Abstract
Cells live in dynamic environments that necessitate perpetual adaptation. Since cells have limited resources to monitor external inputs, they are required to maximize the information content of perceived signals. This challenge is not unique to microscopic life: Animals use senses to perceive inputs and adequately respond. Research showed that sensory-perception is actively shaped by learning and expectation allowing internal cognitive models to “fill in the blanks” in face of limited information. We propose that cells employ analogous strategies and use internal models shaped through the long process of evolutionary adaptation. Given this perspective, we postulate that cells are prone to “misperceptions”, analogous to visual illusions, leading them to incorrectly decode patterns of inputs that lie outside of their evolutionary experience. Mapping cellular misperception can serve as a fundamental approach for dissecting regulatory networks and could be harnessed to modulate cell behavior, a potentially new avenue for therapy.
Keywords: adaptation, cell response, evolution, perception, regulation, signaling
Introduction: Sensory-perception as a framework for cellular responses
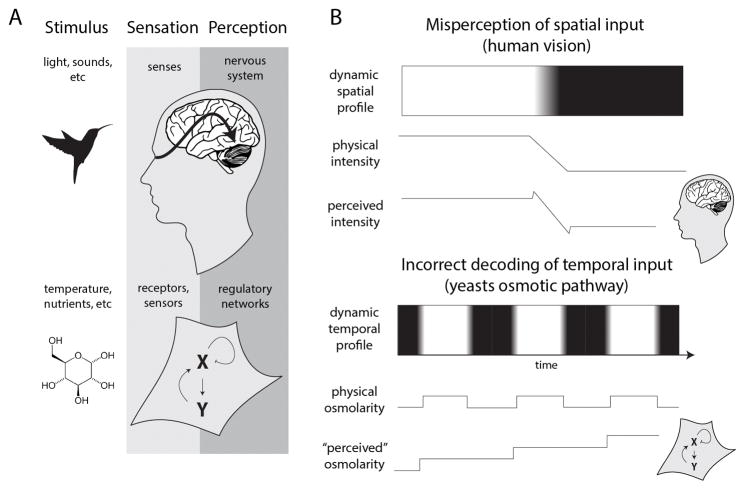
Cells need to process and integrate different, fluctuating and potentially contradictory environmental signals before mounting a suitable response. Moreover, as cellular responses are never instantaneous, an optimal response strategy needs to take into account both the present state of the environment and the projected trajectory of change [1]. From a theoretical perspective, the challenge of maximizing the information content of signals perceived from the environment and mounting an optimal response is by no means unique only to microscopic life. Animals use senses to perceive changes in their surroundings in order to adequately respond to change. Given the similarity in the challenges faced by biological systems across different physical scales it is interesting to explore whether the well-established framework of sensory-perception can help provide novel insights into the strategies of information processing occurring at the cellular level (Figure 1A).
Figure 1.
Analogy between organismal sensory-perception and cellular responses. A: Sensory-perception in humans is not a passive process of registering external stimuli but is actively shaped by learning, memory, and expectation. This intertwined sensation and perception structure allows internal cognitive models to enrich the information content extracted from external inputs and to greatly improve the organism’s response. We propose that the cellular response is an active process of information decoding that is analogous to sensory-perception. B: Incorrect decoding of dynamic inputs reflects misperception of external stimuli due to the underlying information processing network. Visual illusions (such as Mach bands) highlight the constraints inherent to processing of spatial patterns (top panel). The Illusion arises from lateral inhibition and excitation, bands are the illusory bright and dark lines to the left and right of the luminance gradient that connects the two uniform regions [4]. Decoding of temporal patterns of stimuli highlight the potential for “misperceptions” at the cellular level (lower panel). Yeast cells incorrectly interpret osmotic oscillation as a stepwise increase in osmolarity [17].
Over the past centuries, the study of visual perception has greatly advanced our understanding of how animals assimilate information contained in visible light from their surroundings. A major achievement of this longstanding research field is the appreciation that visual perception is not a passive process of registering external stimuli but is actively shaped by learning, memory, and expectation. This intertwined sensation and perception structure allows internal cognitive models to enrich the information content extracted from external inputs and to greatly improve the organism’s response. In this context it is noteworthy to mention the role visual illusions have had on the understanding of the neural architecture as well as its constraints (e.g., in people suffering schizophrenia [2, 3]). These phenomena, thus manifest under unique spatial patterns of visual input, have highlighted the extremes of what our visual system has evolved to handle and frequently stem from the assumptions and internal models of visual perception [4].
