Abstract
IL-17 and IL-17-producing cells have been found in many types of human cancers and murine models. However, the source of tumor-infiltrating IL-17 and IL-17-producing cells in HCC and the prognostic values remain poorly understood.
A total of 57 HCC patients were enrolled in this study, and immunofluorescence double stain was used to evaluate the colocalization of CD3+ T cells, CD4+ T cells, CD56+ NK cells, CD20+ B cells, CD68+ Macrophages, and MCT+ mast cells with IL-17. The prognostic value of IL-17-producing cells was evaluated by Kaplan–Meier analysis and Cox regression model.
MCT+ mast cells, but not other cells, were the predominant IL-17-producing cell type. Overall survival analysis revealed that the increasing intratumoral-infiltrated MCT+ mast cells were significantly associated with poor prognosis. Immunofluorescence double stain showed a positive correlation between the number of MCT+ mast cells and MCVs.
These findings indicated the major IL-17-producing cells in HCC were MCT+ mast cells and these cells infiltration may promote tumor progression by angiogenesis. Increased MCT+ mast cells was associated with a poor prognosis, indicating therapy targeting MCT+ mast cells might be an effective strategy in controlling intratumor IL-17 infiltration and MCVs.
INTRODUCTION
Over the past decade, much attention has been paid on tumor-mediated immunosuppression.1,2 Despite the generalized immunosuppressive status of cancer patients, many malignancies are raised from chronic inflammation and inflammatory mediators produced by inflammatory cells are often participated in.3,4 One of the most important components of inflammatory cytokines associated with cancer has recently been recognized as IL-17. The proinflammatory cytokine interleukin (IL-17) has been identified as a crucial mediator in the pathogenesis of diverse human tumors and is capable of being pro- or antitumorigenic.5,6 IL-17 has been identified in various tumors, including breast cancer,7 gastric cancer,8 colorectal cancer,9 lung cancer,10 intrahepatic cholangiocarcinoma,11 and esophageal squamous cell carcinoma.12
In general, IL-17-producing CD4+ T helper cells (known as Th17 cells) are speculated as the major cellular source of IL-17. However, other cell types including regulatory T cells, γδT cells, NKT cells, NK cells, neutrophils, and eosinophils have been reported to produce IL-17.13,14 Recently, mast cells have been described to express IL-17 and even considered as the main source of IL-17.15 Thus, in tumor, the net IL-17 expression may arise from a broad array of adaptive and innate cells. Thus, to understand the complex tumor immune microenvironment, it is important to define the cellular sources of IL-17 in situ and evaluate their clinical and pathological associations.
HCC is the fifth most common cancer and highly prevalent in the Asia-Pacific region, and due to the dissemination of hepatitis B virus (HBV) infections, its incidence is increasing worldwide.16 HCC is characterized by progressive disease with a poor prognosis.17 Over 80% of HCC cases worldwide have the background of chronic hepatitis B and regenerative nodules and atypical hyperplasia, the immunopathological processes leading to HCC are associated with important changes to the quantity and quality of lymphocyte subsets and inflammatory cytokines in HCC. IL-17 and IL-17-producing cells have been reported in previous studies and the high expression are correlated with poor survival.4,18–21,22 In HCC tissue, IL-17 + cells or IL-17 + cells were speculated as Th1719–21 and there are different kinds of IL-17 + cells too, such as IL-17−producing CD8 + T cells and IL-17-producing γδT cells.4,22 So the source of IL-17 was controversial and did not limited to Th17 cells.
Our aim is to provide the distribution, functional relevance, the source, and predictive value of IL-17-producing cells in HCC. We provide novel insights into the potential mechanism(s) of IL-17 in the tumor microenvironment in patients with HCC by evaluating the relationship between IL-17-producing cells and microvessel density (MCV). We hope our research could provide some clues to understand the tumor microenvironment.
MATERIALS AND METHODS
Patients
Tumor and the corresponding peritumor tissues (at least 3 cm distant from the tumor site) were surgically obtained from 57 HCC patients who received curative resection between 2007 and 2010 at the Hepatobiliary Surgery of the Lishui Central Hospital. The pathological diagnosis of HCC were confirmed by an experienced pathologist under microscope through the standard H.E. sections. None of the HCC patients had received immunosuppressive drugs or chemotherapy before surgery. Overall survival was defined as the interval between the dates of surgery and death or the last follow-up. Liver tissues from 10 patients who received partial liver resection for benign disease were used as normal control. All written informed consents were obtained before the study. The study protocol was approved by the ethics committee of the Lishui Central Hospital.
