Abstract
Background: Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States, with a 5-y survival rate of ∼65%. Therefore, the identification of modifiable health factors to improve CRC survival is crucial.
Objective: We investigated the association of 4 prediagnostic a priori diet quality indexes with CRC-specific and all-cause mortality in the Multiethnic Cohort (MEC).
Methods: The MEC included >215,000 African-American, Native Hawaiian, Japanese-American, Latino, and white adults living in Hawaii and California who completed a validated quantitative food-frequency questionnaire in 1993–1996. CRC cases and deaths were identified through linkages to cancer registries and to state and national vital registries. Sex-specific HRs and 95% CIs were estimated for the Healthy Eating Index (HEI) 2010, the Alternative HEI (AHEI) 2010, the alternate Mediterranean Diet (aMED) score, and the Dietary Approaches to Stop Hypertension (DASH) index with CRC-specific and overall mortality as the primary outcomes. Ethnicity-specific analyses were the secondary outcomes.
Results: Among 4204 MEC participants diagnosed with invasive CRC through 2010, 1976 all-cause and 1095 CRC-specific deaths were identified. A higher aMED score was associated with lower CRC-specific mortality in women [HR continuous pattern score divided by its respective SD (HR1SD): 0.86; 95% CI: 0.77, 0.96] but not in men (HR1SD: 1.01; 95% CI: 0.92, 1.11). A higher aMED score was also associated with lower all-cause mortality in women (HR1SD: 0.88; 95% CI: 0.81, 0.96) but not in men (HR1SD: 1.00; 95% CI: 0.93, 1.07). The HEI-2010, AHEI-2010, and DASH index were not significantly associated with CRC-specific or with all-cause mortality. The inverse relation for the aMED score was limited to African Americans and to colon (compared with rectal) cancer.
Conclusions: The aMED score was related to lower mortality only in African-American women (1 of 5 ethnic groups studied). The results should be interpreted with caution due to the small numbers of cases within ethnic groups and the issue of multiple testing.
Keywords: colorectal cancer, nutrition, Healthy Eating Index, Alternative Healthy Eating Index, alternate Mediterranean Diet score, Dietary Approaches to Stop Hypertension index, dietary patterns, survival, Cox regression, Multiethnic Cohort
Introduction
Colorectal cancer (CRC)8 is the fourth most commonly diagnosed malignancy and the second leading cause of cancer-related death in the United States, with a 5-y survival rate of ∼65% (1). Understanding the impact of modifiable health behaviors, such as physical activity and optimal nutrition (2), on prognosis is therefore critical. In recent years, dietary patterns have been promoted as a way to better capture the complexity of dietary intake than single foods or nutrients (3). A priori indexes evaluate dietary quality on the basis of dietary recommendations and existing scientific evidence, whereas a posteriori–derived dietary patterns are identified through exploratory data-driven approaches (3). Studies investigating diet quality and CRC etiology reported that higher scores on the Healthy Eating Index (HEI) and the Alternative HEI (AHEI), as well as certain a posteriori patterns, were associated with a lower risk of developing CRC (4, 5). The few studies that investigated dietary indexes in relation to CRC survival are contradictory. One US study reported lower CRC-specific mortality among rectal cancer cases with higher prediagnostic HEI-2005 scores but not among colon cancer cases (6). In a European cohort, greater prediagnostic concordance with the World Cancer Research Fund guidelines was associated with a lower CRC-specific mortality (7). Although higher postdiagnostic AHEI-2010 scores predicted lower all-cause mortality in 1201 women with CRC (8), the AHEI-2010, the alternate Mediterranean Diet (aMED) score, the Dietary Approaches to Stop Hypertension (DASH) index, and a posteriori Western and prudent patterns were not significantly related to CRC-specific mortality. In contrast, a posteriori patterns rich in meat as well as higher meat consumption predicted a poorer CRC prognosis and all-cause mortality in several analyses (9–12).
Poor prediagnostic diet quality is linked to a suboptimal micronutrient status that is likely to become even worse after diagnosis (e.g., due to adverse effects from treatment). Certain micronutrients have an impact on oxidative stress (13) and cell differentiation (14), both predictors of CRC risk and progression. Micronutrient status may therefore be a potential underlying biological mechanism of the association of dietary patterns with survival. Recent studies also point toward a role of the human gut microbiome composition as a potential mediator of diet and CRC development and progression (15, 16). Most of the published studies were conducted in relatively homogenous populations composed of non-Hispanic whites. Given that Japanese Americans and African Americans are at a higher risk to develop CRC than whites (17) and that African Americans experience higher CRC mortality (1), research in ethnically diverse populations is of great interest. We therefore investigated the association of 4 prediagnostic a priori indexes—the HEI-2010 (18), the AHEI-2010 (19), the aMED score (20), and the DASH index (21)—with all-cause and CRC-specific mortality among white, African-American, Japanese-American, Native Hawaiian, and Latino participants with CRC in the Multiethnic Cohort (MEC).
Methods
Study population.
The MEC is an ethnically diverse prospective cohort designed to investigate the association of lifestyle and genetic factors with the incidence of cancer. The design and implementation of the MEC have been described elsewhere (22). Briefly, >215,000 men and women aged 45–75 y at recruitment, and residing in Hawaii or California (primarily Los Angeles County), were enrolled in the cohort between 1993 and 1996. To obtain a multiethnic sample of whites, African Americans, Native Hawaiians, Japanese Americans, and Latinos, a population-based sampling frame used drivers’ license files, supplemented with voter registration lists and Health Care Financing Administration (Medicare) files. The institutional review boards at the University of Hawaii and the University of Southern California approved the study protocol.
Incident colon and rectal cancer cases were identified through regular linkages to the Los Angeles County Cancer Surveillance Program, the State of California Cancer Registry, and the statewide Hawaii Tumor Registry, all members of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Dates and causes of death were identified by routine linkages with California and Hawaii vital records and the National Death Index databases. Information on incident cases and/or death ascertainment was available up to 31 December 2010. Among eligible participants of the 5 major ethnic groups who had not developed colon or rectal cancer before cohort entry, 4832 newly diagnosed invasive CRC cases were identified through 2010. After exclusions [272, not adenocarcinoma; 179, invalid diet; 1, did not survive after diagnosis; 69, missing BMI at cohort entry; and 166, with BMI (in kg/m2) <18.5, with some overlap], 4204 cohort members diagnosed with invasive CRC during follow-up were included in the current analysis.
Data collection.
At cohort entry, participants completed a self-administered, 26-page questionnaire [questionnaire at cohort entry (Qx1)] that collected self-reported demographic characteristics, height and body weight, medical history, family history of colon and rectal cancer, physical activity, and a diet history by using a quantitative FFQ (QFFQ). The QFFQ asked participants to report their average frequency of consumption and serving sizes for >180 food items during the past year. A calibration study indicated acceptable correlations between the QFFQ and 24-h recalls for all sex and ethnic groups (23). Between 1999 and 2002, ∼85% of eligible MEC members completed a brief follow-up questionnaire [questionnaire 2 (Qx2)] providing updated information on self-reported body weight. In 2003–2008, a subset of MEC participants responded to a follow-up questionnaire [questionnaire 3 (Qx3)] that included a full QFFQ. Information on stage at diagnosis and first course of treatment but not recurrence was available from the SEER registries in Hawaii and Los Angeles.
Dietary indexes.