In line with the analogy to sensory perception, here we propose to view the internal cellular circuitry as an information-processing network that, similarly to neuronal networks, decodes information gathered from sensors about the environment in order to guide the organism response. At the cellular scale, sensory systems seem to focus to a high degree on interpreting temporal dynamic patterns of stimuli rather than spatial ones. Although it is impossible to directly measure how cells interpret external stimuli, we can infer how they decode environmental perturbations by monitoring their downstream responses (e.g., [5–8]). We postulate that evolutionary adaptation gradually selects for cells that can mount adaptive behaviors that efficiently sense and respond to frequently occurring dynamical stimuli patterns. Indeed, as we will discuss below, evidence gathered in multiple model systems indicates that cells are adapted, and optimally respond to specific anticipated temporal profiles of change. However, we postulate that these biased internal models comes with the cost of incorrect decoding when cells are challenged with highly unnatural temporal stimuli profiles (Figure 1B). Moreover, in some cases we find that such misperception can culminate in self-inflicting harmful responses.
Examples of “assumptions” in cellular perception of dynamic stimuli
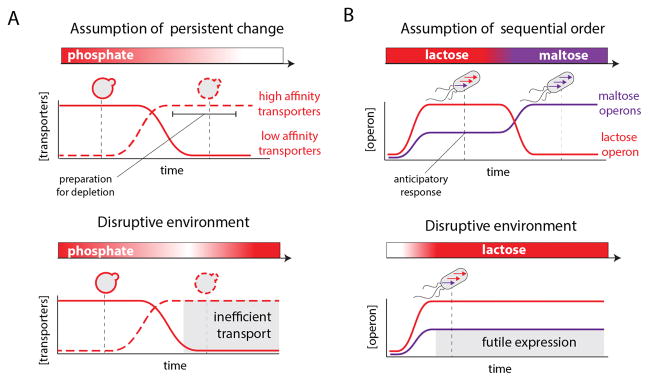
The yeast response to depletion of phosphate from the extracellular environment is a well-characterized example for a response that is highly tuned for specific anticipated dynamics of change (Figure 2A). Saccharomyces cerevisiae employs a dual transporter system to reduce the stressful effects of phosphate depletion by switching low affinity transporters with high affinity ones when intracellular phosphate levels drop below an intermediate threshold [9]. This switch allows the cells to maintain sufficient intracellular levels of phosphate while preparing for its eventual depletion. During this period, cells can trigger, in a timely manner, regulatory programs that prolong cell growth and survival. Interestingly, a study focusing on the regulation of this transporter system discovered that the underlying genetic circuitry behaves as an irreversible toggle switch [10]. Thus, cells that induce this starvation program commit to maintain it for more than ten generations, even if phosphate depletion is only transient [10]. While this cellular commitment is effective in mitigating starvation if limited phosphate availability persists for multiple generations, it also leads to an inappropriate, and potentially maladaptive, activation of a regulatory program when depletion is rapidly reversed [10] (Figure 2A, lower panel).
Figure 2.
Cell response strategies to changing environments highlight the underlying “assumptions” made by cells regarding dynamic changes in their environments. A: The dual phosphate transporter system in yeast is optimized for gradual decrease in availability of extracellular phosphate. The switch between low and high affinity transporters takes place at intermediate concentration of extracellular phosphate due to decline in intracellular levels of phosphate (upper panel). This switch allows cells to prepare to the stress of phosphate depletion before it actually occurs [9]. The underlying regulatory network leads to a maladaptive response if depletion is transient (lower panel). This maladaptive response to transient phosphate depletion underlines the anticipatory aspect of the response. Cells decode short-term depletion, as an indication that starvation will persist. B: The response of wild-type E. coli to different sugars reflects an adaption to the sequential order of nutrient appearance typical to the mammalian digestive tract (lower panel). E. coli induces the maltose operon to an intermediate level upon exposure to lactose as an anticipatory response to the future arrival of maltose [13, 15]. This regulatory circuitry is maladaptive in an environment that lacks maltose and is selected against within a few hundred generations. This maladaptive response to persistent lactose underlines the anticipatory aspect of the response. Cells decode lactose as an environmental cue for the future arrival of maltose.
