Abstract
Background: Iron therapy begun concurrently with antimalarial treatment may not be well absorbed because of malaria-induced inflammation. Delaying the start of iron therapy may permit better iron absorption and distribution.
Objective: We compared erythrocyte iron incorporation in children who started iron supplementation concurrently with antimalarial treatment or 28 d later. We hypothesized that delayed iron supplementation would be associated with greater incorporation and better hematologic recovery.
Methods: We enrolled 100 children aged 6–59 mo with malaria and hemoglobin concentrations of 50.0–99.9 g/L who presented to Mulago Hospital, Kampala, into a randomized trial of iron therapy. All children were administered antimalarial treatment. Children with zinc protoporphyrin (ZPP) ≥80 μmol/mol heme were randomly assigned to start iron supplementation concurrently with the antimalarial treatment [immediate iron (I) group] or 28 d later [delayed iron (D) group]. All children were administered iron-stable isotope 57Fe on day 0 and 58Fe on day 28. We compared the percentage of iron incorporation at the start of supplementation (I group at day 0 compared with D group at day 28, aim 1) and hematologic recovery at day 56 (aim 2).
Results: The percentage of iron incorporation (mean ± SE) was greater at day 28 in the D group (16.5% ± 1.7%) than at day 0 in the I group (7.9% ± 0.5%; P < 0.001). On day 56, concentrations of hemoglobin and ZPP and plasma ferritin, soluble transferrin receptor (sTfR), hepcidin, and C-reactive protein did not differ between the groups. On day 28, the hemoglobin (mean ± SD) and plasma iron markers (geometric mean; 95% CI) reflected poorer iron status in the D group than in the I group at this intervening time as follows: hemoglobin (105 ± 15.9 compared with 112 ± 12.4 g/L; P = 0.04), ferritin (39.3 μg/L; 23.5, 65.7 μg/L compared with 79.9 μg/L; 58.3, 110 μg/L; P = 0.02), sTfR (8.9 mg/L; 7.4, 10.7 mg/L compared with 6.7 mg/L; 6.1, 7.5 mg/L; P = 0.01), and hepcidin (13.3 ng/mL; 8.3, 21.2 ng/mL compared with 38.8 ng/mL; 28.3, 53.3 ng/mL; P < 0.001).
Conclusions: Delaying the start of iron improves incorporation but leads to equivalent hematologic recovery at day 56 in Ugandan children with malaria and anemia. These results do not demonstrate a clear, short-term benefit of delaying iron. This trial was registered at clinicaltrials.gov as NCT01754701.
Keywords: iron, malaria, iron-stable isotopes, Uganda, timing of iron therapy
See corresponding commentary on page 1623.
Introduction
Achieving and maintaining optimal iron status is difficult and potentially risky for children living in malaria-endemic regions. Several studies, beginning with a large, randomized, controlled trial on Pemba Island, Tanzania, in 2006, found that iron supplementation to prevent iron deficiency (1), or simply not being iron deficient (2, 3), predisposes children to more frequent episodes of malaria and other infections and even increased risk of death. Because optimal brain development depends on a sufficient supply of iron during peak periods of brain growth during early childhood (4, 5), identifying strategies to ensure adequate iron for brain development while also protecting children from malaria and other infections is paramount.
Frequently, iron deficiency and malaria co-occur in the same child. The optimal timing for providing iron supplementation to a child being treated for concurrent malaria and iron deficiency is unclear. The standard of care is to prescribe iron supplementation at the onset of antimalarial treatment with antibiotics if needed for other concurrent infection (6). However, studies that followed the regimen have largely reported unresolved anemia, unresolved iron deficiency, and increased frequency of clinical malaria episodes (7–9).
A potential physiologic explanation for this finding is that malaria-induced inflammation and the resulting rise in blood concentrations of the hepatic protein hepcidin impair iron absorption in the gut and release of iron from macrophages, making oral iron therapy given concurrently with antimalarial treatment not optimally absorbed or incorporated into red blood cells. A study that used iron-stable isotopes in Gambian children found that iron incorporation was significantly lower at day 0 and day 15 in children recovering from postmalarial anemia than in children recovering from iron deficiency anemia alone, but children recovering from postmalarial anemia had a greater increase in hemoglobin concentration after 4 wk (10). These findings led the investigators to conclude that children recovering from postmalarial anemia meet their initial iron demands from iron sequestered in storage compartments during the inflammation induced by malaria, rather than from an oral supplement. They concluded that starting iron therapy before 2 wk after successful antimalarial treatment may be futile. Subsequent analysis of the same data set revealed that hepcidin concentration best predicted iron incorporation, with greater hepcidin associated with lower red blood cell iron incorporation (11).