Dietary indexes were a priori defined and based on work conducted by the Dietary Patterns Methods Project (24–26). Scoring was based on food groups from the MyPyramid Equivalents Database. The portion sizes were converted to cup and ounce equivalents as required for MyPyramid Equivalents Databases. The 4 indexes, as described in detail previously (26), use different scoring systems and focus on diverse aspects of the diet, although they share an emphasis on several major food groups (Supplemental Table 1). The HEI-2010 includes 12 components (total fruit, whole fruit, total vegetables, dark green vegetables and legumes, whole grains, dairy, total protein foods, seafood and plant proteins, ratio of PUFAs and MUFAs to SFAs, refined grains, sodium, and empty calories) and reflects the 2010 Dietary Guidelines for Americans, with higher scores reflecting better adherence to federal dietary guidelines (18). The AHEI-2010 includes 11 foods and nutrients [total vegetables excluding potatoes, whole fruit, whole grains, sugar-sweetened beverages and fruit juice, nuts, soy and legumes, trans FAs, long-chain (n–3) FAs (EPA + DHA), PUFAs, sodium, alcohol, and red and processed meat] predictive of chronic diseases such as type 2 diabetes or cardiovascular disease (19).
The aMED score, as developed by Fung et al. (20), includes 9 components (total vegetables excluding potatoes, total fruit, nuts, legumes, fish, whole grains, MUFA to SFA ratio, alcohol, and red and processed meat) and was an adaptation of the Mediterranean Diet Score developed by Trichopoulou et al. (27) that takes into account scientific literature on diet and chronic disease risk. The DASH index as outlined by Fung et al. (21) includes 8 components (total vegetables excluding potatoes; total fruit; nuts, seeds, and legumes; low-fat dairy; whole grains; sodium; sugar-sweetened beverages and fruit juices; and red and processed meat) that are emphasized in the DASH diet designed for hypertension management.
All 4204 CRC cases in this study had information on dietary patterns derived from the questionnaire at cohort study (Qx1; 1993–1996). Of these, 35.8% completed Qx3; 953 participants did so after their CRC diagnosis (with 77 CRC-specific and 212 all-cause deaths) and 552 CRC cases before their CRC diagnosis (with 97 CRC-specific and 151 all-cause deaths). Given the low number of CRC cases with information on postdiagnostic diet, we used prediagnostic dietary index scores derived from Qx1 for the current analysis. Nevertheless, correlations between prediagnostic (Qx1) and postdiagnostic (Qx3) dietary index scores indicated acceptable consistency with the following correlation coefficients: HEI-2010 = 0.50, AHEI = 0.52, aMED = 0.46, and DASH = 0.55 (all P < 0.0001). The mean differences between pre- and postdiagnostic dietary index score points indicated a slight improvement of the HEI-2010 score after diagnosis (HEI-2010: 4.3 score points) and no substantial changes in the AHEI-2010, aMED, and DASH scores (AHEI-2010: 1.3 score points; aMED: −0.21 score points; and DASH: −0.22 score points).
Statistical analysis.
To evaluate the association of dietary indexes with all-cause and CRC-specific death, we computed multivariable-adjusted HRs and 95% CIs with the use of Cox proportional hazards models of mortality separately for men and women as our primary outcome. Given sex differences in a previous study (10), we decided a priori to analyze men and women separately. We formally tested the Schoenfeld residual regression and found that the proportional hazards assumption of the Cox model was fulfilled. Age was used as the time metric, beginning with the age at CRC diagnosis and ending with the age at death or censoring on 31 December 2010. For CRC-specific death, deaths due to other causes were censored. The dietary index scores were divided into quartiles on the basis of the baseline distribution of CRC cases. However, due to the limited range in scores for the aMED and DASH the 4 categories may be slightly different. To evaluate possible dose-response relations and to compare the regression parameters across indexes, trend variables based on the ratio of each index value by its respective standard deviation were tested. In addition, trend tests were performed across index score quartiles while modeling the medians as continuous variable.
Self-reported hypertension, heart disease, and stroke from Qx1 were used to create a variable for comorbidity (0, 1, or 2+). Various covariates were included in the models as potential confounders based on previous publications and on survival analyses within the MEC (28, 29). In the minimally adjusted model, we included age at CRC diagnosis in 10-y age groups, ethnicity, and SEER tumor stage (local, regional, distant, or unknown). In the fully adjusted model, we additionally included education, family history of CRC, BMI, smoking status and number of pack-years, physical activity, total energy intake, comorbidity, SEER tumor stage (local, regional, distant, or unknown), radiation and chemotherapy treatment, and nonsteroidal anti-inflammatory drug (NSAID) use. Physical activity was divided into <0.5 or ≥0.5 h/d spent performing moderate or vigorous activities. Family history of colon or rectal cancer included a self-report of the cancer in the participant’s natural father, mother, or full siblings. Education was coded as high school or less, vocational school or some college, and undergraduate or graduate degree. Cigarette smoking was classified as never, past, or current and pack-years were also computed. On the basis of questions about aspirin or other pain medication, excluding acetaminophen, NSAID use was coded as ever (≥2 times/wk for ≥1 mo) or never. BMI based on self-reported height and weight measures was classified as normal weight (18.5 to <25), overweight (25–29.9), or obese (≥30). For covariates with missing values (i.e., education, smoking status, physical activity, NSAID use, tumor stage, and treatment variables), a missing category was created. BMI was treated as a time-varying exposure by using values from the questionnaire at cohort entry (Qx1) and Qx2, as appropriate (30). BMI at Qx1 was modeled for risk sets before the age at Qx2, and BMI at Qx2 was modeled for risk sets after the age at Qx2. To exclude the possibility that a broad categorization of physical activity and BMI introduced residual confounding, we re-conducted the main analysis with physical activity and BMI as continuous variables. The results were virtually unchanged (data not shown) as were the risk estimates when the year of diagnosis was included to control for cohort effects in dietary patterns and treatment regimens (data not shown).
We investigated the importance of individual score components for the association of the dietary pattern scores with CRC-specific and all-cause mortality separately for men and women by including all individual components simultaneously in a model for each of the 4 indexes. For significant components, we performed confirmatory analyses with the respective individual component only. We examined potential interactions of dietary indexes with ethnicity by using a global Wald test of the cross-product terms modeling dietary indexes as a continuous variable.
In secondary analyses, we explored ethnicity-specific models and performed analyses stratified by postmenopausal estrogen treatment, which was categorized as never estrogen use compared with past/current use as reported at cohort entry (Qx1). In addition, cancers of the colon and the rectum were examined separately in relation to dietary indexes, which were significantly related with mortality in the main analysis. Finally, we stratified the analysis by stage of disease at diagnosis.
For our main analyses, Bonferroni correction was used to adjust for multiple comparison (8 tests: 4 dietary indexes × 2 sexes) for each hypothesis (CRC-specific mortality and all-cause mortality), and P-trend < 0.0065 was deemed significant. For all other analyses, significance was defined as P < 0.05. All of the analyses were conducted in SAS version 9.3 (SAS Institute).
Results
Of the 4204 CRC cases (Table 1), 1441 were Japanese American, 842 African American, 840 white, 805 Latino, and 276 Native Hawaiian. The mean ± SD age at diagnosis was 71.4 ± 8.7 y. Cases were diagnosed between cohort entry (1993–1996) and December 2010, and the mean follow-up time was 6.0 ± 4.7 y. The majority of cases were diagnosed at a localized (n = 1854) or regional (n = 1605) stage compared with a distant (n = 647) or unknown (n = 98) stage. The sample included 1645 men and 1580 women with colon cancer, 591 men and 354 women with rectal cancer, and 22 men and 12 women with a mixed form of cancer. The respective percentages of overweight and obese participants at cohort entry were 40% and 22%, respectively. Men and women in the highest dietary index quartile had lower BMIs, were more likely to be never smokers, and reported higher physical activity. The 4 indexes were strongly associated with each other (Supplemental Table 2), with the highest correlations between HEI-2010 and DASH scores and the lowest between HEI-2010 and aMED scores.
TABLE 1.