Environments with common sequential changes are another instance of habitats that can select for highly optimized dynamic cellular responses [11]. For example, the cycling of E. coli through the mammalian digestive tract exposes the bacteria to different nutrient environments in a set sequential order - for example it is common for the bacteria to first experience a lactose-rich environment followed by a maltose-rich environment [12]. Previous studies have demonstrated that the order in these environmental changes is captured by the wiring of the E. coli regulatory network -- the bacteria actually mount anticipatory responses prior to actual encounter with a subsequent stimulus [13, 14]. The asymmetric cross regulation of lactose and maltose operons is one instance of a conditioned response that fits the order of stimuli in the mammalian digestive tract (Figure 2B). Upon encounter with lactose, cells fully induce the lactose operon but also partly induce the maltose operons, presumably to prepare for future exposure to maltose. Stimulation with maltose, however, does not conversely induce the lactose operon. Interestingly, and in accordance with this interpretation, this conditioned cross regulation can be selected against in a lactose only environment, indicating that this anticipatory response model entails an fitness cost once the typical sequential environment is disrupted [13, 15]. In the context of sensory-perception it is noteworthy to highlight the similarity between these evolutionary dynamics and classical conditioning, and extinction of conditioned responses, observed by Ivan Pavlov almost a hundred years ago [16].
While the two different examples discussed above involve adaptation to different perturbations and include different cellular responses, they both highlight the importance of the temporal context in determining whether the cellular response will be adaptive or maladaptive. This dependency on external dynamics indicates that cellular responses are not only set by the instantaneous extracellular conditions but in fact reflect coping strategies that were shaped by evolutionary selection and are biased by the statistics of past experiences [1]. From the perspective of information processing, this incorporation of past experience into cellular decision is highly beneficial since it maximizes the information content of perceived signals and allows optimization of the cellular response based on incomplete information. This process of evolutionary optimization is analogous to a learning process that shapes sensory perception and optimizes perception for typical stimuli patterns. However, as evolutionary processes rely on natural selection such adaptations arise only after multiple generations and transpire at the lineage level rather than the level of a single organism.
Cellular “misperception” of osmotic changes
While internal sensory models of dynamic changes can be highly beneficial under typical environments they can also backfire when the cell is stimulated with patterns that lie outside of the statistical norm for which the response was optimized. This type of misperception has recently been clearly demonstrated in the yeast hyperosmolarity stress response [17].
At its core, the yeast hyperosmolarity response consists of fast and slow response arms that are facilitated by the master regulator kinase Hog1 (Figure 3A). While the fast response arm helps in quickly increasing the intracellular concentration of glycerol which serves as a counterbalancing osmolyte, the slower response arm induces the expression of dozens of effector genes including ones involved in the general stress response, glycerol synthesis, and remodeling of the cell wall [18].
Figure 3.
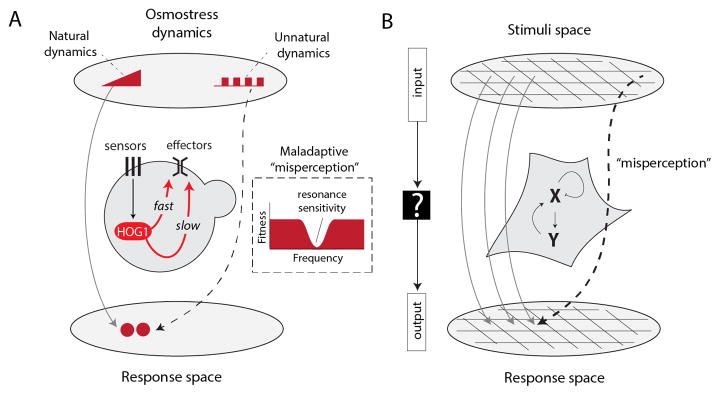
Cellular mapping of external inputs to downstream cell responses can lead to incorrect decoding of the dynamic input analogous to sensory misperceptions. A: An example of incorrect information decoding in the yeast osmotic pathway that leads to the interpretation of osmotic oscillations as gradual increase in osmolarity [17]. This incorrect decoding observed for a wide frequency range of oscillations culminates in severe growth inhibition at a specific resonance frequency [17]. B: Future research efforts should be dedicated to systematically explore how cells map diverse input dynamics to downstream responses. This mapping will help to uncover the rare events of misperception. These efforts require technologies amenable for time-variant input control in high throughput.