Immunohistochemistry
Standard H&E staining was used to confirm the pathological diagnosis. Paraffin-embedded, 4-lm-thick sections of patient were selected for IHC analysis. Sections were dewaxed and then subjected to heat-induced epitope retrieval with preheated epitope retrieval solution (10 mM citrate buffer, pH 6.0). Next, endogenous peroxidase activity was blocked and the sections were incubated overnight with one of the following primary mAbs: Goat anti-IL-17 (1:200, R&D Systems), MCT (1:800, Abcam; UK). After incubation with HRP-conjugated second antibody (Invitrogen, Carlsbad, CA) and development with diaminobenzidine, sections were counterstained with hematoxylin.
Negative control staining was carried out with cold PBS in place of primary antibody.
Immunofluorescence
Sections were dewaxed and then subjected to heat-induced epitope retrieval with preheated epitope retrieval solution (10 mM citrate buffer, pH 6.0). The primary Abs were antibodies cocktail. Goat anti-IL-17 (R&D Systems) was used to detect IL-17 + cells, a panel of antibodies reactive with CD4, CD20, CD57, CD68, CD34 (1/2 working solution, all from Beijing Zhongshan Golden Bridge Biotech), CD4 (1:20; Leica, Germany) and MCT (1:800, Abcam, UK) were used. The sections were then incubated overnight in 4°C. In the next day, a mixture of secondary antibodies (Alexa Fluor 488/568-conjugated donkey anti-mouse or Cy3/Alexa Fluor 488 donkey anti-goat [all from Invitrogen]) were applied and incubated in 37°C for 1 hour. Nucleus were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Images were captured under a fluorescence microscope (Olympus BX51, Japan) coupled to a CCD camera (Nikon DS-Ri1) and analyzed by NIS-Elements BR 3.2 software.
Quantifying Immunostaining Parameters
Positive stained cells in normal, peritumoral, and intratumoral regions were manually counted. The method that identify microvessels has been described in the previous paper.23 Briefly, only vascular endothelial cells or clusters of brown-stained cells had clear boundaries with adjacent structures were counted as microvessels. All the positive-staining cells and the double-staining cells were manual counted by 2 independent blinded investigators.
Statistical Analysis
Immune cell subsets infiltrating into tumor tissue and the corresponding peritumor and normal tissue were compared by Student's t tests. Clinicopathological features between IL-17-producing cells or MCT + mast cells were analyzed by chi-square test. Correlations between microvessel density and IL-17-positive cells were assessed by calculating the Spearman correlation coefficient (r). The associations between IL-17-producing cells and MCT + mast cells with OS were analyzed using Kaplan–Meier curves and compared by the log-rank test. Survival and Spearman correlation analyses were performed by GraphPad Prism (ver. 5.00 for Windows; GraphPad Software, San Diego, CA) and Student's t-tests and chi-square test were by the SPSS (ver. 16.0; SPSS Inc., Chicago, IL) software. P < 0.05 was considered as statistically significant.
RESULTS
Patients
The clinicopathologic characteristics of all patients were summarized in Table 1. Of the 57 patients, male dominated the majority (93%). The mean age was 50 ± 9 years. As for alpha-fetoprotein (AFP), 44% patients were >200 ng/mL, and the median value was 94.8 ng/mL. A total of 26 (45.6%) patients was HBV deoxyribonucleic acid (DNA) possible. Before surgery, 45 (79%) patients were suffered from abnormal of liver function (alanine aminotransferase [ALT] > 40U/L). The pathology showed that the most of HCC were moderately differentiated or poorly differentiated (54/57, 94.7%). As for tumor size, 35 cases were < 5 cm and 24 cases were > 5 cm. The number of tumors was counted at the same time and 29 cases had >1 tumor.
TABLE 1.
