Supplemental Digital Content is available in the text
Abstract
Pancreatic cancer (PC) is known to be frequently associated with venous thromboembolism (VTE). Although treatment and prophylaxis strategies for VTE in PC patients were updated recently, these were mainly based on data from Western populations and were not verified in East Asian ethnic populations.
We investigated the clinical characteristics of VTE in East Asian PC patients. We reviewed electronic medical records (EMR) of 1334 patients diagnosed with pancreatic adenocarcinoma from 2005 to 2010 at single tertiary hospital in Korea. All the patients with newly diagnosed VTE were classified by anatomical site and manifestation of symptoms. The primary outcomes of interest were 2-year cumulative incidence of VTE events. Cox proportional hazards models were used to analyze associations between risk factors and clinical outcomes.
A total of 1115 patients were eligible for enrollment. The 2-year cumulative VTE incidence was 9.2%. Major risk factors associated with VTE event were advanced cancer stage, major surgery, and poor performance status. Risk factors associated with mortality after PC diagnosis included advanced cancer stage, poor performance score, leukocytosis, and lower albumin level. The overall VTE did not affected mortality. However in subgroup analysis, symptomatic VTE and deep vein thrombosis/pulmonary thromboembolism (DVT/PTE) showed worse prognosis than incidental or intra-abdominal VTE.
The overall incidence of VTE events in Korean PC patients was lower than previous studies. Advanced cancer stage was the most important factor for VTE event and mortality. Unlike Western population group, VTE event did not affect overall prognosis after PC diagnosis. However, symptomatic VTE and DVT/PTE showed higher mortality after VTE event.
INTRODUCTION
After Armand Trousseau first reported an association between thrombosis and cancer in 1865, malignancy has become a well-established risk factor for venous thromboembolism (VTE).1–4 Many studies have reported that cancer patients have higher tendency to occur VTE than normal population.5–7 Thrombosis itself is one of the leading cause of death in cancer patients and at the same time, the anticoagulant treatment for VTE can also give rise to bleeding complications.2,6–11 Thus, the stratification of VTE risk and prophylactic anticoagulation strategy in cancer patients has been under debate until recent updates of major international guidelines. In recent few years, updates of American society of clinical oncology, National comprehensive cancer network, American college of chest physicians, and European society for medical oncology guidelines suggest prophylactic anticoagulation to patients with high VTE risk and low bleeding risk in outpatient chemotherapeutic setting.12–15 These updates were supported by recent major trials which include PROTECHT, SAVE-ONCO in common cancers and CONKO-004, FRAGEM-UK in pancreatic cancer (PC).16–19
PC, one of the most lethal malignancies, is known to be frequently associated with VTE events. Many studies reported that VTE incidences are different according to cancer type, and they have a common result that PC is one of the malignancies, which is most highly associated with VTE incidence.20–23 The latest retrospective studies reported that incidence of VTE in PC patients is more than 30%.24–26 In the most commonly used predictive model for chemotherapy-associated thrombosis, PC was categorized in “very high risk” group with gastric cancer.24 Furthermore, even asymptomatic incidental VTE was also associated with mortality in PC.27 These findings suggest that prophylactic anticoagulation should be an important part of treatment for patients with PC.
However, all the aforementioned trials were conducted basically in Western ethnic populations. Because the genetic, somatometric, and dietary differences may impact quite significantly on the VTE biology, these “blanket” guidelines may not be appropriate for all ethnic populations. Actually one retrospective study, although it is small-sized, showed significantly lower incidence of VTE (5.3%) in East Asian patients with PC.28 The low incidence of VTE in East Asians has been reported in other solid malignancies29–31 and the genetic and biological difference associated with thrombosis has been reported in multiple studies.32–34 In this context, it would be meaningful to investigate the characteristics of VTE in East Asian PC patients in large population cohort study.
Therefore, we conducted a retrospective study to address the following objects: (1) to analyze the prevalence of VTE in Korean PC patients, (2) to investigate risk factors associated with the development of VTE after PC diagnosis, (3) to explore risk factors associated with mortality in Korean PC patients, and (4) to analyze the prognosis by subgroups of VTE patients.
