Abstract
It is now broadly accepted that the nutritional environment in early life is a key factor in susceptibility to metabolic diseases. In this study, we evaluated the effects of maternal chromium restriction in vivo on the modulation of lipid metabolism and the mechanisms involved in this process. Sixteen pregnant C57BL mice were randomly divided into two dietary treatments: a control (C) diet group and a low chromium (L) diet group. The diet treatment was maintained through gestation and lactation period. After weaning, some of the pups continued with either the control diet or low chromium diet (CC or LL), whereas other pups switched to another diet (CL or LC). At 32 weeks of age, serum lipid metabolism, proinflammatory indexes, oxidative stress and anti-oxidant markers, and DNA methylation status in adipose tissue were measured. The results indicated that the maternal low chromium diet increased body weight, fat pad weight, serum triglyceride (TG), low-density lipoprotein cholesterol (LDL), tumor necrosis factor-α (TNF-α), malondialdehyde (MDA), and oxidized glutathione (GSSG). There was a decrease in serum reduced/oxidized glutathione (GSH/GSSG) ratio at 32 weeks of age in female offspring. From adipose tissue, we identified 1214 individual hypomethylated CpG sites and 411 individual hypermethylated CpG sites in the LC group when compared to the CC group. Pathway analysis of the differential methylation genes revealed a significant increase in hypomethylated genes in the mitogen-activated protein kinase (MAPK) signaling pathway in the LC group. Our study highlights the importance of the MAPK signaling pathway in epigenetic changes involved in the lipid metabolism of the offspring from chromium-restricted dams.
Keywords: DNA methylation, chromium restriction diet, lipid metabolism, MAPK pathway, metabolic programming
1. Introduction
Recently, studies have implied that nutrition during fetal and neonatal life can have profound effects on lifespan, glucose, and lipid metabolism. Human studies conducted in 1944–1945 revealed that undernutrition during early pregnancy was associated with glucose intolerance and increased serum insulin concentrations later in life (50–58 years-old) [1]. It was the first time that scientists addressed the impact of adverse environmental factors in early life on the occurrence of metabolic diseases in adulthood [2]. Later work confirmed and extended this hypothesis by showing significant opposite correlations between birth weight and the risk of adult obesity [3,4]. One important mechanism believed to be involved in this relationship is DNA methylation. DNA methylation changes normally occur within CpG-rich regions (CpG islands). CpG islands are usually located near the promoter regions of genes. Methylation within the promoter region can negatively affect gene expression [5]. DNA methylation is a key regulator of normal metabolic balance and the occurrence of disease [6,7]. DNA methylation changes are particularly sensitive in “early life window period”. DNA methylation changes occurring in utero may be passed on to offspring and may subsequently lead to metabolic diseases [8].
An increasing number of studies show that chromium (Cr (III)) supplementation is beneficial in maintaining healthy lipid metabolism, regulating appetite, reducing fat mass, and increasing lean body mass [9]. The minimum suggested daily chromium intake is 30 μg. However, the average dietary chromium intake for adults is far below this recommendation in many countries [10,11]. In particular, pregnant women and elderly individuals are more prone to the chromium deficiency [12], due to increased metabolic stress and decreased absorption ratio [13,14]. Vincent et al. report that a chromium-insufficient diet leads to an increase in serum cholesterol, which can be ameliorated by chromium supplementation [15]. A recent study shows that chronic maternal chromium deficiency increases body fat and changes the lipid metabolism in rat pups. The mechanism involved is probably augmented by oxidative stress [16].
We hypothesized that exposure to maternal chromium restriction would have a sustained impact on the methylation of genes involved in lipid metabolism, thus lead to dyslipidemia in mice offspring. To identify this epigenetic alteration, we used a genome-wide DNA methylation approach in adipose tissue and tested whether epigenetic changes were associated with differential gene expression.
2. Materials and Methods
2.1. Animals Protocol
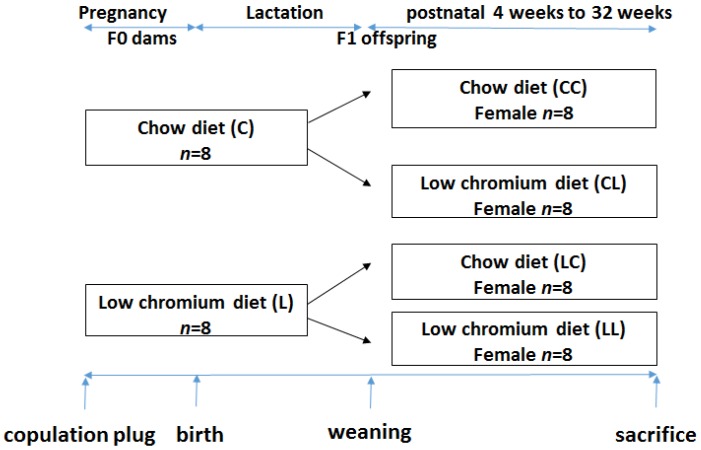
This study was performed in strict accordance with the recommendations given by the Guide for the Care and Use of Laboratory Animals from the National Institute of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Peking Union Medical Hospital (Permit Number: MC-07-6004). All efforts were made to minimize suffering. Seven-week-old C57BL/6J mice (18.5 ± 1.6 g) were acquired from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China, SCXK-2014-0108). After 1 week of adaptation, virgin female C57BL mice were caged with males (2 females to 1 male) overnight. Copulation was confirmed the next morning by establishing the formation of a vaginal plug. Midnight was considered as day 0 of gestation. Pregnant mothers (n = 16) were fed either the control diet (C, n = 8) or low chromium diet (L, n = 8). The control diet was a casein-based diet formulated on the basis of the American Institute of Nutrition AIN-93G diet and contained 1.19 mg/kg chromium. The low chromium diet (reduced only in chromium) contained 0.14 mg/kg chromium (88.23% of chromium restriction compared to control diet). The concentration of dietary chromium was analyzed using an atomic absorption spectrometer (TAS986, Beijing Persee General Corporation, Beijing, China). All diets were produced by Research Diets (New Brunswick, NJ, USA). On day 1 after birth, the litter sizes of both groups were homogenized to six pups (3 male and 3 female mice), to ensure no nutritional bias between litters. The diets were administered throughout gestation and lactation. All offspring was weaned at 3 weeks of age. Following weaning, the offspring were divided into the following sub-groups: CC (control diet-control diet), CL (control diet-low chromium diet), LC (low chromium diet-control diet), and LL (low chromium diet-low chromium diet, n = 8/group, one female pup from each litter was randomly assigned to the experimental groups). The mice were maintained in a light-dark cycle (12 h light and 12 h dark) and were given free access to food and water. Unbalanced maternal nutrition differentially impacted lipid metabolism and phenotypic expression in male and female offspring [17,18]. For this reason, the current study only focused on female offspring. The specific study design is shown in Figure 1. At the end of the experimental period (32 weeks of age), female mice (n = 8/group) were sacrificed. After 10 h of fasting, the mice were anesthetized (ketamine 100 mg/kg i.p., Pharmacia and Upjohn Ltd., Crawley, UK), and blood samples were collected from the intraorbital retrobulbar plexus Adipose tissue of the offspring was quickly collected and stored at −80 °C for further analysis.
Figure 1.
The timeline of the animal experiments.
2.2. Serum Chromium Levels
Serum chromium levels in mothers (at weaning) and in the offspring at 32 weeks were determined using an atomic absorption spectrometer (Atomic Absorption Spectrophotometer, Hitachi, Japan).
2.3. Measurement of Body Weight and Food Intake
The body weight of the offspring was recorded at birth, 3 weeks, and 32 weeks of age. Food consumption of the offspring was recorded at 32 weeks. Food consumption was quantified by subtracting the amount of food remaining at the end of the week from the total amount of food given at the beginning of the week. The average amount of food consumed per mouse was determined by dividing the total amount consumed by the number of mice.