Participant characteristics at baseline by lowest and highest quartiles of the 4 dietary indexes separated by sex in the Multiethnic Cohort1
| HEI-2010 |
AHEI-2010 |
aMED |
DASH |
||||||
| All, n | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Men | |||||||||
| Index score points | 2258 | 51.6 (7.26) | 77.9 (6.29) | 52.4 (6.03) | 75.1 (6.27) | 2 (1) | 6 (1) | 19 (3) | 29 (3) |
| Cases, n | 2258 | 564 | 564 | 564 | 564 | 454 | 528 | 559 | 494 |
| Age at diagnosis, y | 2258 | 70.0 (12.5) | 75.0 (10.5) | 70.0 (13.0) | 74.0 (11.5) | 72.0 (12.0) | 73.0 (11.0) | 68.0 (13.0) | 75.0 (10.0) |
| Ethnicity, % | |||||||||
| White | 449 | 18.1 | 24.1 | 20.6 | 20.0 | 19.2 | 18.9 | 13.2 | 24.5 |
| African-American | 339 | 12.8 | 19.7 | 16.8 | 14.4 | 16.7 | 16.9 | 15.4 | 17.2 |
| Native-Hawaiian | 147 | 6.56 | 6.21 | 6.56 | 6.38 | 4.85 | 7.95 | 7.69 | 4.66 |
| Japanese-American | 845 | 36.5 | 37.1 | 28.9 | 46.3 | 32.6 | 41.1 | 46.0 | 30.4 |
| Latino | 478 | 26.1 | 12.9 | 27.1 | 12.9 | 26.7 | 15.2 | 17.7 | 23.3 |
| BMI (kg/m2), % | |||||||||
| 18.5 to <25 | 807 | 39.5 | 38.1 | 37.8 | 37.6 | 33.7 | 35.0 | 34.2 | 37.9 |
| ≥25 to <30 | 1042 | 40.4 | 46.3 | 42.2 | 46.8 | 46.5 | 45.8 | 45.1 | 47.6 |
| ≥30 | 409 | 20.0 | 15.6 | 20.0 | 15.6 | 19.8 | 19.1 | 20.8 | 14.6 |
| Smoking status,2 % | |||||||||
| Smoking, pack-years | 2258 | 12.0 (30.5) | 3.99 (19.8) | 12.0 (31.2) | 7.75 (27.5) | 10.2 (27.5) | 7.75 (27.5) | 12.0 (30.5) | 3.88 (19.8) |
| Never | 569 | 20.0 | 33.7 | 21.6 | 27.1 | 22.9 | 25.2 | 20.8 | 32.2 |
| Past | 1269 | 49.8 | 57.3 | 50.4 | 61.9 | 53.3 | 62.3 | 49.0 | 59.1 |
| Current | 404 | 29.4 | 8.51 | 27.5 | 10.6 | 23.1 | 12.1 | 29.2 | 8.30 |
| Physical activity (moderate and vigorous),3 % | |||||||||
| <30 min/d | 859 | 45.4 | 31.2 | 45.0 | 30.9 | 48.0 | 29.9 | 43.3 | 30.4 |
| ≥30 min/d | 1363 | 53.6 | 67.0 | 53.4 | 67.9 | 50.7 | 68.9 | 55.5 | 67.4 |
| Education, % | |||||||||
| ≤12 y | 1057 | 52.8 | 37.4 | 50.0 | 42.7 | 49.8 | 42.1 | 50.5 | 39.5 |
| 13–15 y | 652 | 28.9 | 32.1 | 30.1 | 28.9 | 29.3 | 33.3 | 28.6 | 32.0 |
| ≥16 y | 549 | 18.3 | 30.5 | 19.9 | 28.4 | 20.9 | 24.6 | 20.9 | 28.5 |
| Family history of CRC,4 % | 213 | 10.8 | 9.22 | 11.2 | 7.09 | 10.1 | 8.52 | 13.4 | 8.91 |
| Ever NSAID use,5 % | 1041 | 42.7 | 44.7 | 44.7 | 46.5 | 47.1 | 47.9 | 41.9 | 47.6 |
| Stage of disease, % | |||||||||
| Local | 1018 | 42.0 | 48.4 | 42.7 | 48.8 | 40.5 | 46.8 | 44.5 | 47.4 |
| Regional | 842 | 38.7 | 35.3 | 36.7 | 35.8 | 38.1 | 38.1 | 37.9 | 36.4 |
| Distal | 349 | 16.3 | 14.4 | 18.1 | 13.3 | 18.3 | 13.5 | 15.9 | 14.2 |
| Unknown | 49 | 3.01 | 1.95 | 2.48 | 2.13 | 3.08 | 1.70 | 1.61 | 2.02 |
| Radiation therapy,6 % | 244 | 14.0 | 7.45 | 13.1 | 8.33 | 11.01 | 9.47 | 14.3 | 6.68 |
| Chemotherapy,7 % | 724 | 36.7 | 27.3 | 34.6 | 31.0 | 34.4 | 33.0 | 36.0 | 28.5 |
| Alcohol intake, g ethanol/d | 2258 | 4.50 (36.0) | 1.64 (12.4) | 2.52 (45.1) | 4.84 (16.5) | 2.46 (25.9) | 3.65 (16.5) | 6.52 (30.7) | 1.54 (13.2) |
| Red meat consumption, g/d | 2258 | 70.3 (66.9) | 36.0 (43.2) | 68.1 (69.2) | 44.1 (51.7) | 53.9 (50.5) | 56.8 (70.0) | 74.8 (58.8) | 36.2 (45.9) |
| Fruit consumption, g/d | 2258 | 69.3 (95.3) | 307 (262) | 84.8 (105) | 294 (240) | 77.3 (98.0) | 318 (266) | 70.4 (87.0) | 372 (295) |
| Women | |||||||||
| Index score points | 1946 | 56.5 (7.77) | 82.4 (5.08) | 54.1 (5.41) | 75.3 (5.64) | 2 (1) | 6 (1) | 18 (3) | 29 (3) |
| Cases, n | 1946 | 486 | 486 | 486 | 486 | 414 | 464 | 453 | 422 |
| Age at diagnosis, y | 1946 | 69.0 (13.0) | 74.5 (11.0) | 70.0 (13.0) | 73.5 (12.0) | 71.0 (13.0) | 73.0 (12.5) | 69.0 (13.0) | 74.0 (11.0) |
| Ethnicity, % | |||||||||
| White | 391 | 18.3 | 22.0 | 21.2 | 18.3 | 24.9 | 16.8 | 24.9 | 16.8 |
| African-American | 503 | 22.6 | 33.3 | 29.6 | 23.9 | 26.8 | 27.8 | 26.8 | 27.8 |
| Native Hawaiian | 129 | 9.05 | 4.94 | 7.61 | 5.76 | 4.83 | 8.19 | 4.83 | 8.19 |
| Japanese-American | 596 | 26.1 | 29.6 | 19.8 | 44.2 | 22.7 | 36.0 | 22.7 | 36.0 |
| Latina | 327 | 23.9 | 10.1 | 21.8 | 7.82 | 20.8 | 11.2 | 20.8 | 11.2 |
| BMI (kg/m2), % | |||||||||
| 18.5 to <25 | 784 | 32.7 | 44.9 | 30.5 | 49.4 | 33.3 | 45.0 | 33.3 | 45.0 |
| ≥25 to <30 | 632 | 34.0 | 32.7 | 31.1 | 30.5 | 34.5 | 30.8 | 34.5 | 30.8 |
| ≥30 | 530 | 33.3 | 22.4 | 38.5 | 20.2 | 32.1 | 24.1 | 32.1 | 24.1 |
| Smoking status,8 % | |||||||||
| Smoking, pack-years | 1946 | 0 (12.0) | 0 (6.40) | 0 (12.0) | 0 (10.2) | 0 (12.0) | 0 (6.38) | 1.25 (14.2) | 0 (3.88) |
| Never | 1025 | 47.1 | 55.6 | 49.2 | 55.8 | 48.6 | 55.6 | 48.6 | 55.6 |
| Past | 646 | 31.1 | 32.7 | 32.1 | 32.3 | 33.8 | 32.1 | 33.8 | 32.1 |
| Current | 250 | 20.0 | 10.9 | 16.7 | 11.1 | 16.2 | 11.0 | 16.2 | 11.0 |
| Physical activity (moderate and vigorous),9 % | |||||||||
| <30 min/d | 856 | 50.2 | 39.9 | 50.2 | 38.9 | 47.6 | 41.0 | 47.6 | 41.0 |
| ≥30 min/d | 1048 | 46.1 | 58.4 | 46.5 | 60.5 | 47.3 | 58.2 | 47.3 | 58.2 |
| Education, % | |||||||||
| ≤12 y | 1002 | 60.3 | 41.8 | 59.7 | 44.2 | 56.5 | 45.9 | 56.5 | 45.9 |
| 13–15 y | 553 | 28.4 | 29.4 | 26.5 | 31.3 | 28.0 | 29.1 | 28.0 | 29.1 |
| ≥16 y | 391 | 11.3 | 28.8 | 13.8 | 24.5 | 15.5 | 25.0 | 15.5 | 25.0 |