Clinicopathologic Characteristics of the Patients With HCC
MCT+ Mast Cells Were the Major Source of IL-17
The cell morphology of IL-17+ cells was visualized by immunohistochemistry. Both in tumor tissue and in normal tissues, staining of IL-17-positive cells was shown as irregular and different phenotypes, as described previously 15 (Figure 1). Ten samples of intratumor region and the corresponding peritumor tissue from the entire cohort were randomly selected to evaluate the distribution and phenotype of IL-17+ cells. At first, we colocalized IL-17 with the common immune cell markers, such as CD4, CD20, CD56, CD68, and calculated the proportion of IL-17+ cells in each subset. CD4+IL-17+ cells were few and the majority of IL-17+ cells were CD4-negative (Figure 2, positive rate: 0.2–6.8%). This is consistent with that CD4+ Th17 cells account for a minority of the IL-17-producing cells. CD20+IL-17+ and CD56+IL-17+ were barely identified too (Table 2). As mentioned above, IL-17+ cells have the phenotypically distinct subset of IL-17+ cells of irregular shape. This suggested macrophages may have the potential of IL-17-producing population.8,15 Unlike in rheumatoid arthritis or gastric cancer, only 7.6% of IL-17+ cells were CD68 positive (Figure 3). However, the majority of IL-17-producing cells remained unattributed in this study, and additional cellular sources were sought. MCT and IL-17 were colocalized via immunofluorescence (Figure 2 and Figure 4). The percentage of IL-17+ cells simultaneously stained with MCT was 25% to 85.2%, demonstrating that MCT+ mast cells were the key source of IL-17 in HCC (Table 2). Finally, the distribution of IL-17-expressing cells in peritumor tissue was also analyzed and the results as the same in tumor tissue (date not shown).
FIGURE 1.
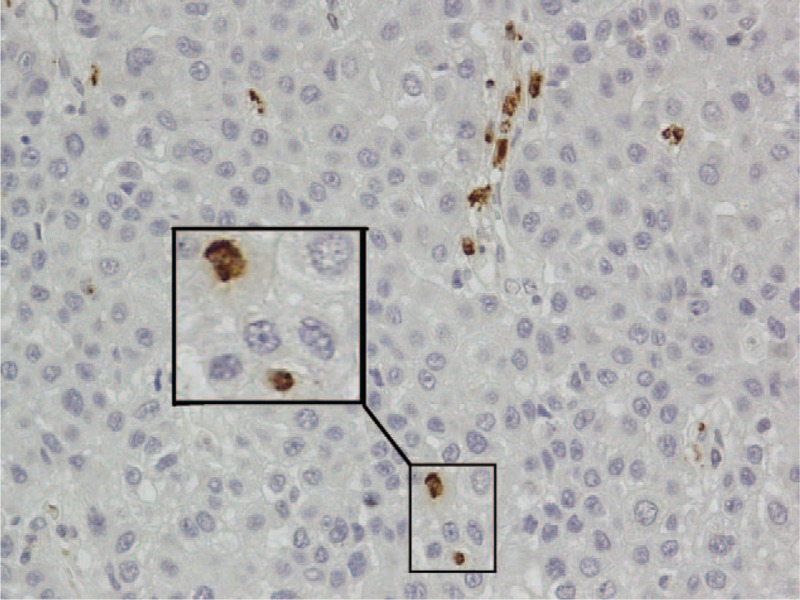
IL-17-positive cells in HCC tissue showed different cell morphologies. The squares indicate different examples (brown, shown at 400× magnification). HCC = hepatocellular carcinoma.
FIGURE 2.
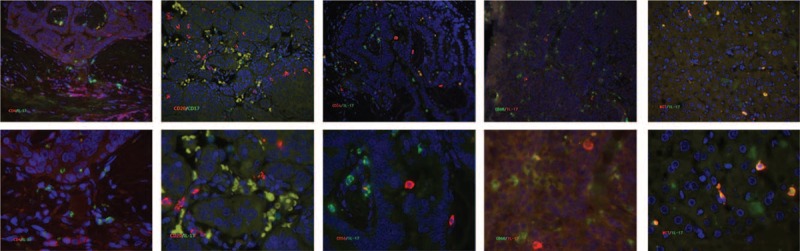
Liver tissue samples (n = 10) were stained for IL-17 (red and green) and indicated markers. Sections were counterstained with DAPI. Markers of CD4 (red) and CD20 (red), CD56 (red) and CD68 (green), as well as MCT (red) are shown. Of note, except MCT/IL-17 was the peritumor tissue; the others were HCC tissue. DAPI = 4′,6-diamidino-2-phenylindole, IL-17 = interleukin-17, HCC = hepatocellular carcinoma, MCT = mast cell tryptase.