2.4. Measurement of Serum Leptin, Adiponectin and Inflammatory Factors
Serum concentrations of leptin, adiponectin, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein 1 (MCP-1), and interleukin-1β (IL-1β) were measured using enzyme-linked immunosorbent assay (ELISA, Abcam, Cambridge, MA, USA).
2.5. Measurement of Serum Oxidative Stress and Antioxidant Markers
Malondialdehyde (MDA) concentration and reduced/oxidized glutathione (GSH/GSSG) were measured using thiobarbituric acid (TBA) and Thiol Green Indicator fluorometric method (Abcam, Cambridge, MA, USA) as oxidative stress and antioxidant markers, respectively.
2.6. Measurement of Serum Total Cholesterol (TC), Triglyceride (TG), High-Density Lipoproterin Cholesterol (HDL), and Low-Density Lipoprotein Cholesterol (LDL)
Serum TC, TG, HDL, and LDL concentrations were determined using an enzyme end-point method via a commercial kit (Roche Diagnostics, GmbH, Mannheim, Germany).
2.7. Measurement of Adipose Tissue Weight
At 32 weeks, mice were sacrificed and retroperitoneal, mesenteric, and ovarian fat were carefully removed and weighed. The adiposity index (AI) was computed as followings [19],
| (1) |
2.8. Methyl-DNA Immunoprecipitation and Microarray Hybridization
Fat collected from three CC mice and LC mice was used for methyl-DNA immunoprecipitation (MeDIP) experiment. Genomic DNA (gDNA) was extracted from fat samples using a DNeasy Blood & Tissue Kit (Qiangen, Fremont, CA, USA). The purified gDNA was then quantified and quality was assessed using Nanodrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). To perform the MeDIP experiment, first, gDNA was sonicated into random 200–1000 bp pieces. Next, 1 µg of fragmented DNA was used for immunoprecipitation with mouse monoclonal anti-5-methylcytidine (Diagenode, Liege, Belgium) at 4 °C overnight. To recover the immunoprecipitated DNA fragments, anti-mouse IgG magnetic beads (ThermoFisher Scientific, Carlsbad, CA, USA) were added and incubated for an additional 2 h at 4 °C with agitation. Then, immunoprecipitated methylated DNA and input gDNA was labeled with Cy5 and Cy3 fluorophores, respectively. Labelled DNA was hybridized to the Arraystar Mouse ReqSeq Promoter Array (Agilent, Waldbronn, Germany). This array contained all well-characterized RefSeq gene (approximately 22,327 genes) promoter regions (from −1300 bp to 500 bp transcription start sites (TSSs)). Finally, arrays were washed and scanned with an Agilent Scanner G2505C (Agilent Technologies, Waldbronn, Germany). After normalization, methylation peaks in the raw data were analyzed using SignalMap software (Roche Diagnostics, GmbH, Mannheim, Germany). We computed the modified Kolmogorov–Smirnov test on the adjacent probes using sliding windows to predict enriched regions across the array. To separate strong CpG islands from weak CpG islands, promoters were categorized into three levels: high CpG promoters/regions (HCP), intermediate CpG promoters/regions (ICP) and low CpG promoters/regions (LCP) [20].
2.9. Differential Methylated Genes Pathway Analysis
To determine the biological meaning to the differentially methylated genes, the subset of methylated genes was analyzed by applying the Gene Ontology (GO) classification system and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways database using DAVID (Database for Annotation, Visualization and Integrated Discovery) software [21].
2.10. Bisulfite Sequencing (BSP)
Bisulfite modification was performed with the EZ DNA Methylation Kit (Zymo Research, Hiss Diagnostics, Germany). The converted DNA was then amplified by PCR with primers detailed in Table 1. Primers were designed using Methyl Primer Express Software version 1.0 (Applied Biosystems, Foster City, CA, USA). PCR products were purified using agarose gels (Invitrogen, Carlsbad, CA, USA) and ligated to the pMD18-T Vector (Takara, Shiga, Japan). The plasmids were then purified using the PureLink Miniprep kit (Invitrogen, Thermo Scientific Inc., Waltham, MA, USA). Positive clones were confirmed by PCR, and a minimum of 10 clones from each mouse (n = 8 mice/group) were sequenced using ABI PRISM 7700 Sequence Detection (Applied Biosystems, Foster City, CA, USA). Sequence analysis was performed using QUMA [22].
Table 1.
PCR Primers for bisulfite sequencing.
| Gene | Accession Number | Primer Sequences (from 5′ to 3′) | Production Size | CpG Number |
|---|---|---|---|---|
| Map3k4 | NM_011948 | F: 5′-AATGATTGAAAGATGTTTTGTT-3′ | 303 | 5 |
| R: 5′-TTCATAAACTAAAACTCAAAATCTC-3′ | ||||
| Mapk14 | NM_001168513 | F: 5′-GGAGTAAGTAGGGTGGTTTTGT-3′ | 382 | 30 |
| R: 5′-CAAACTTTACCCTACAACTCCTC-3′ | ||||
| Map3k5 | NM_008580 | F: 5′-GTAAGGGAGTTGTTGYGGAGTA-3′ | 258 | 35 |
| R: 5′-AAAAAAACAAAACTTCCTCCTCTT-3′ | ||||
| Tab2 | NM_138667 | F: 5′-TGTTGATTAAGGAAAGTTTAGYG-3′ | 252 | 34 |
| R: 5′-CRAAACCCTACAAACCCTAAC-3′ |
PCR: Polymerase Chain Reaction; Map3k4: mitogen-activated protein kinase kinase kinase 4; Mapk14: mitogen-activated protein kinase 14; Map3k5: mitogen-activated protein kinase kinase kinase 5; Tab2: transforming growth factor-β (TGF-β)-activated kinase 1/MAP3K7 binding protein 2.
2.11. Quantitative Real Time RT-PCR
The data (n = 8/group) were further analyzed for the expression of BSP-validated genes and downstream genes. The total RNA was prepared from fat stored at −80 °C using the Qiagen RNeasy Mini Kit (Qiagen, Germantown, MD, USA). cDNA was synthesized from the reverse transcription of the total RNA using an oligodesoxythymidine primer and the TakaRa RT kit (Takara, Shiga, Japan). The experimental real-time PCR signals were normalized to that of Gadph gene. Real-time amplification was performed using the ABI 7900 thermocycler (Applied Biosystems, Foster City, CA, USA). The fold change was calculated using the comparative Ct method. The primer sequences for quantitative real-time PCR are shown in Table 2.
Table 2.
Primer using in real time PCR.
| Gene | Accession Number | Primer Sequences (from 5′ to 3′) | Production Size |
|---|---|---|---|
| Map3k4 | NM_011948 | F: 5′-ATTGGAGAAGGACAGTAT-3′ | 107 |
| R: 5′-ATAGTCTTGTGGTCGTTA-3′ | |||
| Mapk14 | NM_001168513 | F: 5′-TGTTCTGTCTATCTCACTTC-3′ | 75 |
| R: 5′-GAGGCACTTGAATGGTAT-3′ | |||
| Map3k5 | NM_008580 | F: 5′-AATAATGAAGTTGAGGAGAAGACA-3′ | 78 |
| R: 5′-AGAGGAAGCACCGAAGTT-3′ | |||
| Tab2 | NM_138667 | F: 5′-TATCAGTGCTTGGAATGG-3′ | 143 |
| R: 5′-GACCTTCTTAACGCTCAT-3′ | |||
| Pparg | NM_001127330 | F: 5′-GCATCAGGCTTCCACTAT-3′ | 75 |
| R: 5′-CTTCAATCGGATGGTTCTTC-3′ | |||
| Atf2 | NM_001025093 | F: 5′-GGCGTTCAAGCAGGATTC-3′ | 106 |
| R: 5′-TGACACTGAGACCATAGCAATA-3′ |
Map3k4: mitogen-activated protein kinase kinase kinase 4; Mapk14: mitogen-activated protein kinase 14; Map3k5: mitogen-activated protein kinase kinase kinase 5; Tab2: TGF-β-activated kinase 1/MAP3K7 binding protein 2; Pparg: peroxisome proliferator activated receptor gammas; Atf2: activating transcription factor 2.