| Family history of CRC,10 % yes | 228 | 8.85 | 14.8 | 8.85 | 14.8 | 9.18 | 15.1 | 9.18 | 15.1 |
| Ever NSAID use,11 % yes | 992 | 54.5 | 47.1 | 55.4 | 43.2 | 55.3 | 48.3 | 55.3 | 48.3 |
| Stage of disease, % | |||||||||
| Local | 836 | 41.0 | 46.9 | 40.7 | 43.6 | 41.3 | 44.4 | 41.3 | 44.4 |
| Regional | 763 | 38.9 | 36.4 | 38.9 | 36.4 | 39.1 | 39.2 | 39.1 | 39.2 |
| Distal | 298 | 17.7 | 13.6 | 17.1 | 16.1 | 16.9 | 12.9 | 16.9 | 12.9 |
| Unknown | 49 | 2.47 | 3.09 | 3.29 | 3.91 | 2.66 | 3.45 | 2.66 | 3.45 |
| Radiation therapy,12 % yes | 130 | 7.41 | 5.76 | 6.79 | 7.00 | 7.49 | 6.90 | 7.49 | 6.90 |
| Chemotherapy,13 % yes | 574 | 29.2 | 29.0 | 28.6 | 31.9 | 27.5 | 26.3 | 27.5 | 26.3 |
| Alcohol intake, g ethanol/d | 1946 | 0 (1.01) | 0 (0.89) | 0 (1.12) | 0 (1.87) | 0 (0.93) | 0 (1.55) | 0 (1.44) | 0 (0.93) |
| Red meat consumption, g/d | 1946 | 49.5 (46.3) | 22.7 (25.0) | 43.3 (43.3) | 29.2 (31.5) | 36.9 (36.0) | 34.2 (47.3) | 51.5 (42.6) | 22.9 (25.8) |
| Fruit consumption, g/d | 1946 | 99.7 (138) | 338 (287) | 104 (137) | 337 (238) | 108 (119) | 388 (258) | 93.5 (118) | 414 (275) |
Values are medians (IQRs) or percentages unless otherwise indicated. AHEI, Alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; NSAID, nonsteroidal anti-inflammatory drug; Q1, lowest index quartile; Q4, highest index quartile.
Data were missing for n = 216, 336, 4333, 573, 67, 748, 841, 942, 10278, 11100, 126, and 1362 subjects.
In our primary analysis, the multivariable-adjusted model in women but not in men (Tables 2 and 3), continuous aMED score divided by its SD predicted a 14% lower CRC-specific mortality [HR continuous pattern score divided by its respective SD (HR1SD): 0.86; 95% CI: 0.77, 0.96]. The results for all-cause mortality were similar and showed significant associations only for the aMED score in women (HR1SD: 0.88; 95% CI: 0.81, 0.96). The HRs for HEI-2010 indicated a weak inverse association with disease-specific mortality in women that did not reach significance (HR1SD: 0.91; 95% CI: 0.83, 1.00), whereas CRC-specific mortality was not associated with the AHEI-2010 or the DASH index. HEI-2010, AHEI-2010, and DASH scores were not related to all-cause mortality. In the minimally adjusted model, none of the index scores was significantly related with CRC-specific mortality. The continuous HEI-2010 was significantly inversely related to all-cause mortality in both sexes, and 1 SD of aMED score was significantly inversely associated with all-cause mortality in women. After correcting for multiple testing with the Bonferroni method, significant trends were observed across aMED quartiles in women for CRC-specific (P-trend = 0.004) and all-cause (P-trend = 0.0008) mortality in the fully adjusted model and for aMED score and all-cause mortality (P trend = 0.005) in women in the minimally adjusted model. With regard to individual components, the most prominent finding for the aMED score was that fruit consumption was related to better CRC-specific and all-cause mortality in women but not in men (data not shown).
TABLE 2.
HRs (95% CIs) for CRC-specific mortality by quartiles of dietary indexes and for an increase of 1 SD in dietary indexes separately for men and women with a CRC diagnosis in the Multiethnic Cohort1
| Men |
Women |
|||||||||
| Q1 | Q2 | Q3 | Q4 | 1-SD increase | Q1 | Q2 | Q3 | Q4 | 1-SD increase | |
| HEI-2010 | ||||||||||
| Index score points (range) | 28–58 | 58–65 | 65–73 | 73–100 | 29–62 | 62–70 | 70–78 | 78–98 | ||
| Deaths, n | 174 | 143 | 158 | 142 | 617 | 131 | 128 | 113 | 106 | 478 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 0.82 (0.65, 1.02) | 0.94 (0.76, 1.17) | 0.79 (0.63, 1.00) | 0.93 (0.86, 1.01) | 1 (ref) | 1.09 (0.85, 1.40) | 0.91 (0.70, 1.17) | 0.78 (0.60, 1.02) | 0.92 (0.84, 1.00) |
| Fully adjusted HR (95% CI) | 1 (ref) | 0.83 (0.66, 1.05) | 0.98 (0.78, 1.23) | 0.85 (0.66, 1.08) | 0.95 (0.87, 1.04) | 1 (ref) | 1.08 (0.84, 1.39) | 0.89 (0.68, 1.17) | 0.76 (0.58, 1.01) | 0.91 (0.83, 1.00) |
| AHEI-2010 | ||||||||||
| Index score points (range) | 30–58 | 58–64 | 64–71 | 71–92 | 28–58 | 58–65 | 65–71 | 71–90 | ||
| Deaths, n | 170 | 160 | 138 | 149 | 617 | 119 | 131 | 119 | 109 | 478 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 1.04 (0.83, 1.29) | 0.91 (0.72, 1.15) | 1.06 (0.85, 1.34) | 1.02 (0.94, 1.11) | 1 (ref) | 1.04 (0.81, 1.34) | 1.18 (0.91, 1.54) | 0.82 (0.63, 1.08) | 0.97 (0.89, 1.06) |
| Fully adjusted HR (95% CI) | 1 (ref) | 1.04 (0.83, 1.30) | 0.95 (0.75, 1.21) | 1.07 (0.84, 1.36) | 1.03 (0.94, 1.12) | 1 (ref) | 1.03 (0.80, 1.34) | 1.15 (0.87, 1.52) | 0.81 (0.61, 1.07) | 0.97 (0.88, 1.06) |
| aMED | ||||||||||
| Index score points (range) | 0–2 | 3–4 | 5 | 6–9 | 0–2 | 3–4 | 5 | 6–9 | ||
| Deaths, n | 129 | 238 | 104 | 146 | 617 | 115 | 175 | 72 | 116 | 478 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 1.13 (0.91, 1.40) | 1.08 (0.83, 1.40) | 1.22 (0.96, 1.55) | 1.05 (0.97, 1.14) | 1 (ref) | 0.92 (0.72, 1.17) | 0.69 (0.51, 0.93) | 0.84 (0.65, 1.10) | 0.91 (0.84, 1.00) |
| Fully adjusted HR (95% CI) | 1 (ref) | 1.07 (0.85, 1.34) | 0.99 (0.75, 1.31) | 1.07 (0.81, 1.42) | 1.01 (0.92, 1.11) | 1 (ref) | 0.87 (0.68, 1.12) | 0.61 (0.44, 0.85) | 0.74 (0.54, 1.01) | 0.86 (0.77, 0.96) |
