Abstract
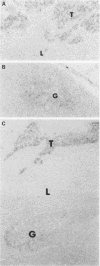
AIMS/BACKGROUND: Telomerase, an enzyme associated with cellular immortality, is expressed by most malignant tumours, but is inactive in normal somatic cells except for male germ cells and proliferating stem cells. Thus, the measurement of telomerase activity in tissue samples may provide useful diagnostic and prognostic information. The aim of this study was to determine whether telomerase expression is useful for the detection of occult malignant cells in lymph nodes. METHODS: Telomerase activity was compared with histological findings in 123 surgically removed lymph nodes submitted for routine or frozen section diagnosis. Telomerase activity was measured using a modified, semi-quantitative PCR-based telomeric repeat amplification protocol (TRAP). The assay was adapted for single 5 microns OCT embedded cryostat sections. In either fresh tissues or cryostat sections, normalised activity was linear when compared with protein concentration. Furthermore, using an in situ hybridisation method, the human telomerase RNA (hTR) component was measured in a subset of negative and positive nodes. RESULTS: Most (96%) of the 97 histologically negative nodes expressed low levels of activity (mean value of positive samples = 3.0 units/microgram protein) which may be derived from activated lymphocytes that express telomerase activity. All 26 malignant nodes (17 metastases, nine lymphomas) expressed telomerase (mean value = 17.8 units/microgram protein). The rank order levels between the two groups differed significantly (p = 0.0002). In situ results showed clearly that the hTR was expressed relatively highly in metastatic cancer cells and at lower levels in germinal centres of secondary follicles. CONCLUSIONS: Although expression of telomerase by activated lymphocytes may limit its usefulness, measurement of enzyme activity combined with detection of hTR using in situ hybridisation may assist in the histopathological diagnosis of lymph nodes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Broccoli D., Young J. W., de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadeneau C., Hay K., Hirte H. W., Gallinger S., Bacchetti S. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res. 1995 Jun 15;55(12):2533–2536. [PubMed] [Google Scholar]
- Chiu C. P., Dragowska W., Kim N. W., Vaziri H., Yui J., Thomas T. E., Harley C. B., Lansdorp P. M. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996 Mar;14(2):239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Gupta J., Harley C. B., Leber B., Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- Counter C. M., Hirte H. W., Bacchetti S., Harley C. B. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutait R., Alves V. A., Lopes L. C., Cutait D. E., Borges J. L., Singer J., da Silva J. H., Goffi F. S. Restaging of colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins. Dis Colon Rectum. 1991 Oct;34(10):917–920. doi: 10.1007/BF02049708. [DOI] [PubMed] [Google Scholar]
- Feng J., Funk W. D., Wang S. S., Weinrich S. L., Avilion A. A., Chiu C. P., Adams R. R., Chang E., Allsopp R. C., Yu J. The RNA component of human telomerase. Science. 1995 Sep 1;269(5228):1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Gunvén P., Maruyama K., Okabayashi K., Sasako M., Kinoshita T. Non-ominous micrometastases of gastric cancer. Br J Surg. 1991 Mar;78(3):352–354. doi: 10.1002/bjs.1800780326. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Arakawa H., Nagase H., Yanagisawa A., Kato Y., Ohta H., Takano S., Ogawa M., Nakamura Y. Genetic diagnosis identifies occult lymph node metastases undetectable by the histopathological method. Cancer Res. 1994 Jul 15;54(14):3853–3856. [PubMed] [Google Scholar]
- Hiyama E., Hiyama K., Yokoyama T., Matsuura Y., Piatyszek M. A., Shay J. W. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat Med. 1995 Mar;1(3):249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- Hiyama E., Yokoyama T., Tatsumoto N., Hiyama K., Imamura Y., Murakami Y., Kodama T., Piatyszek M. A., Shay J. W., Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995 Aug 1;55(15):3258–3262. [PubMed] [Google Scholar]
- Hiyama K., Hirai Y., Kyoizumi S., Akiyama M., Hiyama E., Piatyszek M. A., Shay J. W., Ishioka S., Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995 Oct 15;155(8):3711–3715. [PubMed] [Google Scholar]