TABLE 2.
Percentage of Double-Positive Cells Compared With IL-17+ Cells in HCC Tissue
FIGURE 3.

MCT (red) and IL-17 (green) double positive cells in HCC tissue. Mast cells highly express IL-17 in gastric cancer (merged in yellow, right panel). IL-17 = interleukin-17, HCC = hepatocellular carcinoma, MCT = mast cell tryptase.
FIGURE 4.
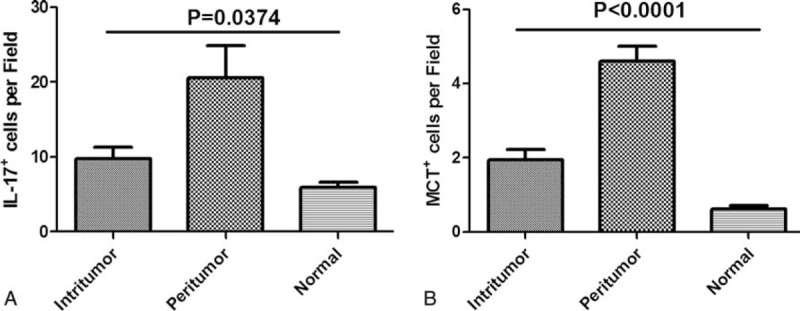
Distribution of IL-17+ cells, MCT+ mast cells. Both IL-17+ cells (A) and MCT+ mast cells (B) were significantly increased in peritumor, followed by intratumor and normal. IL-17 = interleukin-17, MCT+ = mast cell tryptase+.
Distribution of IL-17+ Cells, MCT+ Mast Cells, and the Association With Clinicopathological Features
Immunohistochemistry of intratumor and peritumor sample tissues from 57 patients and 10 normal liver tissues was performed to further investigate the distribution of IL-17+ cells and MCT+ mast cells. Interestingly, the significantly increased numbers of IL-17+ cells or MCT+ mast cells were not in intratumor tissue (6, range[0–68];1.4, range[0–10]), but in peritumor tissues (11.5, range[0–112]; 4.3, range[0–12.5]), and the normal tissue was the least (11.5, range [0–112]; 0.5, range[0–2] P = 0.0374, P < 0.0001, respectively) (Figure 4).
The chi-square test or fisher's exact test was used when appropriated to identify the association between clinicopathological features and MCT+ mast cell or IL-17+ cells. Neither MCT+ mast cell nor IL-17+ cells in the intratumor tissues correlate with any clinical characteristic assessed, including sexual, tumor size, tumor multiplicity, degree of differentiation, and vascular invasion (date not shown).
Increased Intratumoral IL-17-Producing Cell and MCT+ Mast Cell Predicted Poor Survival of HCC Patients
The conventional clinicopathological features, such as gender, age, AFP, HBV DNA, Child-Pugh grade, tumor size, none of them could predict the overall survival in this study. The patients with >1 tumor showed worse prognosis (P = 0.0356). As shown in Figure 5, there was a significant inverse correlation between intratumoral MCT+ mast cell density and patient survival (P = 0.0023). Patients with higher intratumoral MCT+ mast cell density had significantly shorter OS than patients with lower intratumoral MCT+ mast cell density. As intratumoral MCT+ mast cell density, higher IL-17-producing cell predicted poor OS too (P = 0.0125).
FIGURE 5.
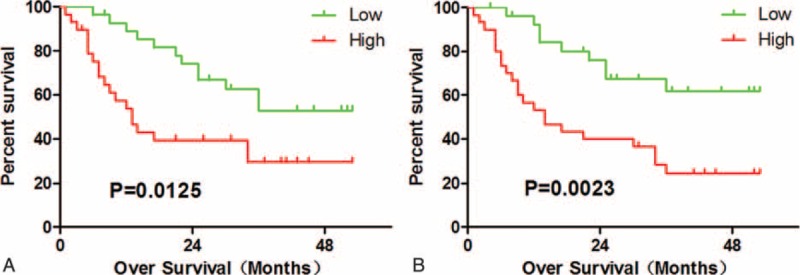
Intratumor IL-17+ cells and MCT+ cells predicts poor survival in HCC. (A) High intratumor IL-17+ cells infiltration conferred a significant high risk of death. (B) Patients with high MCT+ cell intratumor had significant poorer survival than patients with low MCT+ cell. IL-17 = interleukin-17, HCC = hepatocellular carcinoma, MCT+ = mast cell tryptase+.
