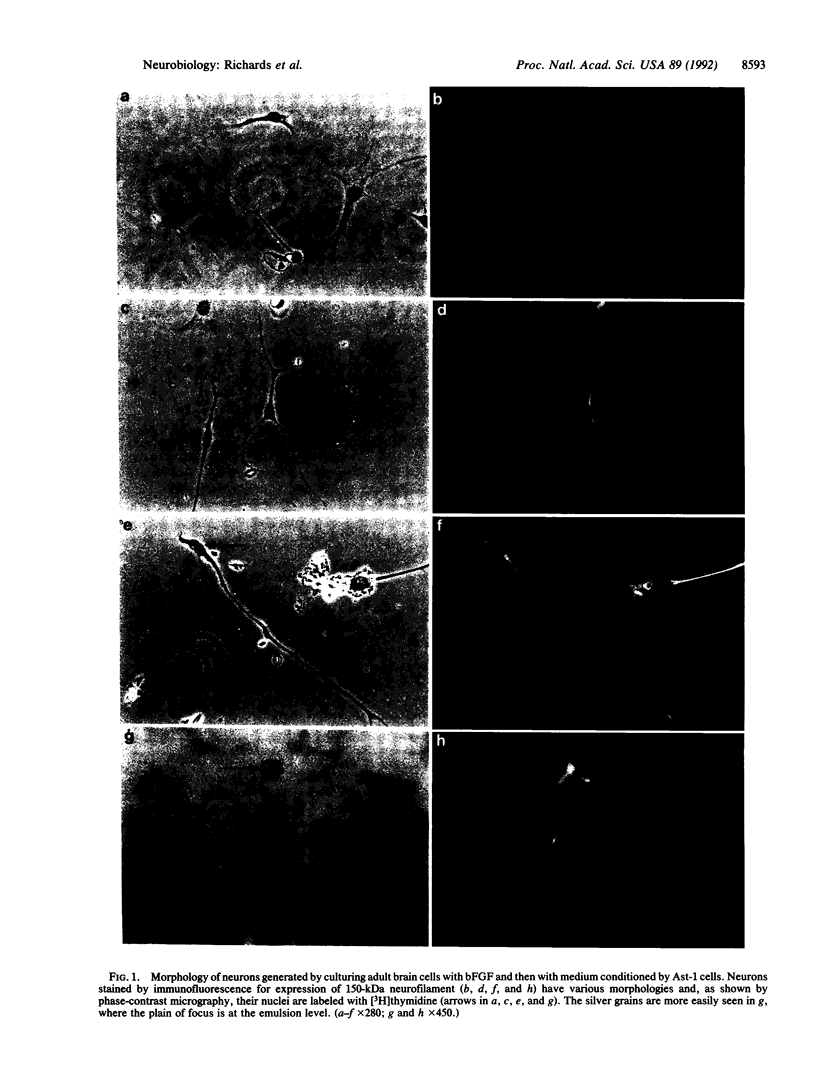
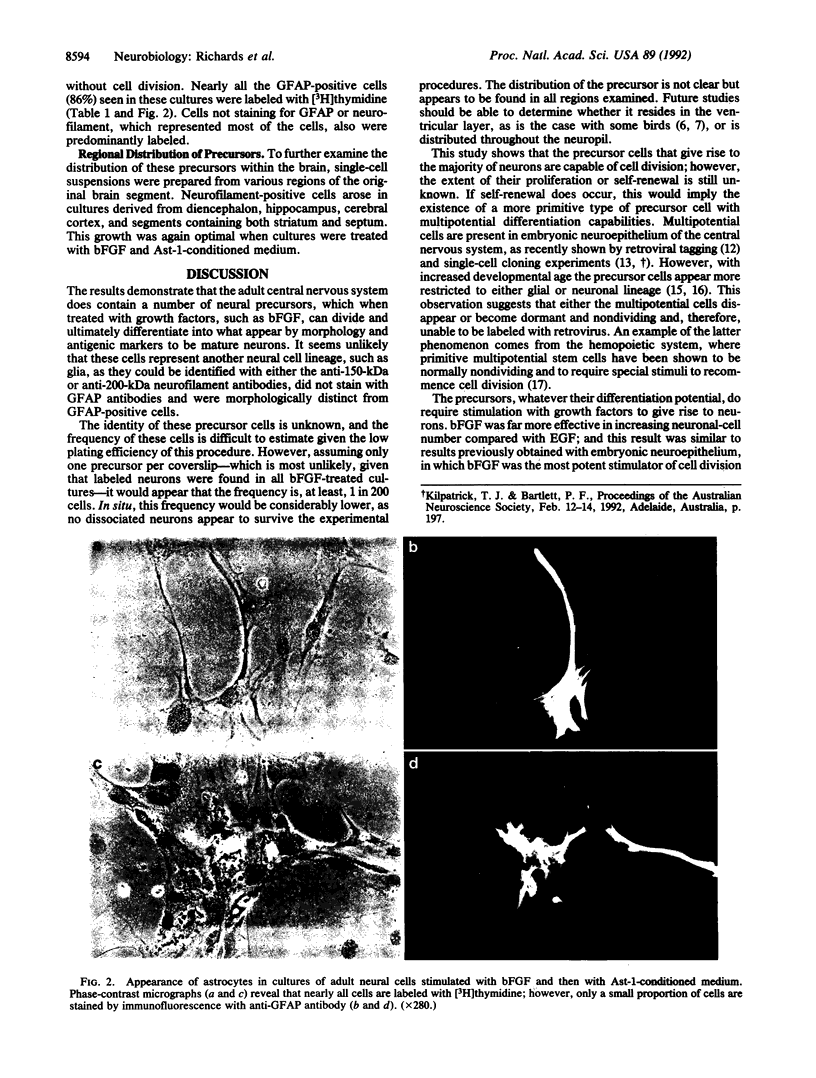
Cells of neuronal morphology, expressing the 150- and 200-kDa neurofilament proteins, were generated in vitro from populations of neural cells dissociated from adult (greater than 60-day-old) mouse brain. Most of these neurons arose from dividing precursors, as demonstrated by the incorporation of [3H]thymidine during the culture period and autoradiography. Neuronal production was optimal under the conditions in which precursors were initially stimulated with basic fibroblast growth factor and then exposed to medium conditioned by an astrocytic cell line, Ast-1, in serum-free medium. Few, if any, neurons arose in control cultures or in cultures kept in serum and fibroblast growth factor. These results suggest that neuronal precursors exist in the adult mammalian brain, but they require discrete epigenetic signals for their proliferation and differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Bartlett P. P., Raff M. C. Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Dev Biol. 1981 Apr 30;83(2):301–310. doi: 10.1016/0012-1606(81)90476-0. [DOI] [PubMed] [Google Scholar]
- Altman J., Bayer S. A. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990 Nov 15;301(3):365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Theelen M., Nottebohm F. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett P. F., Reid H. H., Bailey K. A., Bernard O. Immortalization of mouse neural precursor cells by the c-myc oncogene. Proc Natl Acad Sci U S A. 1988 May;85(9):3255–3259. doi: 10.1073/pnas.85.9.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Reid H. H., Bartlett P. F. Role of the c-myc and the N-myc proto-oncogenes in the immortalization of neural precursors. J Neurosci Res. 1989 Sep;24(1):9–20. doi: 10.1002/jnr.490240104. [DOI] [PubMed] [Google Scholar]