| DASH | ||||||||||
| Index score points (range) | 10–20 | 21–23 | 24–27 | 28–38 | 12–20 | 21–23 | 24–27 | 28–37 | ||
| Deaths, n | 148 | 138 | 197 | 134 | 617 | 116 | 126 | 139 | 97 | 478 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 0.99 (0.78, 1.25) | 1.10 (0.88, 1.37) | 1.06 (0.83, 1.35) | 1.04 (0.96, 1.13) | 1 (ref) | 1.15 (0.89, 1.49) | 0.93 (0.72, 1.19) | 0.91 (0.69, 1.20) | 0.97 (0.89, 1.06) |
| Fully adjusted HR (95% CI) | 1 (ref) | 1.02 (0.81, 1.30) | 1.12 (0.89, 1.41) | 1.05 (0.81, 1.37) | 1.04 (0.95, 1.14) | 1 (ref) | 1.13 (0.87, 1.47) | 0.90 (0.68, 1.17) | 0.88 (0.64, 1.19) | 0.97 (0.87, 1.07) |
HRs (95% CIs) were obtained by Cox regression. Minimally adjusted HRs were adjusted for age at diagnosis, ethnicity, and stage at diagnosis; fully adjusted HRs were additionally adjusted for total energy intake, smoking status, pack-years, physical activity, education, radiation treatment, chemotherapy, NSAID use, family history of CRC, and comorbidities. P-trend tests were performed across index score categories while modeling the medians of the respective index score categories as a continuous variable. A significant P-trend was observed only across aMED score categories in women in the fully adjusted model (P-trend = 0.004) and in the minimally adjusted model (P-trend = 0.03). AHEI, Alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; NSAID, nonsteroidal anti-inflammatory drug; Q, quartile; ref, reference.
TABLE 3.
HRs (95% CIs) for all-cause mortality by quartiles of dietary indexes and for an increase of 1 SD in dietary indexes separately for men and women with a CRC diagnosis in the Multiethnic Cohort1
| Men |
Women |
|||||||||
| Q1 | Q2 | Q3 | Q4 | 1-SD increase | Q1 | Q2 | Q3 | Q4 | 1-SD increase | |
| HEI-2010 | ||||||||||
| Index score points (range) | 28–58 | 58–65 | 65–73 | 73–100 | 29–62 | 62–70 | 70–78 | 78–98 | ||
| Deaths, n | 291 | 273 | 279 | 288 | 1131 | 216 | 213 | 205 | 211 | 845 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 0.90 (0.76, 1.06) | 0.90 (0.76, 1.06) | 0.84 (0.71, 0.99) | 0.93 (0.88, 0.99) | 1 (ref) | 1.07 (0.88, 1.29) | 0.90 (0.74, 1.09) | 0.83 (0.68, 1.01) | 0.92 (0.86, 0.98) |
| Fully adjusted HR (95% CI) | 1 (ref) | 0.91 (0.77, 1.08) | 0.93 (0.79, 1.11) | 0.91 (0.76, 1.09) | 0.96 (0.90, 1.03) | 1 (ref) | 1.09 (0.89, 1.32) | 0.94 (0.76, 1.15) | 0.89 (0.72, 1.09) | 0.94 (0.88, 1.01) |
| AHEI-2010 | ||||||||||
| Index score points (range) | 30–58 | 58–64 | 64–71 | 71–92 | 28–58 | 58–65 | 65–71 | 71–90 | ||
| Deaths, n | 286 | 281 | 273 | 291 | 1131 | 207 | 223 | 217 | 198 | 845 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 1.02 (0.86, 1.20) | 0.95 (0.80, 1.12) | 1.05 (0.89, 1.24) | 1.01 (0.95, 1.07) | 1 (ref) | 0.97 (0.80, 1.18) | 1.08 (0.89, 1.31) | 0.82 (0.67, 1.00) | 0.96 (0.90, 1.03) |
| Fully adjusted HR (95% CI) | 1 (ref) | 1.01 (0.86, 1.20) | 1.00 (0.84, 1.19) | 1.08 (0.90, 1.28) | 1.02 (0.96, 1.09) | 1 (ref) | 0.98 (0.81, 1.19) | 1.07 (0.87, 1.32) | 0.83 (0.67, 1.03) | 0.98 (0.91, 1.05) |
| aMED | ||||||||||
| Index score points (range) | 0–2 | 3–4 | 5 | 6–9 | 0–2 | 3–4 | 5 | 6–9 | ||
| Deaths, n | 230 | 438 | 191 | 272 | 1131 | 193 | 306 | 141 | 205 | 845 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 1.06 (0.90, 1.25) | 1.03 (0.85, 1.25) | 1.12 (0.94, 1.34) | 1.03 (0.97, 1.09) | 1 (ref) | 0.91 (0.76, 1.10) | 0.79 (0.63, 0.98) | 0.82 (0.67, 1.00) | 0.92 (0.86, 0.98) |
| Fully adjusted HR (95% CI) | 1 (ref) | 0.98 (0.83, 1.16) | 0.95 (0.77, 1.17) | 0.99 (0.81, 1.22) | 1.00 (0.93, 1.07) | 1 (ref) | 0.88 (0.73, 1.07) | 0.73 (0.58, 0.93) | 0.74 (0.58, 0.94) | 0.88 (0.81, 0.96) |
| DASH | ||||||||||
| Index score points (range) | 10–20 | 21–23 | 24–27 | 28–38 | 12–20 | 21–23 | 24–27 | 28–37 | ||
| Deaths, n | 263 | 252 | 346 | 270 | 1131 | 190 | 201 | 266 | 188 | 845 |
| Minimally adjusted HR (95% CI) | 1 (ref) | 0.99 (0.83, 1.18) | 0.98 (0.83, 1.15) | 1.03 (0.86, 1.23) | 1.02 (0.96, 1.08) | 1 (ref) | 1.09 (0.89, 1.33) | 0.92 (0.76, 1.11) | 0.93 (0.75, 1.14) | 0.97 (0.90, 1.04) |
| Fully adjusted HR (95% CI) | 1 (ref) | 1.03 (0.86, 1.23) | 1.00 (0.84, 1.19) | 1.06 (0.87, 1.28) | 1.03 (0.97, 1.10) | 1 (ref) | 1.11 (0.91, 1.37) | 0.91 (0.74, 1.11) | 0.97 (0.77, 1.22) | 0.98 (0.90, 1.05) |
HRs (95% CIs) were obtained by Cox regression. Minimally adjusted HRs were adjusted for age at diagnosis, ethnicity, and stage at diagnosis; fully adjusted HRs were additionally adjusted for total energy intake, smoking status, pack-years, physical activity, education, radiation treatment, chemotherapy, NSAID use, family history of CRC, and comorbidities. P-trend tests were performed across index score categories while modeling the medians of the respective index score categories as a continuous variable. A significant P-trend was observed only across aMED score categories in women in the fully adjusted model (P-trend = 0.0008) and in the minimally adjusted model (P-trend = 0.005). AHEI, Alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; NSAID, nonsteroidal anti-inflammatory drug; Q, quartile; ref, reference.