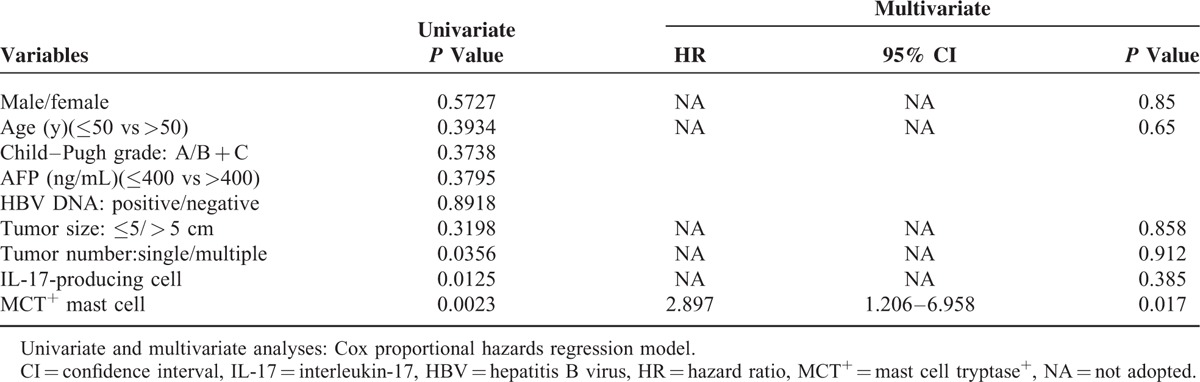
Sexual, age, the size of the tumor, number of tumor, and intratumoral IL-17-producing cell, MCT+ mast cell density were included in a multivariate Cox proportional hazards analysis, we found that only intratumor MCT+ mast cell density (HR = 2.897; 95% CI: 1.206–6.958; P = 0.017) was the independent prognostic factor (Table 3). Indicating that patients with higher numbers of MCT+ mast cell density were nearly 3.1-fold more likely to die than those with lower ratios.
TABLE 3.
Univariate and Multivariate Analyses of Factors Associated With Overall Survival
Positive Correlation Between IL-17-Producing Cell and Microvessels Density
As a pro-inflammatory factor, IL-17 have been reported promote tumor growth by fostering angiogenesis. To explore the potential mechanism(s) of IL-17 accumulation in the HCC microenvironment with poor prognosis, randomly selected 15 intratumor tissue form the entire cohort were performed for IL-17-producing cell and microvessel density (MVD, CD34) immunofluorescence double-staining (Figure 6A). The date showed a significant correlation between IL-17+ cells and CD34+ microvessels (P = 0.0006, r2 = 0.6097, Figure 6B).
FIGURE 6.
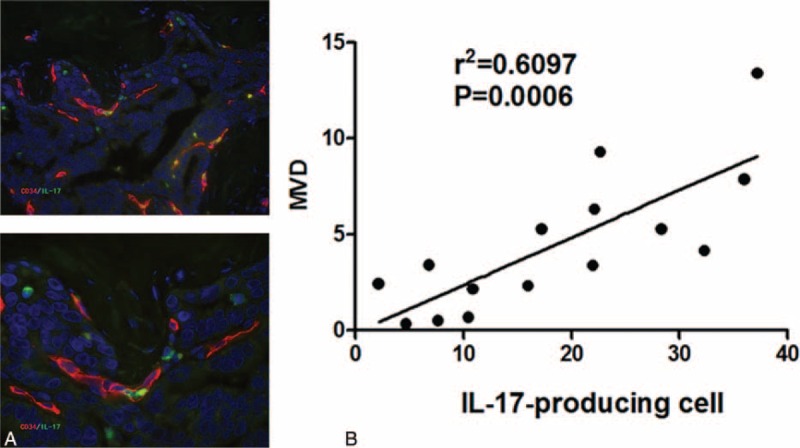
The positive correlation between IL-17-producing cell and microvessels density. (A) Colocalization between IL-17-producing cell (green) and CD34+ vascular endothelial cells (red); (B) Spearman's correlation coefficient analysis showed a positive correlation between IL-17-producing cell and microvessels density. IL-17 = interleukin-17.