In the wild, yeasts are most likely to experience slow ramp-like increases in osmolarity due to evaporation. Not surprisingly, the native response of yeast is optimized to respond to such ramps. In particular, the osmolarity-sensing kinase network shows a hallmark ability to adapt back to is basal activity level, minutes after being triggered by a step increase in osmotic change, allowing it to be retriggered by a further increase in osmolarity. When we systematically monitored stimulated yeast with a highly non-natural oscillatory osmolarity stimulus, however, we observed that growth was severely inhibited at a particular resonance frequency. Focusing on the molecular mechanism, we uncovered that this stress sensitivity arises from the toxic hyperactivation of the transcriptional response that is retriggered again and again with each osmotic oscillation. This retriggering occurs as a result of the adaptive response that normally allows yeast cells to deal with ramps of continuously increasing osmolarity.
These network dynamics can be viewed as a misperception of the external environment - cells interpreted the oscillations as infinite stepwise increases in osmolarity and are driven to respond to this perception, even though the average external osmolarity is in reality relatively modest. In other words, the ability of yeast to mount a robust response optimized for natural inputs leads to the inherent severe fragility under non-natural oscillatory inputs. From the perspective of information processing, this phenomenon demonstrates an example of incorrect mapping between the input space and the cellular response space (Figure 3A). Here, osmotic oscillations are mapped falsely as osmolarity ramps and the harmful effects arise due to a mismatch between the cell’s interpretation of the environment and the actual extracellular conditions. However, since cells never experience these oscillations in their natural environment, this fragility is likely irrelevant for fitness in the wild.
Future perspective: Can cells be “fooled”?
The discovery of the harmful capacity of osmotic oscillations in yeast is exciting since it demonstrates the potential severe implications of cellular misperceptions on fitness. Moreover, the cellular design feature underlying this sensitivity, the ability of the signaling network to adapt and then to be sequentially retriggered, is prevalent in signaling pathways found in diverse organisms, from bacterial chemotaxis [19] to the response of mammalian cells to growth factors [20]. This reoccurring feature suggests that many biological systems may be “fooled” by oscillatory inputs. However, we suspect that misinterpretation of oscillations is only one of many instances that leads to incorrect decoding of dynamic inputs.
The study of cellular misperception can serve as a novel approach for dissecting network behaviors. In this line of research, theoretical and experimental approaches can be used not only to characterize network dynamics under standard conditions but also to identify the rare conditions that lead to network failure and erroneous decoding (Figure 3B). Such studies can add a complementary layer of information for depicting complicated cellular networks that have been at the focal point of research for decades. While misperceptions will likely arise under non-natural dynamics of stimulation, they are still highly valuable since they represent unique points in the stimuli space that have remarkable information content as they illicit highly non-linear responses. Since misperception depends on the structure of the cellular network responsible for information-processing, changes in the stimuli patterns leading to failure will be indicative of the underlying network modification. Such methodologies can be invaluable for dissecting the underlying mechanisms in diseases, such as cancer, that are known to arise due to mutations in signaling and regulatory networks.
The identification of misperceptions that are unique to specific “diseased” networks could potentially have broader therapeutic applications beyond just characterizing changes in information processing. If a specific misperception is restricted only to a group of cells within a mixed population, it can be exploited to single out this subpopulation with minimal effects on surrounding cells. Thus delivering dynamic inputs to a heterogeneous population will allow stratifying different cell states. Moreover, if misperception culminates with toxic effects, as observed for osmotic oscillations in yeast, it can be used to increase the specificity of targeting. It may be possible to drive specific disease cells (e.g. cancer cells) to new states (e.g. differentiation or death) by using patterns of stimulation that selectively exploit their internal sensory models. Tumor cells are known to have rewired signaling behaviors, and thus might have very distinct dynamic sensitivities. More generally, particular dynamic stimuli may provide useful ways to drive many different cell types into desired states.