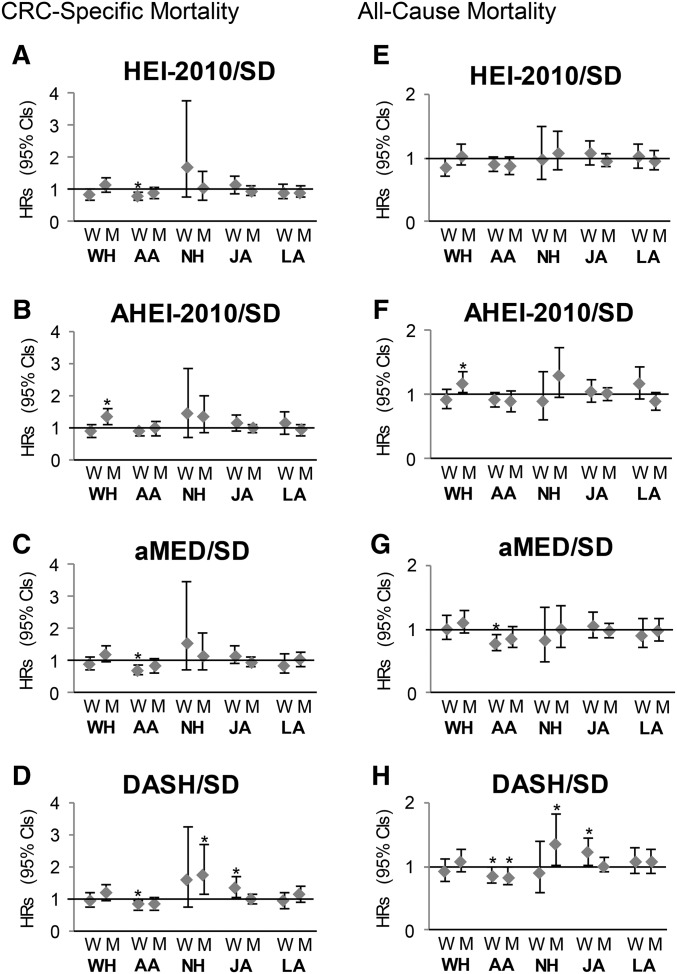
In men, none of the interaction terms for any of the dietary indexes with ethnicity were significant for CRC-specific or all-cause mortality. In women, the interaction terms for the HEI-2010, the AHEI-2010, the aMED score, and the DASH index with ethnicity were significant for CRC-specific mortality (P-interactions = 0.01, 0.02, 0.04, and 0.04, respectively) and the interaction terms for the HEI-2010, the AHEI-2010, and the DASH index with ethnicity were significant for all-cause mortality (P-interactions = 0.002, 0.01, and 0.005, respectively).
In our secondary analysis, ethnicity-specific results (Figure 1) indicated that African Americans experienced lower mortality with higher scores for the HEI-2010, aMED, and DASH, but the risk estimates with regard to CRC-specific mortality were significant only in women. Higher aMED scores predicted lower all-cause mortality among African-American women, whereas higher DASH scores were associated with lower all-cause mortality among African-American women and men. When women were stratified by postmenopausal estrogen treatment at cohort entry, all 4 index scores were significantly related to a lower CRC-specific mortality in past and current users, whereas no associations were observed in nonusers; the results for all-cause mortality were weaker but also reached significance in all index scores except for the AHEI-2010 (Table 4).
FIGURE 1.
Sex- and ethnicity-specific HRs (95% CIs) for a 1-SD increase in diet quality indexes for CRC-specific (A–D) and all-cause (E–H) mortality obtained by Cox regression and adjusted for age at diagnosis, smoking status, pack-years, physical activity, total energy intake, education, stage at diagnosis, radiation, chemotherapy, NSAID use, family history of colorectal cancer, and comorbidities. *The HR reaches significance (the CI of the HR does not include the 1). AA, African American; AHEI, Alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; JA, Japanese American; LA, Latino; NH, Native Hawaiian; M, men, NSAID, nonsteroidal anti-inflammatory drug; W, women; WH, white.
TABLE 4.
HRs (95% CIs) for an increase of 1 SD in dietary indexes for CRC-specific and all-cause mortality by estrogen use at cohort entry for women with a CRC diagnosis in the Multiethnic Cohort1
| CRC-specific mortality | All-cause mortality | |
| Current/past estrogen use (n = 761) | ||
| HEI-2010 | 0.77 (0.65, 0.91) | 0.85 (0.75, 0.95) |
| AHEI-2010 | 0.83 (0.70, 0.98) | 0.89 (0.79, 1.00) |
| aMED | 0.76 (0.63, 0.92) | 0.83 (0.72, 0.95) |
| DASH | 0.79 (0.65, 0.95) | 0.86 (0.75, 0.99) |
| Never estrogen use (n = 1007) | ||
| HEI-2010 | 1.04 (0.91, 1.19) | 1.05 (0.95, 1.16) |
| AHEI-2010 | 1.07 (0.94, 1.22) | 1.04 (0.93, 1.15) |
| aMED | 0.95 (0.82, 1.12) | 0.95 (0.85, 1.07) |
| DASH | 1.09 (0.94, 1.25) | 1.06 (0.95, 1.18) |
HRs (95% CIs) for an increase of 1 SD in the HEI-2010, AHEI-2010, aMED, and DASH scores were obtained by Cox regression and adjusted for age at diagnosis, smoking status, pack-years, physical activity, total energy intake, education, stage at diagnosis, radiation, chemotherapy, NSAID use, family history of CRC, and comorbidities. Data were missing for n = 178 for estrogen use at cohort entry. AHEI, Alternative Healthy Eating Index; aMED, alternate Mediterranean Diet score; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; NSAID, nonsteroidal anti-inflammatory drug.
Separate models for the aMED score by cancer site indicated a lower CRC-specific (HR: 0.85; 95% CI: 0.75, 0.97) and all-cause (HR: 0.86; 95% CI: 0.79, 0.95) mortality for colon but not for rectal cancer in women (data not shown). Stratification by stage of disease at diagnosis showed a significant inverse association of higher aMED scores in women but not in men with distant disease; the respective HRs for women per a 1-SD unit were 0.82 (95% CI: 0.68, 0.98) and 0.84 (95% CI: 0.71, 1.00) for CRC-specific and all-cause mortality, respectively. No significant associations were detected for localized and regional disease (data not shown).
Discussion
In this large multiethnic cohort composed of 5 major ethnic groups, the prediagnostic aMED score was associated with lower CRC-specific mortality and all-cause mortality in all women as a group but not in men. No significant associations of the HEI-2010, the AHEI-2010, or the DASH scores with CRC-specific mortality or all-cause mortality were detected. A number of secondary analyses showed significant associations of the HEI-2010, aMED, and DASH scores with lower CRC-specific mortality in African-American women, inverse associations of all 4 dietary indexes with mortality in estrogen users but not in nonusers, and stronger inverse associations for advanced than for localized stage of disease at diagnosis.