DISCUSSION
Emerging evidence indicates that the specific nature of inflammation and the tissue context may determine the ability of inflammatory response to facilitate or prevent tumor growth. The present study showed that mast cells, but not T cells, were the predominant IL-17-expressing cells in HCC tissues in situ and their associations with the poor prognosis, finally the possible mechanism. These data provide new insights into the significance of proinflammatory response in human tumor progression.
The cytokine interleukin (IL-17) is a proinflammatory cytokine that was identified almost 2 decades ago. More recently, IL-17-producing CD4+ T cells have been shown to be distinct from classical Th1 and Th2 cells, thus designated as Th17 cells and described as the major source of IL-17. In HCC tissue, IL-17+ cells were usually recognized as Th17 cells.18–20 Zhang et al have been found that both in peripheral blood mononuclear and tumor-infiltrating lymphocytes IL-17+ cells contained cells that did not express CD4 molecules and his part of CD4-IL-17+ cells in peripheral blood could be remarkable proportions (20–40%).18,21 In this study, we found that CD4+ IL-17+ lymphocytes comprised only 13.1% of the IL-17-expressing cells. Until lately, more and more researches supported that IL-17 could be produced by other cell types including regulatory T cells, γδT cells, NKT cells, NK cells, neutrophils, and eosinophils.13,14 In murine HCC models, IL-17-producing γδT cells were found and IL-17 produced by which promotes tumor growth. In human HCC, IL-17-producing CD8+ T Cells were also been identified.
Mast cells have recently re-emerged as crucial effectors in innate immune defense, as indicated by the production of a variety of cytokines. Based on the recent description of IL-17 production by mast cells in gastric cancer8 and esophageal squamous cell carcinoma,12 we focused on the expression and production of IL-17 by mast cells in HCC. The crucial observation in our study was the colocalization of IL-17 with MCT, a highly specific marker for mast cells in tissue. As reported in gastric cancer and esophageal squamous cell carcinoma, mast cells, but not T cells, were the predominant (∼54%) IL-17-expressing cells in HCC tissues in situ. In RA, up to 35% of IL-17-producing cells were CD68 + , suggesting that in RA this lineage can also contribute to IL-17 production. However, in HCC, CD68 + macrophages comprised only 7.6% IL-17-producing cells, almost equal to B cells and NK cells.
The distribution of IL-17-producing cells in liver tissue was quite different from previous studies. Yan et al and Zhang et al found the levels of IL17 + cells were significantly increased in tumors when compared with corresponding nontumor regions.18,20 We found the highest number of IL-17-producing cells and MCT + mast cells were in peritumor, followed by intratumor and normal tissue. Our date were consistent with Huang Yong's opinion: there was a decreasing trend from HCC, atypical hyperplasia to chronic hepatitis B infection for the density of IL-17+ T cells.19 The probable reason for this disagreement in each paper was the different define of nontumor regions or peritumor and background disease, such as different kinds of hepatitis virus infection or not.
Until now, evidence of the exact role of IL-17-producing cells in malignant tumors is controversial. As a newly found proinflammatory fact, IL-17 promote tumor progression and associated with the poor prognosis of patients. However, IL-17 + T cells were also identified as antitumor cells. By stimulating CXCL9 and CXCL10 production, IL-17 + T cells recruited effector T cells to the tumor microenvironment and predicted a favorable prognosis. For HCC, the protumor activity of IL-17 + T cells appears greater than antitumor activity. Two clinical study performed by Zhang et al and Huang et al respectively reported that increased intratumoral IL 17-producing cell density was associated with high mortality and reduced survival in patients with HCC,18,19 implying a promoting role of IL-17 in tumor progression. A recent study also found that high expression of IL- 17 and IL-17RE associated with poor prognosis of HCC.18 Consistent with previous studies, we observed both increased intratumoral IL 17-producing cell and MCT + mast cells affected the prognosis.