2.12. Statistical Analysis
Results are shown as means ± SD; n represents the number of mice analyzed. Unpaired Student’s t test was used to compare the two groups, and one-way ANOVA followed by Tukey’s post hoc test was used when more than two groups were analyzed. For GO and KEGG pathway analysis, Fisher’s exact test was used. A p value < 0.05 was considered significant. Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis.
3. Results
3.1. Maternal Body Weight and Serum Chromium Differences
By the end of lactation, there was no significant difference in maternal body weight between the C dams and L dams (Table 3). As expected, the serum chromium levels were significantly decreased in the L group when compared to the C group (p < 0.01, Table 3).
Table 3.
Body weight, fasting blood glucose and serum chromium level in dams at weaning.
| Groups | C | L |
|---|---|---|
| Body weight (g) | 23.1 ± 3.5 | 22.3 ± 3.4 |
| Serum chromium (ng/mL) | 0.89 ± 0.22 | 0.45 ± 0.09 ** |
C, control diet group; L, low chromium diet group. Values are mean ± SD, n = 8 per group. ** p < 0.01 vs. C.
3.2. Effects in Offspring
3.2.1. Effects of Maternal Low Chromium Diet on Serum Chromium Level, Body Weight, Food Intake, and Fat Pad Weight in Offspring
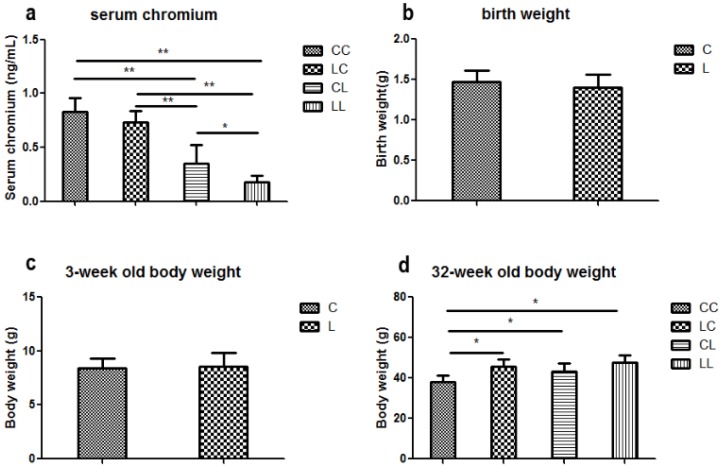
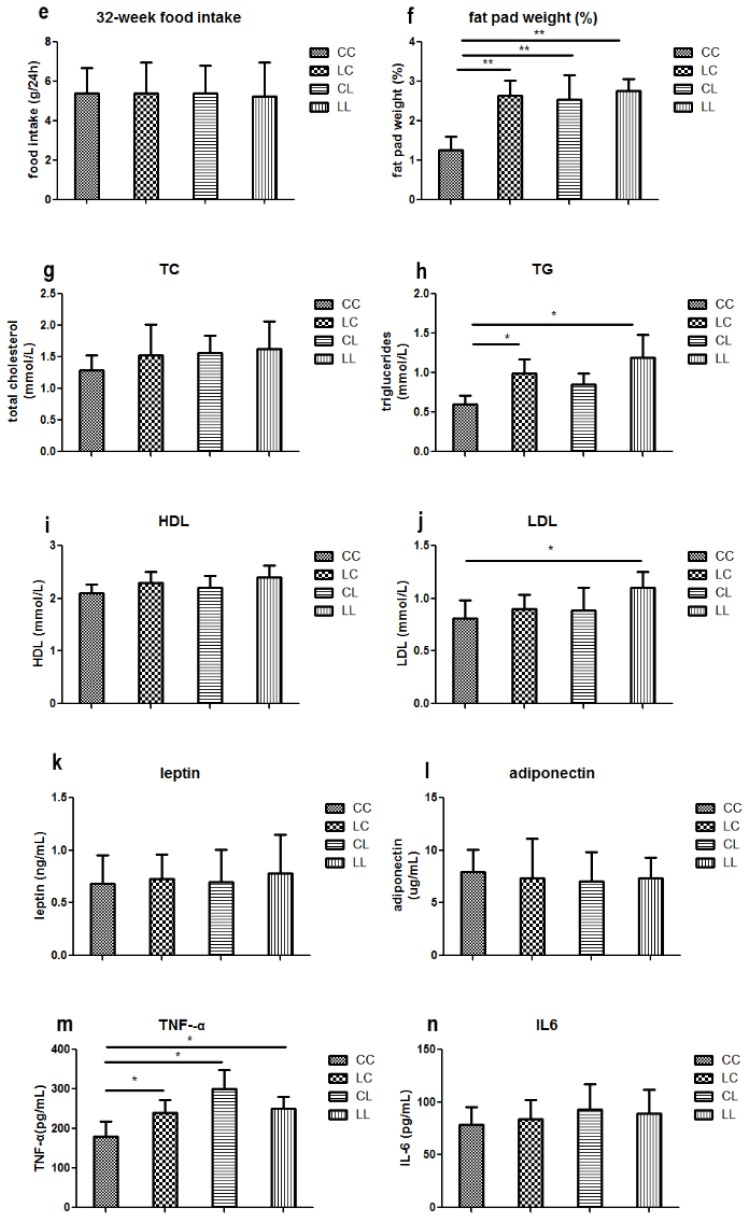
At week 32, serum chromium levels were lower in the LL and CL groups when compared to the CC group (p < 0.01, Figure 2a). Serum chromium levels in the LC group returned to normal (Figure 2a). The birth weight of L group and C group offspring were comparable (Figure 2b). At weaning, there was still no significant difference in body weight between L and C group (Figure 2c). Although food intake was comparable between the groups (Figure 2e), body weight in the LL group and the CL group were higher than those in CC group at 32 weeks of age (p < 0.05, Figure 2d). The body weights of the LC group were higher than those of CC group (p < 0.05, Figure 3d). At 32 weeks of age, body fat pad weights were significantly higher in the LL and CL group offspring when compared to those of the offspring from the CC group (p < 0.01, Figure 2f). Rehabilitation of low chromium diet dams (LC group) did not correct fat pad weight (p < 0.01, Figure 2f).
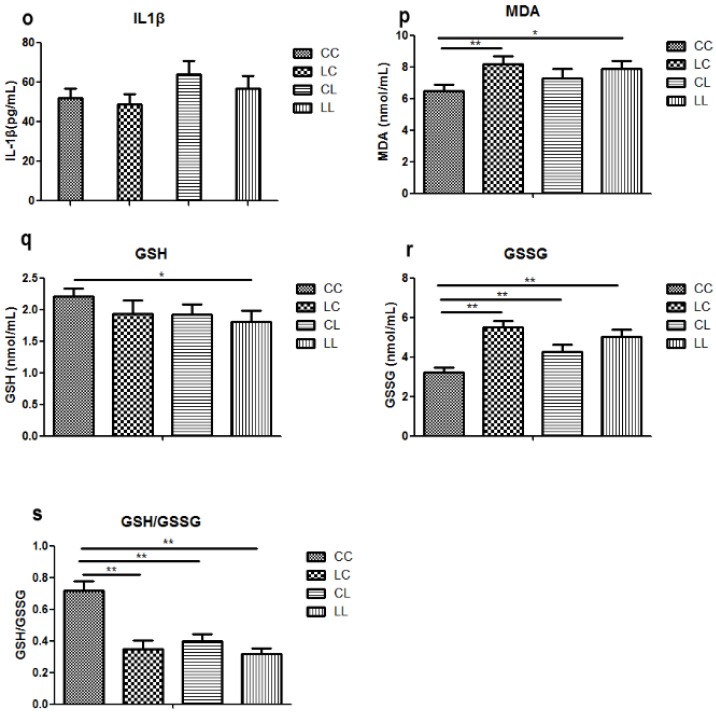
Figure 2.