References
- 1.Perkins TJ, Swain PS. Strategies for cellular decision-making. Mol Syst Biol. 2009;5:326. doi: 10.1038/msb.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dima D, Roiser JP, Dietrich DE, Bonnemann C, et al. Understanding why patients with schizophrenia do not perceive the hollow-mask illusion using dynamic causal modelling. Neuroimage. 2009;46:1180–6. doi: 10.1016/j.neuroimage.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Schneider U, Leweke FM, Sternemann U, Weber MM, et al. Visual 3D illusion: a systems-theoretical approach to psychosis. Eur Arch Psychiatry Clin Neurosci. 1996;246:256–60. doi: 10.1007/BF02190277. [DOI] [PubMed] [Google Scholar]
- 4.Eagleman DM. Visual illusions and neurobiology. Nat Rev Neurosci. 2001;2:920–6. doi: 10.1038/35104092. [DOI] [PubMed] [Google Scholar]
- 5.Bren A, Park JO, Towbin BD, Dekel E, et al. Glucose becomes one of the worst carbon sources for E. coli on poor nitrogen sources due to suboptimal levels of cAMP. Sci Rep. 2016;6:24834. doi: 10.1038/srep24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youk H, van Oudenaarden A. Growth landscape formed by perception and import of glucose in yeast. Nature. 2009;462:875–9. doi: 10.1038/nature08653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Atolia E, Hua B, Savir Y, et al. Natural variation in preparation for nutrient depletion reveals a cost-benefit tradeoff. PLoS Biol. 2015;13:e1002041. doi: 10.1371/journal.pbio.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venturelli OS, Zuleta I, Murray RM, El-Samad H. Population diversification in a yeast metabolic program promotes anticipation of environmental shifts. PLoS Biol. 2015;13:e1002042. doi: 10.1371/journal.pbio.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy S, Kafri M, Carmi M, Barkai N. The competitive advantage of a dual-transporter system. Science. 2011;334:1408–12. doi: 10.1126/science.1207154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vardi N, Levy S, Assaf M, Carmi M, et al. Budding yeast escape commitment to the phosphate starvation program using gene expression noise. Curr Biol. 2013;23:2051–7. doi: 10.1016/j.cub.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Dhar R, Sägesser R, Weikert C, Wagner A. Yeast adapts to a changing stressful environment by evolving cross-protection and anticipatory gene regulation. Molecular Biology and Evolution. 2013;30:573–88. doi: 10.1093/molbev/mss253. [DOI] [PubMed] [Google Scholar]
- 12.Savageau MA. Demand theory of gene regulation. II. Quantitative application to the lactose and maltose operons of Escherichia coli. Genetics. 1998;149:1677–91. doi: 10.1093/genetics/149.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell A, Romano GH, Groisman B, Yona A, et al. Adaptive prediction of environmental changes by microorganisms. Nature. 2009;460:220–80. doi: 10.1038/nature08112. [DOI] [PubMed] [Google Scholar]
- 14.Tagkopoulos I, Liu Y-C, Tavazoie S. Predictive behavior within microbial genetic networks. Science (New York, NY) 2008;320:1313–7. doi: 10.1126/science.1154456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell A, Pilpel Y. A mathematical model for adaptive prediction of environmental changes by microorganisms. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1019754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlov IP. Conditioned Reflexes. Oxford University Press; London: 1927. [Google Scholar]
- 17.Mitchell A, Wei P, Lim WA. Oscillatory stress stimulation uncovers an Achilles’ heel of the yeast MAPK signaling network. Science. 2015;350:1379–83. doi: 10.1126/science.aab0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H, Posas F. Response to hyperosmotic stress. Genetics. 2012;192:289–318. doi: 10.1534/genetics.112.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi TM, Huang Y, Simon MI, Doyle J. Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proc Natl Acad Sci USA. 2000;97:4649–53. doi: 10.1073/pnas.97.9.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirit M, Wang C-C, Haugh JM. Systematic quantification of negative feedback mechanisms in the extracellular signal-regulated kinase (ERK) signaling network. J Biol Chem. 2010;285:36736–44. doi: 10.1074/jbc.M110.148759. [DOI] [PMC free article] [PubMed] [Google Scholar]