These data suggest that delaying iron therapy by some as yet undefined time period after antimalarial therapy rather than providing the treatments concurrently would result in greater iron absorption at the time of initial supplement administration. However, because iron incorporation remained significantly lower at day 15 in children recovering from postmalarial anemia in the Gambian study (10), the question of what is the appropriate time delay remains unanswered. Hepcidin concentrations are shown to normalize by 4 wk after antimalarial treatment (12), suggesting that delaying iron treatment by longer than 2 wk may be more effective.
Here, we present the results of a randomized clinical trial conducted in malaria-endemic Uganda that aimed to determine whether iron supplementation given concurrently with antimalarial treatment compared with 4 wk after antimalarial treatment is better incorporated into red blood cells and improves hematologic recovery after 8 wk. The goal of the study was to use iron-stable isotopes within a randomized design to provide physiologic evidence for the optimal timing of the initiation of iron therapy in children recovering from malaria and anemia.
Methods
Between May 2013 and February 2015, we enrolled a consecutive sample of 100 children from the Pediatric Acute Care Unit at Mulago Hospital in Kampala, Uganda. Inclusion criteria were 1) children between the ages of 6 and 59 mo, 2) hemoglobin concentration between 50.0 and 99.9 g/L, 3) a positive Giemsa smear for Plasmodium falciparum parasitemia, 4) measured fever (temperature, >37.5°C) or a history of fever in the preceding 24 h, and 5) residence within 50 km of the hospital. Exclusion criteria were 1) known sickle cell disease, 2) acute malnutrition, and 3) a history of convulsions or seizures with present illness. We set the lower threshold for hemoglobin at 50.0 g/L because any child with a lower hemoglobin concentration would require transfusion per hospital guidelines, necessitating immediate alternate medical attention.
Once informed consent was obtained from the caregiver, a 5- to 7-mL venous blood sample was drawn. Zinc protoporphyrin (ZPP)8 was measured immediately by front-face hematofluorometer (Aviv Biomedical, Inc.). All children were treated for malaria with parenteral artesunate, per Uganda Ministry of Health guidelines, and were also given a 3-d course of oral artemether-lumefantrine. Children with ZPP ≥80 μmol/mol heme were randomly assigned to start a 27-d course of iron supplementation the day after parenteral antimalarial treatment on day 1 [immediate iron (I) group], or after 4 wk on day 29 [delayed iron (D) group]. Treatment allocation was assigned in permuted blocks of 4 from a computer-generated randomization list produced by a University of Minnesota staff member who was not part of the study. A trained study nurse weighed and measured the length or height of each child.
The daily iron supplement was in the form of liquid oral ferrous sulfate syrup (Rugby Laboratories) and was administered at a daily dose of 2 mg/kg. Children in the I group started the syrup on day 1 and took the syrup daily until day 27. They returned their syrup bottles at the clinic visit on day 28. Children in the D group started taking the supplement on day 29 and continued daily until day 55, returning their bottles on the final visit on day 56. Trained study monitors visited each child at home on day 14 and day 42 to assess supplement adherence as measured by remaining syrup volume and completed iron dosing card and also to record any side effects.
On day 0, after a venous blood draw for baseline laboratory values and a 2-h supervised fast, all children (n = 100) were administered a 4-mg dose of iron-stable isotope 57Fe (Trace Sciences International Corps) in the form of an aqueous solution of ferrous sulfate, prepared with a crushed 50-mg tablet of vitamin C in 30 mL mango juice. The isotope-dosing syringe was weighed before and after injecting the isotope into the mango juice, and the cup from which the child drank the isotope plus juice mixture was also weighed before and after consumption by the child. Any spillage was wiped with a paper napkin that was weighed before and after use. After consuming the isotope, the child again fasted for 2 h under supervision. Any child who vomited or spilled the isotope juice mixture was excluded from the isotopic analysis.