Though the precise underlying mechanism is not yet known, the ascending levels of IL-17-producing cells promote tumor progression by stimulating tissue remodeling and angiogenesis have been reported.8,24,25 Interestingly, the levels of intratumoral IL-17-producing cells were strong positively correlated with microvessel density in HCC. So far as we known, our finding was the first report in HCC. These data strongly indicated that IL-17-producing cells inside tumor may promote angiogenesis in HCC.
In summary, the present study demonstrated the phenotype and distribution of IL-17-producing cells and their clinical relevance, and particularly the prognostic value of intratumor IL-17-producing cell and mast cell in HCC. Our data demonstrate that mast cells occupied the most of IL-17-producing cells, whereas CD4 + IL-17 + lymphocytes were a small part of them. Furthermore, we found that intratumoral IL-17 + cells and intratumoral MCT + mast cells were correlated with worse survival and the later was the independent prognostic factor. Our results might provide a novel strategy for the rational design of HCC therapies.
Footnotes
Abbreviations: HBV = hepatitis B virus, HCC = hepatocellular carcinoma, IL-17 = interleukin-17, MCT = mast cell tryptase, MCT+ = mast cell tryptase+ , MCVs = mast cells microvessels.
Authors’ contributions—J-fT, HZ planned the article and contributed to data collection, discussing content, writing, and reviewing the article. JL, J-SJ, X-HY conceived the study and participated in its design, study supervision, and helping to writing the article.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Leung J, Suh WK. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw 2014; 14:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podojil JR, Miller SD. Targeting the B7 family of co-stimulatory molecules: successes and challenges. BioDrugs 2013; 27:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009; 457:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuang DM, Peng C, Zhao Q, et al. Tumor-activated monocytes promote expansion of IL-17-producing CD8 + T cells in hepatocellular carcinoma patients. J Immunol 2010; 185:1544–1549. [DOI] [PubMed] [Google Scholar]
- 5.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009; 183:4169–4175. [DOI] [PubMed] [Google Scholar]
- 6.Huang G, Wang Y, Chi H, et al. Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol 2012; 9:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X, Mulcahy LA, Mohammed RA, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res 2008; 10:R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Jin H, Zhang G, et al. Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients. PLoS One 2014; 9:e106834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst M, Putoczki T. IL-17 cuts to the chase in colon cancer. Immunity 2014; 41:880–882. [DOI] [PubMed] [Google Scholar]
- 10.Gu K, Li MM, Shen J, et al. Interleukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-kappaB/ZEB1 signal pathway. Am J Cancer Res 2015; 5:1169–1179. [PMC free article] [PubMed] [Google Scholar]
- 11.Gu FM, Gao Q, Shi GM, et al. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2012; 19:2506–2514. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Li L, Liao Y, et al. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother 2013; 62:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med 2009; 361:888–898. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27:485–517. [DOI] [PubMed] [Google Scholar]
- 15.Hueber AJ, Asquith DL, Miller AM, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol 2010; 184:3336–3340. [DOI] [PubMed] [Google Scholar]
- 16.Fitzmorris P, Shoreibah M, Anand BS, et al. Management of hepatocellular carcinoma. J Cancer Res Clin Oncol 2015; 141:861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 18.Liao R, Sun J, Wu H, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res 2013; 32:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Wang F, Wang Y, et al. Intrahepatic interleukin-17 + T cells and FoxP3 + regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol 2014; 29:851–859. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Liu XL, Xiao G, et al. Prevalence and clinical relevance of T-helper cells, Th17 and Th1, in hepatitis B virus-related hepatocellular carcinoma. PLoS One 2014; 9:e96080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009; 50:980–989. [DOI] [PubMed] [Google Scholar]
- 22.Ma S, Cheng Q, Cai Y, et al. IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res 2014; 74:1969–1982. [DOI] [PubMed] [Google Scholar]
- 23.Fang J, Shing Y, Wiederschain D, et al. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A 2000; 97:3884–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantovani A, Romero P, Palucka AK, et al. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 2008; 371:771–783. [DOI] [PubMed] [Google Scholar]
- 25.He D, Li H, Yusuf N, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol 2010; 184:2281–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]