In the current analysis, higher aMED scores but none of the other examined dietary indexes were related to a lower CRC-specific and all-cause mortality in women. None of the dietary indexes were related to all-cause or CRC-specific mortality in men. Note that the dietary indexes investigated in this analysis were not developed specifically for cancer survival but for other chronic conditions such as hypertension. The high correlations of the dietary indexes in this study suggest some level of agreement. Still, the less than perfect correlations confirm that each index represents a unique combination of dietary components. The aMED score differs in many important ways from the other indexes (Supplemental Table 1). The scores are more determined by foods than nutrients, only 9 components are emphasized, vegetables exclude potatoes, and alcohol intake is part of the score. Another distinct property is that the consumption of nuts and legumes makes a stronger contribution than to any other index. Each is counted separately as “1” whereas they are scored together in the AHEI-2010 and the DASH index and nuts are not present in the HEI-2010. Legumes include soy beans, a source of isoflavones that might affect cancer initiation and progression through estrogenic and antiestrogenic activities (31). Nuts are sources of bioactive compounds, including phytoestrogens and MUFAs. Only the aMED score includes the component the ratio of MUFAs to SFAs, which was related to lower all-cause mortality in a meta-analysis of cohort studies (32). In addition, dichotomous scoring in the aMED may lead to greater contrasts and better discrimination of eating patterns within the population and provide more power for detecting differences. It should be noted that due to differences in the median intakes of foods, aMED results from studies conducted in the United States are likely to differ from those of European studies.
The single-component analyses from our study suggest that consumption of fruit may be a crucial component to lower mortality in women. As a potential biological mechanism, fruit is rich in phytochemicals (e.g., carotenoids), which were related to a lower mortality in patients with CRC in the MEC (33) but showed mixed results in other studies (34, 35). With regard to other specific micronutrients, prediagnostic plasma concentrations of the biologically active form of vitamin B-6 were not associated with CRC-specific or all-cause mortality (36), whereas high prediagnostic serum folate was associated with lower CRC-specific and all-cause mortality (37), flavonoid supplements reduced CRC recurrence (38), and patients with CRC with high circulating 25-hydroxyvitamin D had a lower risk of CRC-specific and all-cause mortality in a meta-analysis of cohort studies (39). As another potential mediator, the composition of the human gut microbiome is known to be influenced by diet (15) and might be linked to CRC development and progression (16).
The Mediterranean Diet Score was previously inversely related with CRC risk and all-cause cancer mortality in a meta-analysis of cohort and case-control studies (40). However, no associations of the postdiagnostic AHEI-2010, aMED, and DASH scores with CRC-specific mortality were detected among 1201 women from the Nurses’ Health Study (NHS) diagnosed with stage I–III CRC (8). The index versions were similar and cannot explain the observed differences across studies, but the NHS used postdiagnostic diet, whereas we used prediagnostic diet due to the small sample size of MEC participants with information on postdiagnostic diet. Because pre- and postdiagnostic dietary index scores showed minimal differences and significant correlations, it appears that no major dietary changes occurred.
Significant findings were mainly restricted to women in this study. Sex differences were also reported in an a posteriori pattern and with CRC-specific survival analysis: for instance, the adverse influence of the processed-meat pattern on survival was more pronounced among women than men (10). The results of the stratified analysis by postmenopausal estrogen use might partly explain the observed sex differences, because inverse associations for all 4 dietary indexes were limited to current or past postmenopausal estrogen users. These findings point toward a synergistic effect of diet and estrogen use as also seen in research from the Women’s Health Initiative Estrogen-plus-Progestin Study in which women taking hormone therapy had a lower risk of CRC than did women taking a placebo (41). Current postmenopausal estrogen use before CRC diagnosis was also associated with improved CRC-specific and all-cause survival in the NHS (42). There are several mechanisms for hormone exposure to protect against development and progression of colon cancer. For example, cell studies suggest that exogenous estrogens could lead to slower disease progression (43, 44).
Significant associations of the HEI-2010, aMED, and DASH scores with a lower CRC-specific mortality were seen in African-American women only. Considering that most dietary indexes were originally created and tested among participants of European and African-American (for DASH) heritage, food preferences of Japanese Americans, Native Hawaiians, and Latinos might not be as well represented in the indexes. This may partly explain the lack of associations among these ethnic groups but not among white participants. Our findings are of particular relevance given that African Americans are more likely to be diagnosed with CRC than whites and have lower survival rates (1). To our knowledge, no study has investigated by using an ethnicity-specific design the associations of a priori indexes with CRC-specific survival. In previous studies, white and African-American participants showed different results in analyses of exploratory dietary patterns with the risk of colon (45) and rectal (46) cancer; for instance, the “Western-Southern,” “fruit-vegetable,” and “metropolitan” intake patterns were identified in both ethnic groups, but the “fruit-vegetable” pattern was associated with colon cancer risk in whites only (45). In rectal cancer, the “high fat/meat/potatoes” intake pattern was identified in both ethnic groups; however, this was associated with risk only in whites (46). Associations between single foods and CRC risk also differed by ethnicity [e.g., fiber consumption was significantly associated with lower CRC risk in African Americans but not in whites (47)]. Ethnicity-specific differences in the bacterial colonization of the gut (48) and the frequency of genetic polymorphisms (49) may play a role in these findings.
In contrast to the associations among colon and not rectal cancer cases in the current study, the predominantly white NIH-AARP Diet and Health Study reported better CRC-specific survival for rectal cancer cases with higher prediagnostic HEI-2005 scores, whereas no association was observed among colon cancer cases (6). Discrepancies in the results might be explained by different HEI versions (HEI-2005 compared with HEI-2010), which differ, for instance, by the introduction of the food groups “seafood and plant proteins” and “refined grains” in the HEI-2010 and the replacement of the food group “oils and saturated fat” in the HEI-2005 by the food group “ratio of unsaturated fatty acids to SFAs” in the HEI-2010. Different findings might also be explained by differences in sample size and ethnic composition of the study populations. The latter may be particularly relevant because of the higher relative proportions of African Americans diagnosed with colon cancer (22.0%) than rectal cancer (13.9%) in the MEC.
To our knowledge, this study is the first to investigate the association of 4 a priori–defined dietary indexes with survival among participants diagnosed with CRC from different ethnic backgrounds. A strength of this study was its prospective design. Because of the large number of cases, we were able to examine tumors at specific anatomic sites. In addition, the use of a QFFQ designed for the relevant ethnic populations enabled us to study heterogeneous populations with wide variations in dietary habits and allowed for differences in usual portion sizes. Although the validation of the QFFQ with 24-h recalls indicated acceptable results (23), self-reported diet by QFFQ is always a limitation that may result in nondifferential misclassification and attenuated risk estimates (50). Small sample sizes in some ethnic groups may have limited our ability to detect associations or led to spurious findings due to multiple testing. However, when Bonferroni-corrected, the reported dietary score associations of our main analysis in women of all ethnic groups combined remained significant. Given the possibility of false-positive results due to multiple testing, the findings of our secondary analyses (i.e., by ethnic group, hormone treatment, and disease stage) should be interpreted with caution. These analyses are hypothesis-generating only and need to be replicated in other cohorts.
Given that dietary patterns may change after cancer diagnosis, another weakness of this study is that the exposure assessment was distant to the outcomes. Significant correlations and small differences between pre- and postdiagnostic dietary index scores in a subset of 953 patients in our study indicate an acceptable consistency, however. Covariate exposures, such as smoking status, may also change after cancer diagnosis. However, in our study subset with information on postdiagnostic confounders, the vast majority of prediagnosis nonsmokers and former smokers remained in the respective group after diagnosis, whereas 83 current smokers stopped smoking after the diagnosis and pre- and postdiagnostic BMI correlated well (r = 0.82).
In this multiethnic cohort, African-American women diagnosed with CRC whose prediagnostic diet at cohort entry was more closely aligned with the aMED experienced lower CRC mortality. The observed ethnicity-specific associations could be a result of true biological differences in metabolism, genetics, and eating patterns or due to the smaller sample sizes for ethnicity-specific analysis, particularly for Native Hawaiians. Our findings highlight the importance of examining relations between dietary patterns and CRC mortality in ethnically diverse populations but also indicate that the associations between prediagnostic diet quality and prognosis appear to be fairly weak. Given the multiple testing issues and small numbers of cases within ethnic groups, the current finding of an inverse association between the aMED and mortality in 1 of 5 ethnic groups may be due to chance and needs replication in other cohorts.
