Abstract
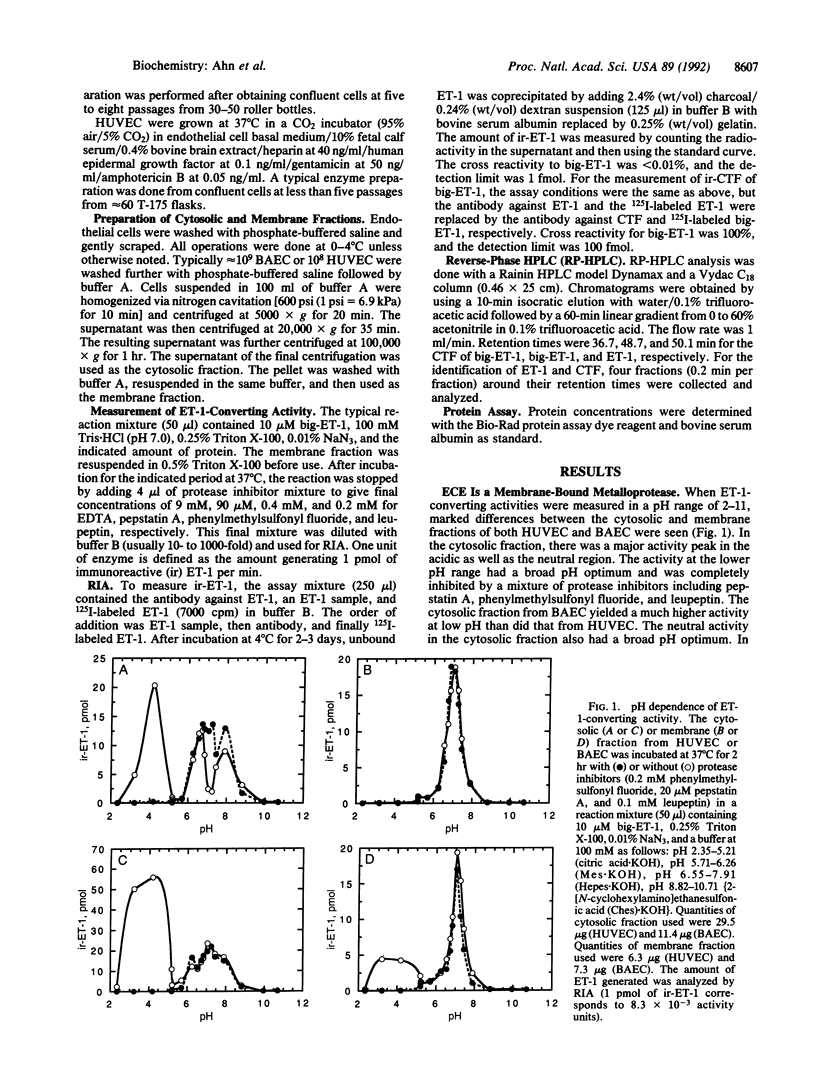
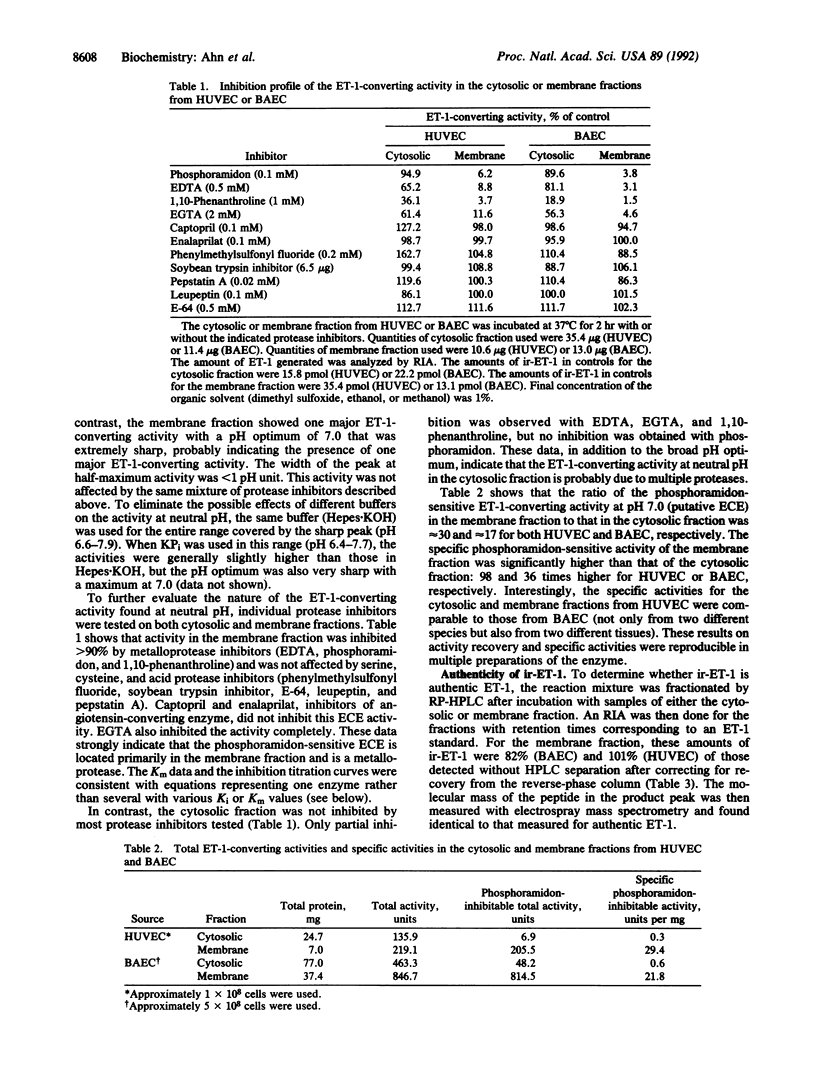
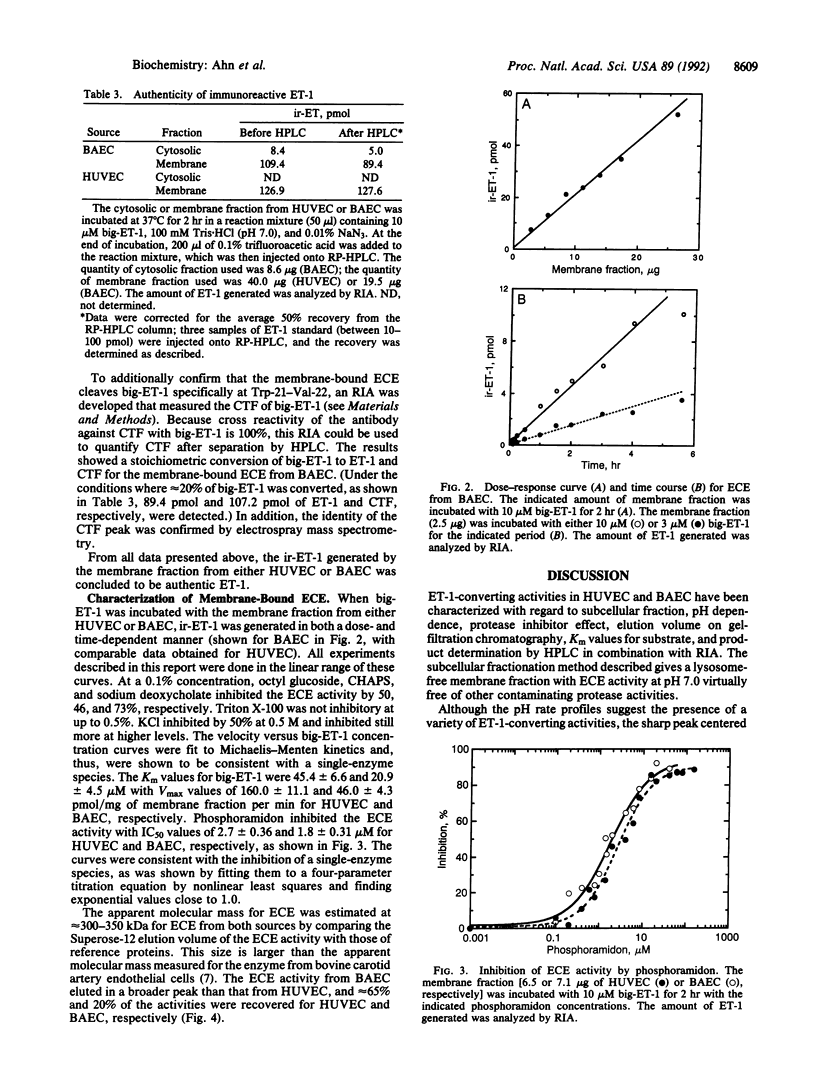
A phosphoramidon-sensitive, membrane-bound metalloprotease that cleaves big endothelin 1 (big-ET-1) to ET-1 was obtained from human umbilical vein endothelial cells and also from bovine aortic endothelial cells by isolation of plasma-membrane vesicles free of lysosomes. The enzyme was characterized by RIA with an antibody specific for ET-1 and also by reverse-phase HPLC. For both sources, the pH rate profile of the membrane fraction had a very sharp maximum at pH 7.0; little or no activity was seen at more acidic pH values. In contrast, the cytosolic fraction had a major peak at acidic pH values, as well as a broad peak in the neutral region. The activity at pH 7.0 in the membrane fraction was shown by reverse-phase HPLC to produce ET-1 and C-terminal fragment as products. This activity was abolished by phosphoramidon, EDTA, and 1,10-phenanthroline but was not inhibited by pepstatin A, phenylmethylsulfonyl fluoride, soybean trypsin inhibitor, leupeptin, or E-64--consistent with the characteristics of a metalloprotease. These results suggest that this activity is from the physiologically relevant, phosphoramidon-inhibitable, endothelin-converting enzyme. The activity found at neutral pH values in the cytosolic fraction was only partially inhibited by EDTA and 1,10-phenanthroline but was not inhibited by phosphoramidon. The membrane-bound endothelin-converting enzyme from human umbilical vein endothelial cells and bovine aortic endothelial cells showed marked similarities, including IC50 values for phosphoramidon of 2.7 and 1.8 microM and Km values for