- Hiyama K., Hiyama E., Ishioka S., Yamakido M., Inai K., Gazdar A. F., Piatyszek M. A., Shay J. W. Telomerase activity in small-cell and non-small-cell lung cancers. J Natl Cancer Inst. 1995 Jun 21;87(12):895–902. doi: 10.1093/jnci/87.12.895. [DOI] [PubMed] [Google Scholar]
- Härle-Bachor C., Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi H., Sakaguchi N. Telomerase activity is induced by the stimulation to antigen receptor in human peripheral lymphocytes. Biochem Biophys Res Commun. 1996 Feb 15;219(2):649–655. doi: 10.1006/bbrc.1996.0288. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford L. A., Piatyszek M. A., Xu R., Schold S. C., Jr, Shay J. W. Telomerase activity in human brain tumours. Lancet. 1995 Nov 11;346(8985):1267–1268. doi: 10.1016/s0140-6736(95)91865-5. [DOI] [PubMed] [Google Scholar]
- Makarov V. L., Lejnine S., Bedoyan J., Langmore J. P. Nucleosomal organization of telomere-specific chromatin in rat. Cell. 1993 May 21;73(4):775–787. doi: 10.1016/0092-8674(93)90256-p. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Mimori K., Inoue H., Barnard G. F., Tsuji K., Nanbara S., Ueo H., Akiyoshi T. Detection of cancer micrometastases in lymph nodes by reverse transcriptase-polymerase chain reaction. Cancer Res. 1995 Aug 1;55(15):3417–3420. [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson P., Mehle C., Remes K., Roos G. Telomerase activity in vivo in human malignant hematopoietic cells. Oncogene. 1994 Oct;9(10):3043–3048. [PubMed] [Google Scholar]
- Noguchi S., Aihara T., Nakamori S., Motomura K., Inaji H., Imaoka S., Koyama H. The detection of breast carcinoma micrometastases in axillary lymph nodes by means of reverse transcriptase-polymerase chain reaction. Cancer. 1994 Sep 1;74(5):1595–1600. doi: 10.1002/1097-0142(19940901)74:5<1595::aid-cncr2820740516>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Norrback K. F., Dahlenborg K., Carlsson R., Roos G. Telomerase activation in normal B lymphocytes and non-Hodgkin's lymphomas. Blood. 1996 Jul 1;88(1):222–229. [PubMed] [Google Scholar]
- Olovnikov A. M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973 Sep 14;41(1):181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- Passlick B., Izbicki J. R., Kubuschok B., Nathrath W., Thetter O., Pichlmeier U., Schweiberer L., Riethmüller G., Pantel K. Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with non-small-cell lung cancer. J Clin Oncol. 1994 Sep;12(9):1827–1832. doi: 10.1200/JCO.1994.12.9.1827. [DOI] [PubMed] [Google Scholar]
- Schoenfeld A., Luqmani Y., Smith D., O'Reilly S., Shousha S., Sinnett H. D., Coombes R. C. Detection of breast cancer micrometastases in axillary lymph nodes by using polymerase chain reaction. Cancer Res. 1994 Jun 1;54(11):2986–2990. [PubMed] [Google Scholar]
- Shay J. W., Wright W. E., Werbin H. Defining the molecular mechanisms of human cell immortalization. Biochim Biophys Acta. 1991 Apr 16;1072(1):1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- Tahara H., Nakanishi T., Kitamoto M., Nakashio R., Shay J. W., Tahara E., Kajiyama G., Ide T. Telomerase activity in human liver tissues: comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res. 1995 Jul 1;55(13):2734–2736. [PubMed] [Google Scholar]
- Taylor R. S., Ramirez R. D., Ogoshi M., Chaffins M., Piatyszek M. A., Shay J. W. Detection of telomerase activity in malignant and nonmalignant skin conditions. J Invest Dermatol. 1996 Apr;106(4):759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- Wang X., Heller R., VanVoorhis N., Cruse C. W., Glass F., Fenske N., Berman C., Leo-Messina J., Rappaport D., Wells K. Detection of submicroscopic lymph node metastases with polymerase chain reaction in patients with malignant melanoma. Ann Surg. 1994 Dec;220(6):768–774. doi: 10.1097/00000658-199412000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Weng N. P., Levine B. L., June C. H., Hodes R. J. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996 Jun 1;183(6):2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Piatyszek M. A., Rainey W. E., Byrd W., Shay J. W. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W., Piatyszek M. A. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995 Sep 25;23(18):3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992 Jun;8(6):193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]