Children returned to the clinic on day 28, at which time children in the I iron group returned their iron syrup bottles, and children in the D group were given iron syrup to be taken home and started the next day. A venous blood sample was collected from each child, and all children were administered an oral 1.5-mg dose of 58Fe, prepared and administered in analogous manner as 57Fe on day 0. Each 1.5-mg dose of 58Fe was enriched with 2.5 mg iron (0.75 mL of the liquid ferrous sulfate syrup) to make the total iron content of each dose of 57Fe and 58Fe equivalent at 4 mg (10). All children returned to the clinic on day 56 for their final phlebotomy to assess iron status.
Venous blood collected at day 0, day 28, and day 56 was centrifuged immediately at 1500 × g for 10 min at room temperature. Plasma and red blood cells were stored at −80°C until shipment to the United States. Plasma concentrations of ferritin (Ramco Laboratories), soluble transferrin receptor (sTfR; Ramco Laboratories), hepcidin (Peninsula Laboratories), and C-reactive protein (CRP; R&D Systems) were measured by an ELISA assay at the University of Minnesota. Isotopic enrichment of red blood cells was calculated by inductively coupled plasma mass spectrometry at Baylor College of Medicine to provide a direct estimate of the proportion of iron isotope incorporated into red blood cells on day 0 (57Fe) and day 28 (58Fe). For the purpose of these calculations, each child’s blood volume was assumed to be 80 mL/kg body weight (13).
Ethical review.
Written informed consent was obtained from parents or guardians of all study participants. Ethics approval for the study was granted by the Institutional Review Board of the University of Minnesota, the Research and Ethics Committee of Makerere University School of Medicine, the Uganda National Council of Science and Technology, and the Ugandan National Drug Authority. This study was registered at clinical trials.gov as NCT01754701.
Statistical analysis.
All analyses were intention to treat. We compared baseline characteristics, including age, sex, and anthropometric indexes, between the treatment groups by t test for continuous outcomes and χ2 test for dichotomous outcomes. Because it was not normally distributed, malaria parasite density was compared with Wilcoxon’s signed rank test. To accomplish the primary aim of comparing iron incorporation at the start of supplementation in the I compared with D groups, we compared the mean percentage of iron incorporation at day 0 in the I group with the mean percentage of iron incorporation at day 28 in the D group by t test. Adjusted analysis controlled for day 0 plasma ferritin concentration. To determine whether hematologic recovery was different at day 56 in the I compared with D groups, we compared day 56 values of iron biomarkers by t test. We repeated this analysis to test intervening values of iron biomarkers at day 28. The distributions of iron markers other than hemoglobin were not normally distributed. Accordingly, they were log-transformed before analysis, and geometric means were calculated. To prevent negative logarithms, we added 1 to all values of CRP, hepcidin, and ferritin before log transformation. Anthropometric indexes (z scores for height-for-age, weight-for-age, and weight-for-height) were calculated with the 2006 WHO Growth Standards (14).
To identify iron and inflammatory markers associated with iron incorporation at the start of supplementation (day 0 or day 28), we constructed 2 multiple linear regression models. In the first, we included the entire sample of children to identify factors associated with iron incorporation at baseline, that is, after a clinical malaria episode. In the second model, to identify factors associated with iron incorporation when iron is started 28 d after antimalarial treatment, we included only children in the D group. For each model, iron incorporation percentage (day 0 or day 28) was the outcome variable, with iron, inflammatory, and anthropometric markers as predictors. We constructed each model with a stepwise approach, with the use of forward selection and retaining in the model those variables that remained statistically significant at P < 0.05.
We based our sample size of 100 children on the reported variation among children in 57Fe incorporation reported in Doherty et al. (10), with 40 children needed in each group to provide a clinically significant, detectable difference of 4.5% in red cell incorporation between groups, assuming 80% power and α = 0.05. We recruited 100 children, that is, 50 children in each group, to allow for up to 20% loss to follow-up.