big-ET-1 of 45.4 and 20.9 microM, respectively. The apparent molecular mass by gel filtration was approximately 300-350 kDa for the enzyme from either source. This report characterizes human endothelin-converting enzyme, which may be an important therapeutic target for cardiovascular disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird J. E., Waldron T. L., Little D. K., Asaad M. M., Dorso C. R., DiDonato G., Norman J. A. The effects of novel cathepsin E inhibitors on the big endothelin pressor response in conscious rats. Biochem Biophys Res Commun. 1992 Jan 15;182(1):224–231. doi: 10.1016/s0006-291x(05)80134-2. [DOI] [PubMed] [Google Scholar]
- Fujitani Y., Oda K., Takimoto M., Inui T., Okada T., Urade Y. Autocrine receptors for endothelins in the primary culture of endothelial cells of human umbilical vein. FEBS Lett. 1992 Feb 17;298(1):79–83. doi: 10.1016/0014-5793(92)80026-d. [DOI] [PubMed] [Google Scholar]
- Fukuroda T., Noguchi K., Tsuchida S., Nishikibe M., Ikemoto F., Okada K., Yano M. Inhibition of biological actions of big endothelin-1 by phosphoramidon. Biochem Biophys Res Commun. 1990 Oct 30;172(2):390–395. doi: 10.1016/0006-291x(90)90685-g. [DOI] [PubMed] [Google Scholar]
- Hioki Y., Okada K., Ito H., Matsuyama K., Yano M. Endothelin converting enzyme of bovine carotid artery smooth muscles. Biochem Biophys Res Commun. 1991 Jan 31;174(2):446–451. doi: 10.1016/0006-291x(91)91436-g. [DOI] [PubMed] [Google Scholar]
- Ikegawa R., Matsumura Y., Takaoka M., Morimoto S. Evidence for pepstatin-sensitive conversion of porcine big endothelin-1 to endothelin-1 by the endothelial cell extract. Biochem Biophys Res Commun. 1990 Mar 16;167(2):860–866. doi: 10.1016/0006-291x(90)92104-8. [DOI] [PubMed] [Google Scholar]