- Drago J., Murphy M., Carroll S. M., Harvey R. P., Bartlett P. F. Fibroblast growth factor-mediated proliferation of central nervous system precursors depends on endogenous production of insulin-like growth factor I. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2199–2203. doi: 10.1073/pnas.88.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei G. A., Graziadei P. P. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979 Apr;8(2):197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Murphy M., Drago J., Bartlett P. F. Fibroblast growth factor stimulates the proliferation and differentiation of neural precursor cells in vitro. J Neurosci Res. 1990 Apr;25(4):463–475. doi: 10.1002/jnr.490250404. [DOI] [PubMed] [Google Scholar]
- Nordeen E. J., Nordeen K. W. Estrogen stimulates the incorporation of new neurons into avian song nuclei during adolescence. Brain Res Dev Brain Res. 1989 Sep 1;49(1):27–32. doi: 10.1016/0165-3806(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Price J., Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988 Nov;104(3):473–482. doi: 10.1242/dev.104.3.473. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Bartlett P. F., Gavrilovic J., Lisak R. P., Rattray S. Mitogens for glial cells: a comparison of the response of cultured astrocytes, oligodendrocytes and Schwann cells. Brain Res. 1981 Aug;254(1):19–35. doi: 10.1016/0165-3806(81)90056-0. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989 Aug 10;340(6233):471–473. doi: 10.1038/340471a0. [DOI] [PubMed] [Google Scholar]
- Walsh C., Cepko C. L. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science. 1992 Jan 24;255(5043):434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- Williams B. P., Read J., Price J. The generation of neurons and oligodendrocytes from a common precursor cell. Neuron. 1991 Oct;7(4):685–693. doi: 10.1016/0896-6273(91)90381-9. [DOI] [PubMed] [Google Scholar]
- Wolswijk G., Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989 Feb;105(2):387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Ziller C., Dupin E., Brazeau P., Paulin D., Le Douarin N. M. Early segregation of a neuronal precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell. 1983 Feb;32(2):627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]