Results
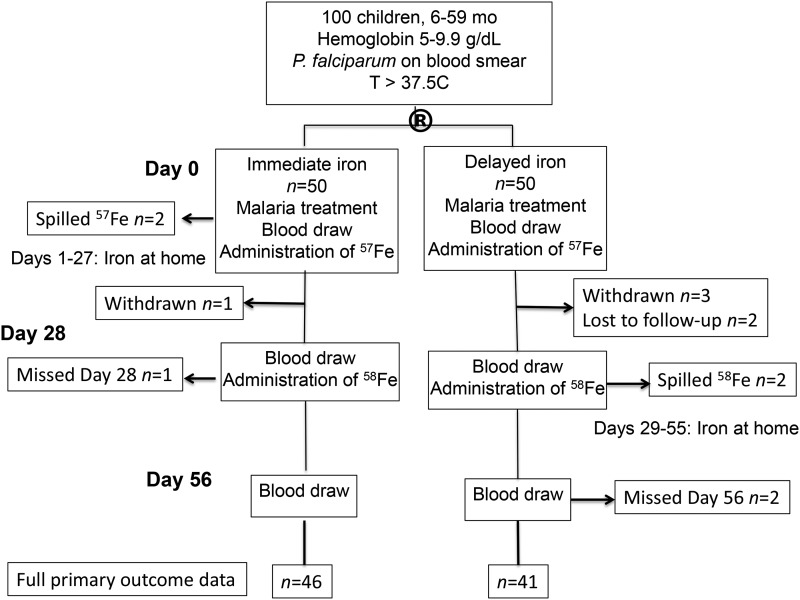
Of the 100 children enrolled, all had ZPP ≥80 μmol/mol heme and were randomly assigned to the I or D iron groups. Four children were withdrawn (1 in the I group; 3 in the D group) and 2 were lost to follow-up (both in the D group; Figure 1). Two children in the I group spilled 57Fe on day 0, and 1 child missed the clinic visit on day 28, whereas 2 children in the D group spilled 58Fe, and 2 children missed the clinic visit on day 56, permitting assessment of the primary aim of incorporation at the time of initial iron supplementation possible for 46 children in the I group and 41 children in the D group. The study groups did not differ significantly in age or sex (Table 1). Approximately 20% of children in each group were stunted (height-for-age z score <−2) and more than one-third were underweight (weight-for-age z score <−2). Median malaria parasite density was equivalent between the groups.
FIGURE 1.
Consort flow diagram. The circled R indicates random assignment.
TABLE 1.
Baseline characteristics of Ugandan children with malaria and anemia by study group1
| Immediate (n = 50) | Delayed (n = 50) | P | |
| Age, y | 2.2 ± 1.2 | 2.2 ± 1.1 | 0.792 |
| Sex, M | 30 (60) | 27 (54) | 0.623 |
| HAZ4 | −1.4 ± 1.1 | −1.3 ± 1.0 | 0.57 |
| HAZ <−2 | 11 (22.9) | 10 (21.7) | 0.89 |
| WHZ4 | −0.85 ± 1.2 | −0.78 ± 1.1 | 0.76 |
| WHZ <−2 | 8 (16.7) | 5 (10.8) | 0.42 |
| WAZ5 | −1.5 ± 1.1 | −0.78 ± 1.1 | 0.59 |
| WAZ <−2 | 19 (38.0) | 17 (34.7) | 0.73 |
| Malaria parasite density,6 parasites/μL | 46,700 [4600; 111,000] | 31,300 [1240; 94,100] | 0.407 |
Values are means ± SDs, medians [IQRs], or n (%). HAZ, height-for-age z score; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
Determined by t test that compared immediate with delayed groups (all means).
Determined by χ2 test (all proportions).
Immediate, n = 48; delayed, n = 46.
Delayed, n = 49.
Immediate, n = 46; delayed, n = 43.
Wilcoxon’s rank sum test compared immediate with delayed groups.
Iron incorporation (mean ± SE) was equivalent between children in the I and D treatment groups on day 0 (I group: 7.9% ± 0.5%; D group: 9.5% ± 1.0%; P = 0.15). On day 28, mean iron incorporation among children in the I group was 6.2% ± 0.9%, and mean incorporation in the D group was 16.5% ± 1.7% (P < 0.001). Starting iron therapy 28 d after antimalarial treatment rather than concurrently with antimalarial treatment was thus associated with a statistically significant, 2 times greater percent of iron incorporation at the time of initial supplement administration than concurrent administration of antimalarial treatment and iron therapy (I group compared with D group: 7.9% compared with 16.5%; P < 0.001). This difference remained significant after controlling for baseline plasma ferritin concentration (P < 0.001). Exclusion of 6 children (4 in the I group; 2 in the D group) within the inclusion hemoglobin range, but still necessitating blood transfusion, also did not affect these results (P-difference < 0.001).