- Ikegawa R., Matsumura Y., Tsukahara Y., Takaoka M., Morimoto S. Phosphoramidon, a metalloproteinase inhibitor, suppresses the secretion of endothelin-1 from cultured endothelial cells by inhibiting a big endothelin-1 converting enzyme. Biochem Biophys Res Commun. 1990 Sep 14;171(2):669–675. doi: 10.1016/0006-291x(90)91198-2. [DOI] [PubMed] [Google Scholar]
- Kashiwabara T., Inagaki Y., Ohta H., Iwamatsu A., Nomizu M., Morita A., Nishikori K. Putative precursors of endothelin have less vasoconstrictor activity in vitro but a potent pressor effect in vivo. FEBS Lett. 1989 Apr 10;247(1):73–76. doi: 10.1016/0014-5793(89)81243-8. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Hisaki K., Takaoka M., Morimoto S. Phosphoramidon, a metalloproteinase inhibitor, suppresses the hypertensive effect of big endothelin-1. Eur J Pharmacol. 1990 Aug 21;185(1):103–106. doi: 10.1016/0014-2999(90)90216-s. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Ikegawa R., Tsukahara Y., Takaoka M., Morimoto S. Conversion of big endothelin-1 to endothelin-1 by two types of metalloproteinases derived from porcine aortic endothelial cells. FEBS Lett. 1990 Oct 15;272(1-2):166–170. doi: 10.1016/0014-5793(90)80475-x. [DOI] [PubMed] [Google Scholar]
- Ohnaka K., Takayanagi R., Yamauchi T., Okazaki H., Ohashi M., Umeda F., Nawata H. Identification and characterization of endothelin converting activity in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1990 May 16;168(3):1128–1136. doi: 10.1016/0006-291x(90)91146-j. [DOI] [PubMed] [Google Scholar]