Effects of maternal low chromium diet on serum chromium (a); body weight (d); food intake (e); fat pad weight (f); serum total cholesterol (g); triglyceride (h); HDL (i) and LDL (j); leptin (k); adiponectin (l); TNF-α(m); IL-6 (n); IL-1β (o); MDA (p); GSH (q); GSSG (r); GSH/GSSG (s) at 32 weeks of age; birth weight (b) and weaning weight (c). Values are mean ± SD, n = 8 per group. * p < 0.05, ** p < 0.01.
Figure 3.
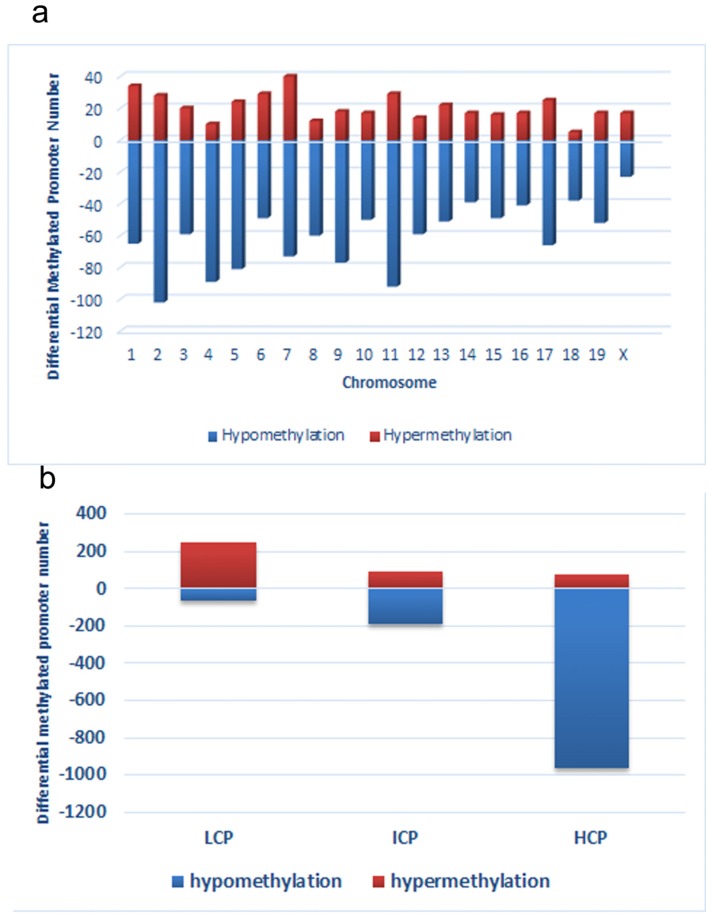
Distribution of differentially methylated promoters on chromosomes (a) and promoters with respect to the cytosine polyguanine (CpG) content (b), including high CpG promoters/regions (HCP), intermediate CpG promoters/regions (ICP), and low CpG promoters/regions (LCP)in low chromium diet-control diet (LC) group.
3.2.2. Effects of Maternal Low Chromium Diet on Serum Lipid Profile in Offspring
Serum TG levels were significantly higher in the LL and LC groups’ offspring when compared to those of the CC group (Figure 2h, p < 0.05). LDL in the LL group was higher than the CC group (p < 0.05, Figure 2j). However, LDL levels were corrected in the LC group. At 32 weeks of age, serum TC and HDL were not significantly different among the offspring across all groups (Figure 2g,i).
3.2.3. Effects of Maternal Low Chromium Diet on Serum Adiponectin, Leptin, and Pre-Inflammation Cytokines in Offspring
There was no significant differences among the groups in serum adiponectin, leptin, IL-6, and IL-1β values at 32 weeks of age (Figure 2k,l,n,o). Only serum TNF-α levels were significantly higher in the LL, LC, and CL groups when compared to that of the CC group (Figure 2m, p < 0.05).
3.2.4. Effects of Maternal Low Chromium Diet on Serum Oxidative Stress and Antioxidant Markers in Offspring
Serum MDA levels were significantly higher in the LC and LL group when compared to that of the CC group at 32 weeks of age (Figure 2p, p < 0.01 and p < 0.05, respectively). There was no significant difference among the groups in serum GSH (Figure 2q). Serum GSSG levels were significantly higher in LC, CL, and LL groups than that in CC group (Figure 2r, p < 0.01). Thus, serum GSH/GSSG ratio was significant lower in LC, CL, and LL groups than CC group (Figure 2s, p < 0.01).
3.2.5. Effects of Maternal Low Chromium Diet on Genome-Wide DNA Methylation in Adipose Tissue Collected from Offspring
First, we obtained a CpG promoter methylation profile of the LC group and compared it to that of the CC group (GEO database: GSE82030). This comparison showed that 4.8% (1214 loci) of promoters in 1145 genes were hypomethylated in the LC group, particularly on chromosomes 2, 4, 5, 7, 9, 11, and 17, which have more promoters with differential methylation (Peak Score > 2). In contrast, fewer promoters showed a trend toward hypermethylation in the LC group, just 1.8% (411 loci) of promoters (Figure 3a). Among the hypomethylated promoters, 963 (79.3%) were located in HCP, 188 (15.5%) in ICP, and 63 (5.2%) in LCP. And among hypermethylated promoters, 73 (17.8%) were located in HCP, 90 (21.9%) in ICP, and 248 (60.3%) in LCP (Figure 3b).
3.2.6. Functional Analysis Using DAVID
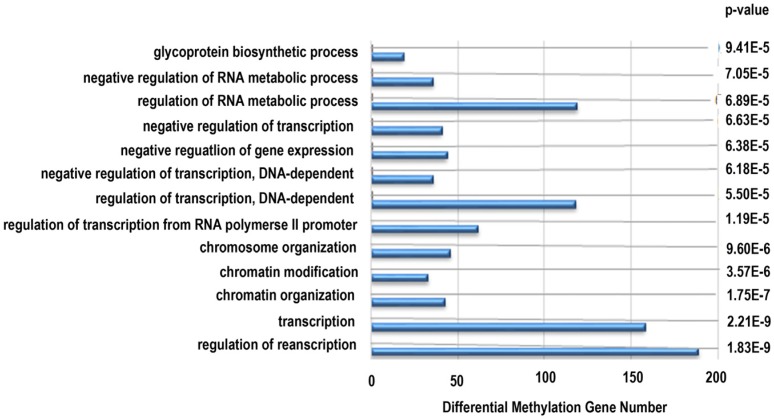
Next, functional analysis of hypomethylated genes was performed. Function analysis revealed that hypomethylated genes were involved in the regulation of transcript, transcription, chromatin organization, and so on (Table 4, Figure 4). Biological pathway impacted by hypomethylated genes include gap junction and the mitogen-activated protein kinase (MAPK) signaling pathway (Table 5). Figure 5 and Table 6 illustrate that the MAPK signaling pathway in the LC group affected genes exhibiting promoter hypomethylation in the LC group; this was in contrast to what was observed in the CC group.
Table 4.