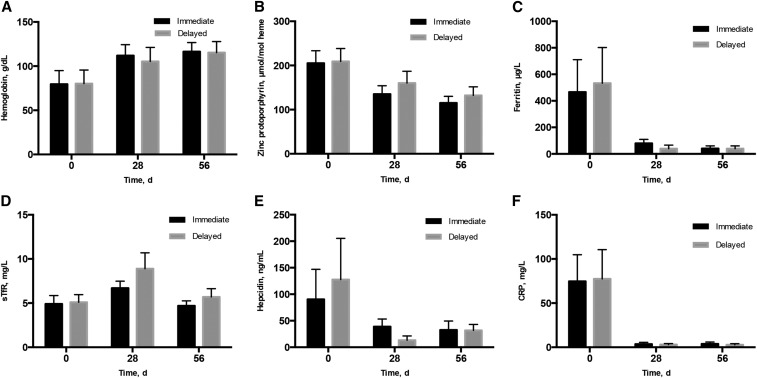
Hematologic, iron status, and inflammatory markers were equivalent between the treatment groups at baseline and collectively reflected inflammation and functional iron deficiency (Figure 2). All children were anemic (hemoglobin: <100.0 g/L) by inclusion criteria. Mean hemoglobin was ∼80.0 g/L, and geometric mean ZPP was just >200 μmol/mol heme in each group. Geometric mean plasma ferritin was ∼500 μg/L in each group, and geometric mean plasma concentrations of CRP and hepcidin were correspondingly high, at >70 mg/L and >90 ng/mL, respectively. Geometric mean plasma sTfR concentration was similar between the groups and was below the iron deficiency threshold of 8.3 mg/L, although 14 children (7 in the I group; 7 in the D group) did have a plasma TfR concentration >8.3 mg/L.
FIGURE 2.
Plasma iron and inflammatory markers at day 0, day 28, and day 56 in 100 Ugandan children aged 6–59 mo with malaria and anemia who started iron therapy concurrently with antimalarial treatment (immediate, I) or 28 d after antimalarial treatment (delayed, D). Error bars reflect SD for arithmetic mean and 95% CI for geometric means. Sample size for indicators (day 0, day 28, day 56) were as follows: hemoglobin (I group: 50, 43, 42; D group: 49, 41, 42), zinc protoporphyrin (I group: 50, 46, 45; D group: 49, 43, 42), plasma ferritin (I group: 50, 45, 43; D group: 48, 40, 43), plasma sTfR (I group: 50, 45, 43; D group: 48, 41, 43), plasma hepcidin (I group: 48, 45, 42; D group: 47, 39, 43), and plasma CRP (I group: 48, 45, 43; D group: 47, 41, 41). CRP, C-reactive protein; sTfR, soluble transferrin receptor.
At day 28, after all children had been treated for malaria, but only children in the I group were administered daily iron supplementation for 27 d, inflammation, as measured by CRP, was largely resolved in both groups. Hemoglobin increased in both groups but was significantly higher among children in the I group than in the D group (112 ± 12.4 compared with 105 ± 15.9 g/dL; P = 0.04). A total of 18 children (5 in the I group; 13 in the D group) remained anemic with hemoglobin <100.0 g/L (χ2 P = 0.18). ZPP declined by day 28, but all children still had a ZPP value >80 μmol/mol heme, and ZPP [geometric mean (95% CI)] was not statistically significantly different between the treatment groups [I group: 135 μmol/mol heme (119, 154 μmol/mol heme) compared with D group: 160 μmol/mol heme (137, 187 μmol/mol heme); P = 0.10]. Remaining plasma markers [geometric mean (95% CI)] suggested better iron status among children in the I group than in the D group at day 28 as follows: ferritin was greater [79.9 μg/L (58.3, 109.6 μg/L) compared with 39.3 μg/L (23.5, 65.7 μg/L); P = 0.02], sTfR was lower [6.7 mg/L (6.1, 7.5 mg/L) compared with 8.9 mg/L (7.4, 10.5 mg/L); P = 0.01], and hepcidin was higher [38.8 ng/mL (28.3, 53.3 ng/mL) compared with 13.3 ng/mL (8.3, 21.2 ng/mL); P < 0.001].