- Okada K., Miyazaki Y., Takada J., Matsuyama K., Yamaki T., Yano M. Conversion of big endothelin-1 by membrane-bound metalloendopeptidase in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1192–1198. doi: 10.1016/0006-291x(90)90811-z. [DOI] [PubMed] [Google Scholar]
- Pollock D. M., Opgenorth T. J. Evidence for metalloprotease involvement in the in vivo effects of big endothelin 1. Am J Physiol. 1991 Jul;261(1 Pt 2):R257–R263. doi: 10.1152/ajpregu.1991.261.1.R257. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Kasuya Y., Matsushita Y., Suzuki N., Shinmi O., Kishi N., Sugita Y., Yanagisawa M., Goto K., Masaki T. Phosphoramidon inhibits the intracellular conversion of big endothelin-1 to endothelin-1 in cultured endothelial cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):779–784. doi: 10.1016/0006-291x(91)91485-u. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Kimura S., Shinmi O., Sugita Y., Kobayashi M., Mitsui Y., Yanagisawa M., Goto K., Masaki T. Characterization of endothelin converting enzyme activities in soluble fraction of bovine cultured endothelial cells. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1138–1144. doi: 10.1016/0006-291x(90)92014-q. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Shinmi O., Kishi N., Sugita Y., Yanagisawa M., Goto K., Masaki T., Kimura S. Analysis of big endothelin-1 digestion by cathepsin D. Biochem Biophys Res Commun. 1990 Oct 30;172(2):883–889. doi: 10.1016/0006-291x(90)90758-f. [DOI] [PubMed] [Google Scholar]
- Takada J., Okada K., Ikenaga T., Matsuyama K., Yano M. Phosphoramidon-sensitive endothelin-converting enzyme in the cytosol of cultured bovine endothelial cells. Biochem Biophys Res Commun. 1991 Apr 30;176(2):860–865. doi: 10.1016/s0006-291x(05)80265-7. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