Annotation of LC group hypomethylated promoter genes (p value < 0.00001).
| Term | Term Number | Count | p-Value | Fold Enrichment |
|---|---|---|---|---|
| Biological process | - | - | - | - |
| regulation of transcription | GO:0045449 | 189 | 1.5015 | |
| transcription | GO:0006350 | 158 | 1.5775 | |
| chromatin organization | GO:0006325 | 43 | 2.4151 | |
| chromatin modification | GO:0016568 | 33 | 2.4737 | |
| chromosome organization | GO:0051276 | 46 | 2.0145 | |
| regulation of transcription from RNA polymerase II promoter | GO:0006357 | 62 | 1.7807 | |
| regulation of transcription, DNA-dependent | GO:0006355 | 118 | 1.4250 | |
| negative regulation of transcription, DNA-dependent | GO:0045892 | 36 | 2.0679 | |
| negative regulation of gene expression | GO:0010629 | 44 | 1.8987 | |
| negative regulation of transcription | GO:0016481 | 41 | 1.9500 | |
| regulation of RNA metabolic process | GO:0051252 | 119 | 1.4149 | |
| negative regulation of RNA metabolic process | GO:0051253 | 36 | 2.0546 | |
| glycoprotein biosynthetic process | GO:0009101 | 19 | 2.8731 | |
| Cellular componts | - | - | - | - |
| nuclear lumen | GO:0031981 | 86 | 1.8592 | |
| intracellular organelle lumen | GO:0070013 | 101 | 1.7017 | |
| organelle lumen | GO:0043233 | 101 | 1.6972 | |
| endomembrane system | GO:0012505 | 58 | 2.0695 | |
| membrane-enclosed lumen | GO:0031974 | 102 | 1.6585 | |
| Golgi apparatus | GO:0005794 | 68 | 1.9034 | |
| nucleoplasm | GO:0005654 | 57 | 1.8165 | |
| Golgi apparatus part | GO:0044431 | 29 | 2.4388 | |
| Golgi membrane | GO:0000139 | 20 | 2.7868 | |
| Molecular Function | - | - | - | - |
| DNA binding | GO:0003677 | 167 | 1.6952 | |
| transcription regulator activity | GO:0030528 | 113 | 1.6939 | |
| transcription factor activity | GO:0003700 | 76 | 1.7706 | |
| metal ion binding | GO:0046872 | 260 | 1.2209 | |
| cation binding | GO:0043169 | 262 | 1.2192 |
Figure 4.
Gene ontology classifications in biology process for hypomethylated genes. The gene ontology (GO) term is on the y-axis, number of genes is on the x-axis, and the p-value indicating significance of enrichment is on the right side.
Table 5.
KEGG pathway in LC group hypomethylated genes (p < 0.01).
| Pathway Name | Pathway Term | Gene Count | Fold Enrichment | p-Value |
|---|---|---|---|---|
| Gap junction | mmu04540 | 11 | 2.6025 | 0.008845 |
| MAPK signaling pathway | mmu04010 | 23 | 1.7660 | 0.009097 |
Figure 5.
Promoter-specific DNA methylation of genes in the Kyoto Encyclopedia of Genes and Genomes (KEGG) mitogen-activated protein kinase (MAPK) signaling pathway. The red box indicates hypomethylated gene promoters and the map was provided by KEGG Pathway Database.
Table 6.
LC group hypomethylation genes in MAPK signaling pathway (peak score > 2).
| Gene Name | Accession | Chromosome | Peak to TSS | Promoter Classification | Peak Score | Peak M Value |
|---|---|---|---|---|---|---|
| Rac1 | NM_009007 | chr5 | 264 | HCP | 2.42 | 0.550 |
| Rela | NM_009045 | chr19 | 349 | HCP | 2.65 | 0.477 |
| Prkcb | NM_008855 | chr7 | 350 | HCP | 2.96 | 0.370 |
| Cacna1h | NM_001163691 | chr17 | 37 | HCP | 3.1 | 0.338 |
| Map3k4 | NM_011948 | chr17 | −422 | HCP | 3.48 | 0.334 |
| Tab2 | NM_138667 | chr10 | −175 | HCP | 3.68 | 0.312 |
| Fgf3 | NM_008007 | chr7 | 336 | HCP | 2.08 | 0.305 |
| Braf | NM_139294 | chr6 | 105 | HCP | 2.48 | 0.265 |
| Mapk14 | NM_001168513 | chr17 | −833 | HCP | 3.38 | 0.259 |
| Mapk14 | NM_001168514 | chr17 | 393 | HCP | 3.38 | 0.259 |
| Fgf18 | NM_008005 | chr11 | 85 | HCP | 2.93 | 0.258 |
| Map3k5 | NM_008580 | chr10 | −291 | HCP | 2.6 | 0.254 |
| Cacna2d3 | NM_009785 | chr14 | −314 | HCP | 2.94 | 0.251 |
| Pdgfa | NM_008808 | chr5 | −301 | HCP | 3.42 | 0.247 |
| Fgf8 | NM_010205 | chr19 | −231 | HCP | 2.59 | 0.218 |
| Cacnb4 | NM_001037099 | chr2 | 145 | ICP | 2.27 | 0.169 |
| Fgf11 | NM_010198 | chr11 | 117 | ICP | 2.11 | 0.144 |
| Hras1 | NM_001130444 | chr7 | −254 | HCP | 2.36 | 0.121 |
| Sos1 | NM_009231 | chr17 | −350 | HCP | 2.49 | 0.109 |
| Cacna1g | NM_001177890 | chr11 | −401 | HCP | 2.15 | 0.060 |
| 1500003O03Rik | NM_019769 | chr2 | 437 | HCP | 2.37 | 0.053 |
| Dusp16 | NM_130447 | chr6 | −410 | HCP | 2.18 | 0.038 |
| Prkx | NM_016979 | chrX | −253 | HCP | 2.22 | 0.035 |
| Nfkb2 | NM_001177370 | chr19 | −536 | ICP | 2.1 | 0.012 |
TSS, transcription start sites; Peak to TSS, the distance from the center of the peak to the TSS. (“-”: peak center in upstream of the TSS); Peak Score, the average of −log10p−value from the probes within the peak. The score reflects the probability of positive enrichment. (cut-off = 2); Peak M Value, the median of log2−ratio from the probes within the peak. The score reflects the methylation degree; HCP, high CpG promoters/regions; ICP, intermediate CpG promoters/regions; LCP, low CpG promoters/regions.
3.2.7. Bisulfite Sequencing (BSP)
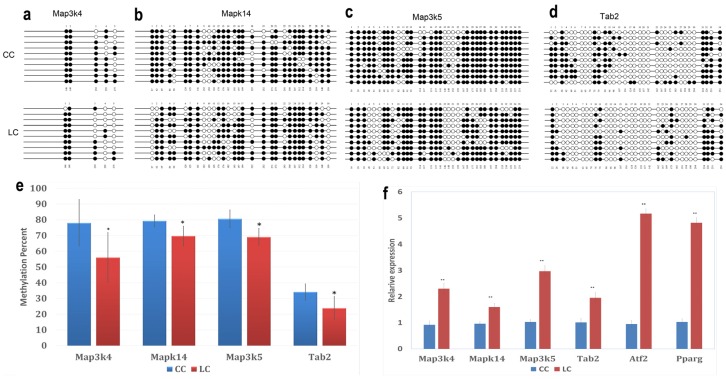
Bisulfite Sequencing (BSP) on four hypomethylated promoters was performed. Five to 35 CpGs for each gene were involved in the analysis. The methylation percentages of these promoters are shown in Figure 6a–e. Mitogen-activated protein kinase kinase kinase 4 (Map3k4), mitogen-activated protein kinase kinase kinase 5 (Map3k5), mitogen-activated protein kinase 14 (Mapk14), and TGF-β-activated kinase 1/MAP3K7 binding protein 2 (Tab2) were significantly hypomethylated in the LC group, compared to the CC group (p < 0.05). This result confirmed the results acquired using the DNA methylation array.
Figure 6.
Effects of maternal low chromium diet on methylation of Map3k4 (a); Mapk14 (b); Map3k5 (c); and Tab2 (d) and relative expression (f) of genes in adipose of offspring. For bisulfite sequencing (BSP) results, white circles represent unmethylated CpGs, and black circles represent methylated CpGs (a–d); figure e shows the analysis of DNA methylation status. Values are expressed as means ± SD, n = 8 per group. For BSP, pooled DNA of eight mice adipose for each group is what we used for analysis. For each gene in each group, 10 clones randomly selected were analyzed and represent the average methylation level for the indicated genes. * p < 0.05, **p < 0.01, vs. CC. Map3k4: mitogen-activated protein kinase kinase kinase 4; Mapk14: mitogen-activated protein kinase 14; Map3k5: mitogen-activated protein kinase kinase kinase 5; Tab2: TGF-β-activated kinase 1/MAP3K7 binding protein 2; Pparg: peroxisome proliferator activated receptor gammas; Atf2: activating transcription factor 2.