By day 56, after children in the D group were administered 27 d of iron supplementation but children in the I group were administered no additional iron supplementation, all markers were equivalent between the 2 treatment groups. Hemoglobin was >100.0 g/L in all but 5 children (2 in the I group; 3 in the D group). ZPP concentrations declined further from day 28, but ZPP [geometric mean (95% CI)] remained elevated in each group [I group: 115 μmol/mol heme (101, 130 μmol/mol heme); D group: 132 μmol/mol heme (114, 152 μmol/mol heme); P = 0.14], and only 14 children (9 in the I group; 5 in the D group) had a ZPP concentration <80 μmol/mol heme. Mean plasma ferritin was ∼40 μg/L in both groups (P = 0.94). Mean (95% CI) plasma sTfR tended (P = 0.05) to be higher among children in the D group [I group: 4.7 mg/L (4.3, 5.3 mg/L); D group: 5.7 mg/L (4.9, 6.6 mg/L)]. Eleven children (7 in the I group; 4 in the D group) had a plasma ferritin concentration <12 μg/L, whereas 11 children had a plasma sTfR concentration >8.3 mg/L (2 in the I group; 9 in the D group). Two children, both in the D group, had both low plasma ferritin and elevated sTfR. Both plasma hepcidin and CRP had normalized by day 56 and were equivalent between the groups.
In bivariate analysis, baseline log sTfR (β = 6.4, R2 = 10.1%, P < 0.01), log hepcidin (β = −2.2, R2 = 8.9%, P < 0.01), and log ZPP (β = 7.2, R2 = 7.5%, P = 0.01) concentrations significantly predicted iron incorporation of 57Fe. Anthropometric indexes, hemoglobin, log ferritin, log CRP, or log malaria parasite density were not significantly associated with incorporation of 57Fe (all P > 0.05). Age was marginally significant when added alone (P = 0.07), but in multivariate models sTfR concentration along with age best predicted 57Fe incorporation (R2 = 18.9%), with greater 57Fe incorporation associated with both older age and greater concentrations of sTfR.
On day 28, concentrations of hepcidin (β = −10.1, R2 = 37.9%, P = <0.001), ferritin (β = −7.5, R2 = 19.9%, P < 0.001), CRP (β = −4.5, R2 = 5.7%, P = 0.04), ZPP (β = 11.0, R2 = 5.8%, P = 0.03), and sTfR (β = 15.0, R2 = 11.3%, P < 0.01) all significantly predicted 58Fe incorporation among children who did not receive iron until day 28, that is, among children in the D group. Hemoglobin concentration at day 28 did not significantly predict 58Fe incorporation, but baseline hemoglobin did, with greater baseline hemoglobin associated (β = 1.4, R2 = 5.3%, P = 0.04) with higher iron incorporation at day 28. Multivariate analysis revealed that lower concentrations of hepcidin at day 28 and greater day 0 hemoglobin concentrations together best predicted 58Fe, that is, iron incorporation begun 28 d after antimalarial treatment, accounting for 41.2% of the variability in 58Fe incorporation.
Forty-one of the 100 children (25 in the I group; 16 in the D group) visited the study clinic during the 8 wk of enrollment for ≥1 illness. Twenty children had a diagnosis of malaria (12 in the I group; 8 in the D group). Neither the number of children who had ≥1 sick child visit (P = 0.07) nor the number of children with a malaria diagnosis during a sick child visit (P = 0.32) differed significantly between the treatment groups.
Discussion
We aimed to determine whether oral iron therapy started concurrently with antimalarial treatment according to the WHO standard of care or iron therapy started 28 d after antimalarial treatment resulted in better iron incorporation at the time of initial supplement administration and improved hematologic recovery at day 56 in Ugandan children with anemia and malaria. Our primary findings were 1) iron incorporation was twice as great if iron supplementation was started 28 d after antimalarial treatment compared with concurrently with antimalarial treatment; 2) values of iron and inflammatory markers were equivalent at day 56 regardless of whether iron supplementation was started immediately or after 28 d; and 3) intervening day 28 values of iron and inflammatory markers suggest that poorer iron status, rather than lower inflammation, among children in the D group explained the greater iron incorporation at the start of supplementation, that is, at day 28, in that group.