Acknowledgments
SJ and GM conducted the statistical analysis and interpreted the results and finalized the manuscript; SJ wrote the first draft of the manuscript; LRW contributed to the statistical analysis and interpretation of the results; BEH, NJO, LRW, KRM, LNK, LLM, and CJB critically reviewed the manuscript draft and contributed to the revised draft; LRW, LNK, and LLM designed the overall cohort study and were responsible for the study design; and GM had primary responsibility for final content and was responsible for the integrity of the work as a whole. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AHEI, Alternative Healthy Eating Index; aMED, alternate Mediterranean Diet; CRC, colorectal cancer; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; HR1SD, HR continuous pattern score divided by its respective SD; MEC, Multiethnic Cohort; NHS, Nurses’ Health Study; NSAID, nonsteroidal anti-inflammatory drug; QFFQ, quantitative FFQ; Qx1, questionnaire at cohort entry; Qx2, questionnaire 2; Qx3, questionnaire 3; SEER, Surveillance, Epidemiology, and End Results.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute [cited 2016 Jun 20]. Available from: http://seer.cancer.gov/csr/1975_2013/.
- 2.Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol 2015;33:1825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 4.Fung TT, Brown LS. Dietary patterns and the risk of colorectal cancer. Curr Nutr Rep 2013;2:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steck SE, Guinter M, Zheng J, Thomson CA. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr 2015;6:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelser C, Arem H, Pfeiffer RM, Elena JW, Alfano CM, Hollenbeck AR, Park Y. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 2014;120:1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romaguera D, Ward H, Wark PA, Vergnaud AC, Peeters PH, van Gils CH, Ferrari P, Fedirko V, Jenab M, Boutron-Ruault MC, et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Med 2015;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung TT, Kashambwa R, Sato K, Chiuve SE, Fuchs CS, Wu K, Giovannucci E, Ogino S, Hu FB, Meyerhardt JA. Post diagnosis diet quality and colorectal cancer survival in women. PLoS One 2014;9:e115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 2007;298:754–64. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Wu H, Wang PP, Savas S, Woodrow J, Wish T, Jin R, Green R, Woods M, Roebothan B, et al. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open 2013;3: pii: e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT. Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol 2013;31:2773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zell JA, Ignatenko NA, Yerushalmi HF, Ziogas A, Besselsen DG, Gerner EW, Anton-Culver H. Risk and risk reduction involving arginine intake and meat consumption in colorectal tumorigenesis and survival. Int J Cancer 2007;120:459–68. [DOI] [PubMed] [Google Scholar]
- 13.Amir Aslani B, Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci 2016;146:163–73. [DOI] [PubMed] [Google Scholar]
- 14.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev 2008;66:S116–24. [DOI] [PubMed] [Google Scholar]
- 15.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns MB, Lynch J, Starr TK, Knights D, Blekhman R. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Med 2015;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ollberding NJ, Nomura AM, Wilkens LR, Henderson BE, Kolonel LN. Racial/ethnic differences in colorectal cancer risk: the Multiethnic Cohort Study. Int J Cancer 2011;129:1899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013;113:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 21.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 22.Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stram DO, Hankin JH, Wilkens LR, Henderson B, Kolonel LN. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol 2000;151:358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014;180:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le ML, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr 2015;101:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 28.Leo QJ, Ollberding NJ, Wilkens LR, Kolonel LN, Henderson BE, Le ML, Maskarinec G. Obesity and non-Hodgkin lymphoma survival in an ethnically diverse population: the Multiethnic Cohort Study. Cancer Causes Control 2014;25:1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat 2011;129:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maskarinec G, Harmon BE, Little MA, Ollberding NJ, Kolonel LN, Henderson BE, Le ML, Wilkens LR. Excess body weight and colorectal cancer survival: the Multiethnic Cohort. Cancer Causes Control 2015;26:1709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Crit Rev Toxicol 2011;41:463–506. [DOI] [PubMed] [Google Scholar]
- 32.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis 2014;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooney RV, Chai W, Franke AA, Wilkens LR, Kolonel LN, Le ML. C-reactive protein, lipid-soluble micronutrients, and survival in colorectal cancer patients. Cancer Epidemiol Biomarkers Prev 2013;22:1278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev 2006;7:533–46. [PubMed] [Google Scholar]
- 35.Leung EY, Crozier JE, Talwar D, O’Reilly DS, McKee RF, Horgan PG, McMillan DC. Vitamin antioxidants, lipid peroxidation, tumour stage, the systemic inflammatory response and survival in patients with colorectal cancer. Int J Cancer 2008;123:2460–4. [DOI] [PubMed] [Google Scholar]
- 36.Je Y, Lee JE, Ma J, Zhang X, Cho E, Rosner B, Selhub J, Fuchs CS, Meyerhardt J, Giovannucci E. Prediagnostic plasma vitamin B6 (pyridoxal 5′-phosphate) and survival in patients with colorectal cancer. Cancer Causes Control 2013;24:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolpin BM, Wei EK, Ng K, Meyerhardt JA, Chan JA, Selhub J, Giovannucci EL, Fuchs CS. Prediagnostic plasma folate and the risk of death in patients with colorectal cancer. J Clin Oncol 2008;26:3222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoensch H, Groh B, Edler L, Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J Gastroenterol 2008;14:2187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maalmi H, Ordonez-Mena JM, Schottker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer 2014;50:1510–21. [DOI] [PubMed] [Google Scholar]
- 40.Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer 2014;135:1884–97. [DOI] [PubMed] [Google Scholar]
- 41.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 42.Chan JA, Meyerhardt JA, Chan AT, Giovannucci EL, Colditz GA, Fuchs CS. Hormone replacement therapy and survival after colorectal cancer diagnosis. J Clin Oncol 2006;24:5680–6. [DOI] [PubMed] [Google Scholar]
- 43.Singh S, Paraskeva C, Gallimore PH, Sheppard MC, Langman MJ. Differential growth response to oestrogen of premalignant and malignant colonic cell lines. Anticancer Res 1994;14:1037–41. [PubMed] [Google Scholar]
- 44.Lointier P, Wildrick DM, Boman BM. The effects of steroid hormones on a human colon cancer cell line in vitro. Anticancer Res 1992;12:1327–30. [PubMed] [Google Scholar]
- 45.Satia JA, Tseng M, Galanko JA, Martin C, Sandler RS. Dietary patterns and colon cancer risk in whites and African Americans in the North Carolina Colon Cancer Study. Nutr Cancer 2009;61:179–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, Sandler RS. Dietary patterns, food groups, and rectal cancer risk in whites and African-Americans. Cancer Epidemiol Biomarkers Prev 2009;18:1552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satia-Abouta J, Galanko JA, Potter JD, Ammerman A, Martin CF, Sandler RS. Associations of total energy and macronutrients with colon cancer risk in African Americans and whites: results from the North Carolina Colon Cancer Study. Am J Epidemiol 2003;158:951–62. [DOI] [PubMed] [Google Scholar]
- 48.O’Keefe SJ, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J, Adada H, van der Merwe T. Why do African Americans get more colon cancer than Native Africans? J Nutr 2007;137(Suppl):175S–82S. [DOI] [PubMed] [Google Scholar]
- 49.Slattery ML, Herrick J, Wolff RK, Caan BJ, Potter JD, Sweeney C. CDX2 VDR polymorphism and colorectal cancer. Cancer Epidemiol Biomarkers Prev 2007;16:2752–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]