3.2.8. Real-Time RT PCR Assay for BSP-Validated Hypomethylated Genes and Downstream Genes
To explore whether hypomethylated promoters can affect gene expression, the expression of four genes (Map3k4, Map3k5, Mapk14, and Tab2) regulated by these hypomethylated promoters and two downstream genes (Atf2, Pparg) was analyzed by real-time PCR. The results showed that the LC group had significantly high expression levels of Map3k4, Map3k5, Mapk14, Tab2, Atf2, and Pparg compared to CC group (p < 0.01, Figure 6f).
4. Discussion
Most importantly, in this study we found that body weight was comparable among the different groups at birth and at 3 weeks of age. However, at 32 weeks, pups from low chromium diet dams were heavier than those of the control group. Switching to the control diet did not correct the body weight increase. Other studies have reported that maternal chromium restriction significantly increased body weight from 12 months of age. Rehabilitation regimes did not correct the change [16]. It has also been reported that dietary vitamin B12 restriction increased body weight from weaning [23].
In our study, food intake was comparable among all groups at 32 weeks. However, maternal chromium restriction increased fat pad weight in offspring at 32 weeks of age. Reversing the diet did not correct this change. Venu et al. found that offspring from vitamin-restricted dams had a significantly higher percentage of body adipose from day 100 [24].
In addition, maternal chromium restriction may increase serum TG and LDL in offspring at 32 weeks of age; reversing the diet corrected LDL levels only. Other groups have found that maternal vitamin or chromium restriction may also significantly increase circulating triglyceride levels [16,24].
Moreover, maternal chromium restriction may increase serum TNF-α levels. TNF-α modulates lipid metabolism and is associated with obesity. The long-term effect of maternal magnesium restriction increases plasma TNF-α in offspring at 18 months [25]. Several groups found that maternal protein restriction upregulated TNF-α expression in the liver and spleen, and increased serum TNF-α from fetal age to adulthood [26,27,28].
Early nutritional exposures can affect later disease risk through the epigenomic changes (DNA methylation, histone modifications, and so on). Although these epigenomic changes do not directly affect the DNA nucleotide sequence, it can be passed on to subsequent generations [8]. DNA methylation, which usually occurs at cytosines within CpG islands, is a key regulator in gene transcription. CpG islands are usually located in or near the promoters of the genes [29,30]. The DNA methylation changes in promoter regions are negatively related to gene expression [31].
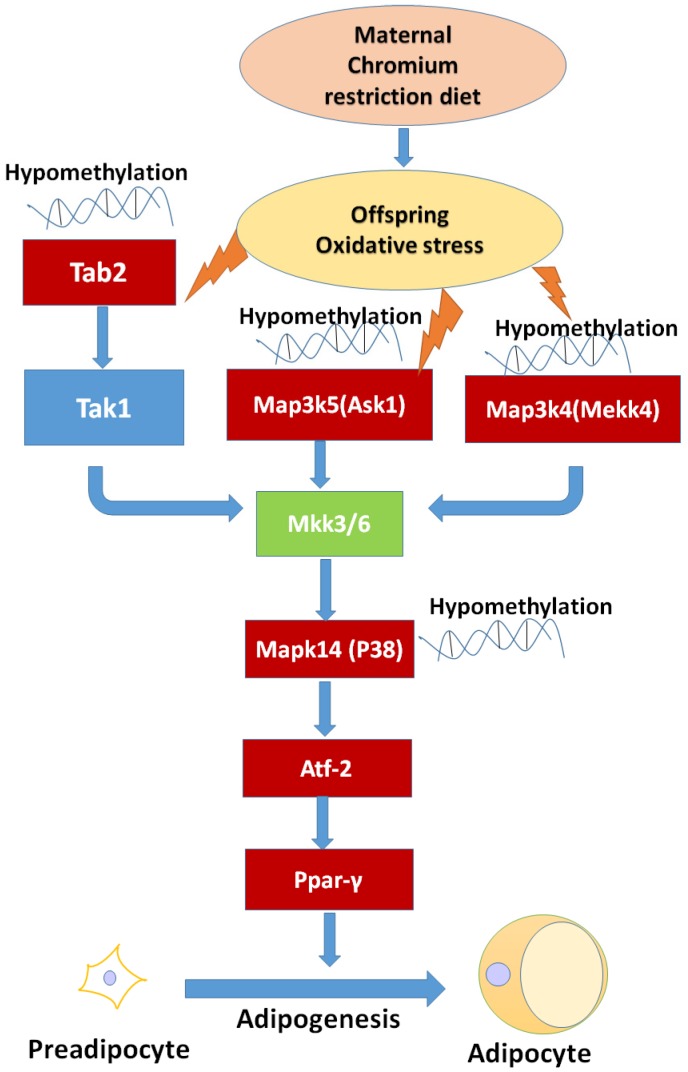
After genome-wide screening for differentially methylated promoters, we found that MAPK signaling was located at the center of many different methylated genes (Figure 7), such as Map3k4, Mapk14, Map3k5, Tab2. MAPKs can regulate many cellular processes, such as normal cell proliferation, differentiation, and apoptosis [32]. More and more evidence shows that MAPK-dependent signal transduction is related to adipogenesis [33,34]. The MAPK family consists of three main members: extracellular signal-regulated protein kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 kinases. Inflammatory cytokines and many other cellular stresses may activate the p38 MAPK pathway. The p38 MAPK takes part in apoptosis and cell cycle regulation [35]. Transforming growth factor-β-activated kinase 1 (TAK1) belongs to the MAPK family [36]. TAK1 activity is regulated by TAB1, TAB2, TAB3, and TAB4 [37,38]. The activated TAK1 induces the activation of p38 MAPK. Liu et al. found TNF-α markedly promotes the interaction between TAB2 and TAK1, leading to the phosphorylation of p38 MAPK. Pre-treatment with docosahexaenoic acid (DHA) can block the interaction between TAB2 and TAK1 and can attenuate the TNF-α-induced phosphorylation of ERK [39]. We found that maternal low chromium diet could lead to the demethylation of TAB2 and p38, and activation of Tab2 and p38 expression. Finer et al. found that maternal gestational diabetes leads to genome-wide DNA methylation difference in the placenta and cord blood of exposed offspring. Pathways involved in MAPK signaling are enhanced in methylation genes [40]. Nyggf4, which is a newly discovered obesity candidate gene, may regulate the methylation levels of various isoforms of the MAPK subfamily [41].
Figure 7.
The possible mechanism of maternal chromium restriction on offspring. Maternal chromium restriction induces oxidative stress, then leads to hypomethylate in Map3k5 (Ask1), Tab2, Map3k4 (Mekk4), Mapk14 (p38 MAPK) promoter, activates Ppar-γ through Atf-2 to accelerate adipogenesis in adipose of mice offspring. The red box indicates hypomethylated gene promoters, and upregulated gene expression. Map3k4: mitogen-activated protein kinase kinase kinase 4; Mapk14: mitogen-activated protein kinase 14; Map3k5: mitogen-activated protein kinase kinase kinase 5; Tab2: TGF-beta activated kinase 1/MAP3K7 binding protein 2; Tak1: Transforming growth factor-β-activated kinase 1; Ask1: apoptosis signal-regulated kinase 1; Mkk3/6: mitogen-activated protein kinase kinase 3/6; Pparγ: peroxisome proliferator activated receptor gammas; Atf2: activating transcription factor 2.