Our iron incorporation value of ∼8% at baseline is consistent with 2 studies of iron incorporation in young children with malaria (10, 15). However, our findings that iron incorporation is 2 times greater if the start of oral iron supplementation is delayed by 4 wk after antimalarial treatment are in contrast to those of Glinz et al. (15) who found no significant difference in iron incorporation in Malawian children aged 12–24 mo who were administered iron supplements at the same time compared with 2 wk after antimalarial treatment. Potential explanations for the difference between that study and our own include the difference in the time of delay of iron supplementation, that is, 2 wk compared with 4 wk, and also the iron dose, which at 30 mg for the 12- to 24-mo-old children in the Malawian study was considerably greater than ours and could have obscured differences in the regulation of iron absorption that occur with a lower dose. In a separate study in Ivorian school-age children, Glinz et al. (16) did find that afebrile P. falciparum infection significantly reduced iron absorption by one-half, although it did not affect systemic iron utilization.
Although we did find that iron incorporation is significantly greater if iron supplementation is started 28 d after antimalarial treatment, our data do not support our original hypothesis that greater incorporation at day 28 would be due to lower inflammation and more complete liberation of body iron from storage compartments. Rather, as evidenced by the lower hemoglobin, ferritin, and hepcidin concentrations and greater sTfR concentrations, children in the D group appeared to be more iron deficient at day 28, which likely drove the greater isotopic iron incorporation in this group at this time. Concentrations of CRP were equivalent between the groups at day 28, suggesting that differences in iron biomarkers at this time point were attributable to iron status alone and not to inflammation. However, all iron markers were equivalent between the groups by day 56.
We based the 28-d delay in the start of iron supplementation in the present study on the results of Doherty et al. (10), who found that iron incorporation remained significantly suppressed 15 d after antimalarial treatment in children recovering from postmalarial anemia compared with children recovering from iron deficiency anemia alone. Further, P. falciparum-induced increases in hepcidin concentration only normalized after 4 wk in young Tanzanian children recovering from malaria (12). However, on the basis of the results from the present study, it appears that in children with malaria and anemia a 4-wk delay may be longer than necessary. The degree of inflammation and resulting amount of iron trapped in reticuloendothelial stores might be an important factor to consider. We recently reported a study with the same randomization design of immediate compared with 28-d delayed iron, but without stable isotope assessment, in Ugandan children with severe malaria (cerebral malaria or severe malarial anemia) (17). In that study, hepcidin was lower and ferritin was higher at day 28 in children who were administered iron supplements immediately, as in the present study, but hemoglobin, ZPP, and sTfR were not statistically significantly different between the I and D groups. It is possible that because children with severe malaria in the previous study were considerably more inflamed, having a mean CRP concentration >500 mg/L compared with ∼70 mg/L in in the present study, their iron delivery could be sustained longer by the liberation of iron from stores after antimalarial treatment than children in the present study whose iron stores were seemingly depleted by day 28.
With our study design, we are unable to determine when hepcidin and CRP declined between day 0 and day 28, permitting absorption and distribution of the iron supplement and improvement of iron status in children in the I group. It is possible that hepcidin and CRP concentrations decreased after 2 wk, permitting iron to be absorbed for 2 wk before the assessment point on day 28 when the children in the D group first were administered iron supplements. By nearly all biomarkers, however, children in the D group had poorer iron status at this point, with lower hemoglobin, ferritin, and hepcidin and greater sTfR. All values were equivalent, at day 56, after children in the D group were administered iron for 27 d.
A consideration that we are unable to address with the present study design is long-term outcomes associated with immediate compared with delayed iron therapy. For example, despite having initially poorer iron status at day 28, it remains unknown if children in the D group have better long-term iron status or fewer infections. Morbidity was not significantly different in the present study between the treatment groups, and, although there was a trend toward more children with ≥1 return visit to the health clinic in the I group, this study was not powered for those outcomes nor was the follow-up time ample for full surveillance. If long-term morbidity was mitigated in children whose iron supplementation was delayed by 28 d, it remains unclear if having transiently poorer iron status might be acceptable. Only adequately powered clinical trials with longer follow-up can answer these questions. The results from the present study do not demonstrate a clear benefit in delaying iron therapy by 4 wk in children with malaria in terms of iron status after 8 wk, despite suboptimal iron incorporation when iron therapy is started concurrently with antimalarial treatment, but they do demonstrate that iron status at 8 wk is no different if iron therapy is started immediately with antimalarial treatment or is delayed.
Acknowledgments
SEC, ROO, SAA, CCJ, MKG, and EM designed the research; SEC, ROO, SAA, and EM conducted the research; SAA and CCJ provided essential reagents or materials; SEC analyzed the data, performed the statistical analysis, wrote the paper, and has responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; D, delayed iron group; I, immediate iron group; sTfR, soluble transferrin receptor; ZPP, zinc protoporphyrin.
References
- 1.Sazawal S, Black RE, Ramsan M, Chwaya HM, Dutta A, Dhingra U, Stoltzfus RJ, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006;367:133–43. [DOI] [PubMed] [Google Scholar]
- 2.Gwamaka M, Kurtis JD, Sorensen BE, Holte S, Morrison R, Mutabingwa TK, Fried M, Duffy PE. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis 2012;54:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, Alexander ND, Bäck R, Kortok M, Chemtai AK, Marsh K, Williams TN. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis 2004;190:439–47. [DOI] [PubMed] [Google Scholar]
- 4.Cusick SE, Georgieff MK. Nutrient supplementation and neurodevelopment: timing is the key. Arch Pediatr Adolesc Med 2012;166:481–2. [DOI] [PubMed] [Google Scholar]
- 5.Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci 2014;1308:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull 2007;28(4 Suppl):S621–7. [DOI] [PubMed] [Google Scholar]
- 7.Desai MR, Mei JV, Kariuki SK, Wannemuehler KA, Phillips-Howard PA, Nahlen BL, Vulule JM, ter Kuile FO. Randomized, controlled trial of daily iron supplementation and intermittent sulfadoxine-pyramethamine for the treatment of mild childhood anemia in western Kenya. J Infect Dis 2003;187:658–66. [DOI] [PubMed] [Google Scholar]
- 8.Nwanyanwu OC, Ziba C, Kazembe PN, Gamadzi G, Gandwe J, Redd SC. The effect of oral iron therapy during treatment for Plasmodium falciparum malaria with sulphadoxine-pyramethamine on Malawian children under 5 years of age. Ann Trop Med Parasitol 1996;90:589–95. [DOI] [PubMed] [Google Scholar]
- 9.Verhoef H, West CE, Nzyuko SM, de Vogel S, van der Valk R, Wanga MA, Kuijsten A, Veenemans J, Kok FJ. Intermittent administration of iron and sulfadoxine-pyramethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet 2002;360:908–14. [DOI] [PubMed] [Google Scholar]
- 10.Doherty CP, Cox SE, Fulford AJ, Austin S, Hilmers DC, Abrams SA, Prentice AM. Iron incorporation and post-malaria anaemia. PLoS One 2008;3:e2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, Armitage AE, Drakesmith H. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood 2012;119:1922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Mast Q, Nadjm B, Reyburn H, Kemna EH, Amos B, Laarakkers CM, Silalya S, Verhoef H, Sauerwein RW, Swinkels DW, et al. . Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis 2009;199:253–62. [DOI] [PubMed] [Google Scholar]
- 13.Abrams SA. Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr 1999;70:955–64. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization [Internet]. Basel: The WHO Child Growth Standards [cited 2016 Feb 18]. Available from: http://www.who.int/childgrowth/standards/en/.
- 15.Glinz D, Kamiyango M, Phiri KS, Munthali F, Zeder C, Zimmermann MB, Hurrell RF, Wegmüller R. The effect of timing of iron supplementation on iron absorption and haemoglobin in post-malaria anaemia: a longitudinal stable isotope study in Malawian toddlers. Malar J 2014;13:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glinz D, Hurrell RF, Righetti AA, Zeder C, Adiossan LG, Tjalsma H, Utzinger J, Zimmermann MB, N’Goran E, Wegmüller R. In Ivorian school-age children, infection with hookworm does not reduce dietary iron absorption or systemic iron utilization, whereas afebrile Plasmodium falciparum infection reduces iron absorption by half. Am J Clin Nutr 2015;101:462–70. [DOI] [PubMed] [Google Scholar]
- 17.Cusick SE, Opoka RO, Ssemata AS, Georgiegg MK, John CC. Comparison of iron status 28 d after provision of antimalarial treatment with iron therapy compared with antimalarial treatment alone in Ugandan children with severe malaria. Am J Clin Nutr 2016;103:919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]