MAPK family is sensible to imbalance of antioxidant system [42]. Our research found that maternal chromium restriction increased serum MDA level and reduced GSH/GSSG ratio in offspring. Indeed, the measurement of oxidative stress and antioxidant markers is often disturbed by interference from the assay or overestimation derived form stressing analysis conditions. However, MDA is an end-product of lipid hydroperoxide and a widely used marker of the status of oxidative stress [43]. And GSH is a critical antioxidants in vivo. It can neutralize reactive oxygen species through converting to GSSG. Reduction of the GSH/GSSH ratio is an important biomarker of oxidative stress [44]. Saben et al. identified pathways affected by oxidative stress significantly increased in placenta from obese women [45]. Maternal low protein diet increases oxidative stress and reduces anti-oxidant enzyme activity in placenta and fetal livers of rats [46]. Oxidative stress is one mechanism programming offspring metabolic outcomes.
P38 MAPK can activate PPARγ through activating transcription factor-2 (ATF-2) [47,48]. PPARγ is a key transcription factor medicating adipocyte differentiation [49]. Our result also showed that maternal chromium restriction activate the expression of Atp2 and Pparg in adipose of mice offspring. Activated Pparg induces the expression of lipolytic (lipoprotein lipase) and lipogenic (fatty acid synthase) enzymes, which modulate fatty acid uptake and synthesis [50,51]. Increased PPARγ levels are observed in the adipose tissue of obese animals and humans [52,53,54]. Ahmad et al. found a significant upregulation of hepatic PPARγ expression in 12 months old rat pups born to vitamin B12-deficient mothers [55].
5. Conclusions
In summary, this study is the first to demonstrate a linkage among maternal low chromium diet, the regulation of methylation levels in MAPK pathway genes, and dysfunctional lipid metabolism in offspring. This could lead to adipogenesis and lipogenesis and could contribute to an enhanced susceptibility to the development of obesity in later life. Identifying the biological underpinnings of these mechanisms could help us find early interventional treatments for offspring due to maternal undernutrition. Based on the results revealed by this study, the MAPK pathway genes methylation could be considered as a possible target to modulate the development of lipid dysfunction in the offspring born from mothers with chromium restriction diet during pregnancy.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81170736, 81570715), National Natural Science Foundation for Young Scholars of China (81300649), China Scholarship Council foundation (201308110443), PUMC Youth Fund (33320140022), Fundamental Research Funds for the Central Universities, and the Scientific Activities Foundation for Selected Returned Overseas Professionals of Human Resources and Social Security Ministry.
Author Contributions
Xinhua Xiao conceived and designed the experiments; Qian Zhang, Xiaofang Sun, Jia Zheng, Tong Wang, Cuijuan Qi, Zhixin Wang and Xiaojing Wang performed the experiments; Miao Yu, Ming Li and Fan Ping analyzed the data; Xinhua Xiao contributed reagents/materials/analysis tools; Qian Zhang wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.De Rooij S.R., Painter R.C., Phillips D.I., Osmond C., Michels R.P., Godsland I.F., Bossuyt P.M., Bleker O.P., Roseboom T.J. Impaired insulin secretion after prenatal exposure to the dutch famine. Diabetes Care. 2006;29:1897–1901. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- 2.Barker D.J., Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 3.Oken E., Gillman M.W. Fetal origins of obesity. Obes. Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 4.Desai M., Gayle D., Babu J., Ross M.G. Programmed obesity in intrauterine growth-restricted newborns: Modulation by newborn nutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 5.Razin A. Cpg methylation, chromatin structure and gene silencing—A three-way connection. Embo J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Razin A., Shemer R. Dna methylation in early development. Hum. Mol. Genet. 1995;4(Suppl. 1):1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- 7.Wu G., Bazer F.W., Cudd T.A., Meininger C.J., Spencer T.E. Maternal nutrition and fetal development. J. Nutr. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey K.M., Lillycrop K.A., Burdge G.C., Gluckman P.D., Hanson M.A. Epigenetic mechanisms and the mismatch concept of the Developmental Origins of Health and Disease. Pediatr. Res. 2007;61:5r–10r. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- 9.Onakpoya I., Posadzki P., Ernst E. Chromium supplementation in overweight and obesity: A systematic review and meta-analysis of randomized clinical trials. Obes. Rev. 2013;14:496–507. doi: 10.1111/obr.12026. [DOI] [PubMed] [Google Scholar]
- 10.Anderson R.A., Kozlovsky A.S. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am. J. Clin. Nutr. 1985;41:1177–1183. doi: 10.1093/ajcn/41.6.1177. [DOI] [PubMed] [Google Scholar]
- 11.Anderson R.A. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000;26:22–27. [PubMed] [Google Scholar]
- 12.Offenbacher E.G., Pi-Sunyer F.X. Beneficial effect of chromium-rich yeast on glucose tolerance and blood lipids in elderly subjects. Diabetes. 1980;29:919–925. doi: 10.2337/diab.29.11.919. [DOI] [PubMed] [Google Scholar]
- 13.Suksomboon N., Poolsup N., Yuwanakorn A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J. Clin. Pharm. Ther. 2014;39:292–306. doi: 10.1111/jcpt.12147. [DOI] [PubMed] [Google Scholar]
- 14.Lefavi R.G., Anderson R.A., Keith R.E., Wilson G.D., Mcmillan J.L., Stone M.H. Efficacy of chromium supplementation in athletes: Emphasis on anabolism. Int. J. Sport Nutr. 1992;2:111–122. doi: 10.1123/ijsn.2.2.111. [DOI] [PubMed] [Google Scholar]
- 15.Vincent J.B. Recent advances in the nutritional biochemistry of trivalent chromium. Proc. Nutr. Soc. 2004;63:41–47. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 16.Padmavathi I.J., Rao K.R., Venu L., Ganeshan M., Kumar K.A., Rao Ch N., Harishankar N., Ismail A., Raghunath M. Chronic maternal dietary chromium restriction modulates visceral adiposity: Probable underlying mechanisms. Diabetes. 2010;59:98–104. doi: 10.2337/db09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiken C.E., Ozanne S.E. Sex differences in developmental programming models. Reproduction. 2013;145:R1–R13. doi: 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- 18.Dabelea D., Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60:1849–1855. doi: 10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor B.A., Phillips S.J. Detection of obesity QTLS on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics. 1996;34:389–398. doi: 10.1006/geno.1996.0302. [DOI] [PubMed] [Google Scholar]
- 20.Weber M., Hellmann I., Stadler M.B., Ramos L., Paabo S., Rebhan M., Schubeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat. Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 21.Dennis G., Jr., Sherman B.T., Hosack D.A., Yang J., Gao W., Lane H.C., Lempicki R.A. David: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 22.Kumaki Y., Oda M., Okano M. Quma: Quantification tool for methylation analysis. Nucleic Acids Res. 2008;36:W170–W175. doi: 10.1093/nar/gkn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar K.A., Lalitha A., Pavithra D., Padmavathi I.J., Ganeshan M., Rao K.R., Venu L., Balakrishna N., Shanker N.H., Reddy S.U., et al. Maternal dietary folate and/or vitamin b12 restrictions alter body composition (adiposity) and lipid metabolism in wistar rat offspring. J. Nutr. Biochem. 2013;24:25–31. doi: 10.1016/j.jnutbio.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Venu L., Harishankar N., Prasanna Krishna T., Raghunath M. Maternal dietary vitamin restriction increases body fat content but not insulin resistance in wnin rat offspring up to 6 months of age. Diabetologia. 2004;47:1493–1501. doi: 10.1007/s00125-004-1506-4. [DOI] [PubMed] [Google Scholar]
- 25.Venu L., Padmavathi I.J., Kishore Y.D., Bhanu N.V., Rao K.R., Sainath P.B., Ganeshan M., Raghunath M. Long-term effects of maternal magnesium restriction on adiposity and insulin resistance in rat pups. Obesity (Silver Spring) 2008;16:1270–1276. doi: 10.1038/oby.2008.72. [DOI] [PubMed] [Google Scholar]
- 26.Liu X., Qi Y., Tian B., Chen D., Gao H., Xi C., Xing Y., Yuan Z. Maternal protein restriction induces alterations in hepatic tumor necrosis Factor-Alpha/Cyp7a1 signaling and disorders regulation of cholesterol metabolism in the adult rat offspring. J. Clin. Biochem. Nutr. 2014;55:40–47. doi: 10.3164/jcbn.13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva S.V., Garcia-Souza E.P., Moura A.S., Barja-Fidalgo C. Maternal protein restriction during early lactation induces changes on neutrophil activation and tnf-alpha production of adult offspring. Inflammation. 2010;33:65–75. doi: 10.1007/s10753-009-9159-6. [DOI] [PubMed] [Google Scholar]
- 28.Bringhenti I., Schultz A., Rachid T., Bomfim M.A., Mandarim-De-Lacerda C.A., Aguila M.B. An early fish oil-enriched diet reverses biochemical, liver and adipose tissue alterations in male offspring from maternal protein restriction in mice. J. Nutr. Biochem. 2011;22:1009–1014. doi: 10.1016/j.jnutbio.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Caiafa P., Zampieri M. DNA Methylation and chromatin structure: The puzzling cpg Islands. J. Cell Biochem. 2005;94:257–265. doi: 10.1002/jcb.20325. [DOI] [PubMed] [Google Scholar]
- 30.Campion J., Milagro F.I., Martinez J.A. Individuality and epigenetics in obesity. Obes. Rev. 2009;10:383–392. doi: 10.1111/j.1467-789X.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 31.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 32.Kyosseva S.V. Mitogen-activated protein kinase signaling. Int. Rev. Neurobiol. 2004;59:201–220. doi: 10.1016/S0074-7742(04)59008-6. [DOI] [PubMed] [Google Scholar]
- 33.Sale E.M., Atkinson P.G., Sale G.J. Requirement of map kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 s6 kinase and for insulin or serum stimulation of DNA synthesis. Embo J. 1995;14:674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bost F., Aouadi M., Caron L., Even P., Belmonte N., Prot M., Dani C., Hofman P., Pages G., Pouyssegur J., et al. The extracellular signal-regulated kinase isoform erk1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- 35.Kummer J.L., Rao P.K., Heidenreich K.A. Apoptosis induced by withdrawal of trophic factors is mediated by P38 mitogen-activated protein kinase. J. Biol. Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi K., Shirakabe K., Shibuya H., Irie K., Oishi I., Ueno N., Taniguchi T., Nishida E., Matsumoto K. Identification of a member of the mapkkk family as a potential mediator of tgf-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 37.Cheung P.C., Nebreda A.R., Cohen P. Tab3, A new binding partner of the protein kinase tak1. Biochem. J. 2004;378:27–34. doi: 10.1042/bj20031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prickett T.D., Ninomiya-Tsuji J., Broglie P., Muratore-Schroeder T.L., Shabanowitz J., Hunt D.F., Brautigan D.L. Tab4 stimulates tak1-tab1 phosphorylation and binds polyubiquitin to direct signaling to Nf-Kappab. J. Biol. Chem. 2008;283:19245–19254. doi: 10.1074/jbc.M800943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu K.L., Yang Y.C., Yao H.T., Chia T.W., Lu C.Y., Li C.C., Tsai H.J., Lii C.K., Chen H.W. Docosahexaenoic acid inhibits inflammation via free fatty acid receptor ffa4, disruption of tab2 interaction with tak1/tab1 and downregulation of Erk-dependent Egr-1 expression in Ea.Hy926 cells. Mol. Nutr. Food Res. 2016;60:430–443. doi: 10.1002/mnfr.201500178. [DOI] [PubMed] [Google Scholar]
- 40.Finer S., Mathews C., Lowe R., Smart M., Hillman S., Foo L., Sinha A., Williams D., Rakyan V.K., Hitman G.A. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum. Mol. Genet. 2015;24:3021–3029. doi: 10.1093/hmg/ddv013. [DOI] [PubMed] [Google Scholar]
- 41.Yang L., Tong M.L., Chi X., Zhang M., Zhang C.M., Guo X.R. Genomic DNA methylation changes in Nyggf4-Overexpression 3t3-L1 adipocytes. Int. J. Mol. Sci. 2012;13:15575–15587. doi: 10.3390/ijms131215575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mccubrey J.A., Lahair M.M., Franklin R.A. Reactive oxygen species-induced activation of the map kinase signaling pathways. Antioxid. Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 43.You Y., Yoo S., Yoon H.G., Park J., Lee Y.H., Kim S., Oh K.T., Lee J., Cho H.Y., Jun W. In vitro and in vivo hepatoprotective effects of the aqueous extract from taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010;48:1632–1637. doi: 10.1016/j.fct.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Sentellas S., Morales-Ibanez O., Zanuy M., Alberti J.J. Gssg/Gsh ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol. Vitro. 2014;28:1006–1015. doi: 10.1016/j.tiv.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Saben J., Lindsey F., Zhong Y., Thakali K., Badger T.M., Andres A., Gomez-Acevedo H., Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35:171–177. doi: 10.1016/j.placenta.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vega C.C., Reyes-Castro L.A., Rodriguez-Gonzalez G.L., Bautista C.J., Vazquez-Martinez M., Larrea F., Chamorro-Cevallos G.A., Nathanielsz P.W., Zambrano E. Resveratrol partially prevents oxidative stress and metabolic dysfunction in pregnant rats fed a low protein diet and their offspring. J. Physiol. 2016;594:1483–1499. doi: 10.1113/JP271543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maekawa T., Jin W., Ishii S. The role of Atf-2 family transcription factors in adipocyte differentiation: Antiobesity effects of P38 inhibitors. Mol. Cell Biol. 2010;30:613–625. doi: 10.1128/MCB.00685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Figuera-Losada M., Lograsso P.V. Enzyme kinetics and interaction studies for human jnk1beta1 and substrates activating transcription factor 2 (Atf2) and C-Jun N-Terminal kinase (C-Jun) J. Biol. Chem. 2012;287:13291–13302. doi: 10.1074/jbc.M111.323766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hata K., Nishimura R., Ikeda F., Yamashita K., Matsubara T., Nokubi T., Yoneda T. Differential roles of Smad1 and P38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol. Biol. Cell. 2003;14:545–555. doi: 10.1091/mbc.E02-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fajas L., Schoonjans K., Gelman L., Kim J.B., Najib J., Martin G., Fruchart J.C., Briggs M., Spiegelman B.M., Auwerx J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell Biol. 1999;19:5495–5503. doi: 10.1128/MCB.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J.B., Spiegelman B.M. Add1/Srebp1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 52.Tontonoz P., Spiegelman B.M. Fat and beyond: The diverse biology of ppargamma. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 53.Joss-Moore L.A., Wang Y., Campbell M.S., Moore B., Yu X., Callaway C.W., Mcknight R.A., Desai M., Moyer-Mileur L.J., Lane R.H. Uteroplacental insufficiency increases visceral adiposity and visceral adipose ppargamma2 expression in male rat offspring prior to the onset of obesity. Early Hum. Dev. 2010;86:179–185. doi: 10.1016/j.earlhumdev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janesick A., Blumberg B. Minireview: Ppargamma as the target of obesogens. J. Steroid. Biochem. Mol. Biol. 2011;127:4–8. doi: 10.1016/j.jsbmb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmad S., Kumar K.A., Basak T., Bhardwaj G., Yadav D.K., Lalitha A., Chandak G.R., Raghunath M., Sengupta S. Ppar signaling pathway is a key modulator of liver proteome in pups born to vitamin B(12) deficient rats. J. Proteomics. 2013;91:297–308. doi: 10.1016/j.jprot.2013.07.027. [DOI] [PubMed] [Google Scholar]