METHODS
Patients
We retrospectively reviewed all the electronic medical records (EMR) of patients diagnosed with PC at Yonsei University Severance Hospital, Seoul, Korea. A total of 1334 patients were detected between January 2005 and December 2010. Among the detected patients, eligible patients were sorted out by following criteria: (1) patients diagnosed with histologically proven pancreatic adenocarcinoma, (2) more than 18 years old, and (3) patients who were followed up more than 2 times on outpatient clinic or in hospitalization. This study was approved by the institutional review board of the Severance Hospital, Seoul, Korea (IRB approval number, 4–2016–0024).
Variables
All the eligible patients were reviewed for demographic and clinical variables including age, sex, cancer stage, primary tumor site, number of metastatic site, initial Eastern Cooperative Oncology Group (ECOG) performance status, body mass index (BMI), initial lab findings, comorbidity, and treatment modality. The stage of PC was evaluated according to American Joint Committee on Cancer 7th guideline.35 All the continuous variables were converted into categorical variables to perform univariate and multivariate analysis for VTE incidence and death rate. The cutoff levels of each laboratory value were basically determined according to relevant studies. The cutoff levels of leukocyte (11 × 109/L), hemoglobin (10 g/dL), platelet (350 × 109/L) were originated from the Khorana's predictive model for VTE in ambulatory PC patients.26 The cutoff level of albumin (3.5 g/dL) was originated from normal range of serum albumin level.36 The cutoff level of hemoglobin A1c (HbA1c) was originated from, 2015 American Diabetes Association guideline.37 In regard of cancer antigen 19–9 (CA19–9) level, almost patients had higher level than normal range, and the previous studies’ cutoff levels vary. Therefore, we exceptively used median level in our data as a cutoff level of CA19–9. Because the definitions of overweight and obesity are different from those of Western population, we applied the different BMI cutoff level which is recommended by the guideline of Korean Society for the Study of Obesity.38–40
The date of cancer diagnosis was defined as the date of first positive pathological confirmation of pancreatic adenocarcinoma. The date VTE diagnosis was defined the date of VTE detection by imaging studies regardless of symptomatic or incidental. For the date of death, if patient expired in hospital, the biological death date was used and if not, the patient's disqualification date of the Korean National Health Insurance Service was used. For the life testing, the termination date of analysis was August 15, 2012.
Identification and Classification and Identification of VTE
Venous thromboembolisms were diagnosed with imaging modalities including Doppler ultrasonography, computed tomography (CT), or pulmonary angiography, depending on the anatomical sites. The asymptomatic and incidental VTE on the regular imaging follow-up was included to the VTE event, whereas central venous catheter-related VTE and superficial phlebitis were excluded. Because VTE group means “new VTE group,” the VTE group only include patients who were detected VTE after cancer diagnosis. For the patients who were diagnosed VTE with PC simultaneously in the first imaging study, we investigated additional chart review whether there had been previous imaging study before cancer diagnosis.
By the anatomical sites, we divided the overall VTE events into two categories: deep vein thrombosis or pulmonary thromboembolism (DVT/PTE) and intra-abdominal venous thromboembolism (IVT). The DVT/PTE group includes PTE with or without DVT, and DVT only. The rest VTE events occurred in the intra-abdominal vessels—portal vein, superior mesenteric vein (SMV), inferior vena cava (IVC), splenic vein, and others—were defined as IVT.
Statistical Analyses
The incidence rate per 100 person-years and the cumulative incidence rates with 95% confidence intervals (CIs) for thromboembolism and mortality were calculated. Kaplan–Meier plots were generated to estimate the influence of patients’ various clinical factors, including cancer stage, ECOG performance status, and the effect of VTE type, on incidence of VTE and survival. Differences in probability among comparison cohorts were assessed using the log-rank test. Cox proportional hazards models were used to analyze the effect of specified risk factors on outcomes for VTE or death within 2 years of cancer diagnosis. Analyses were performed using STATA (Version 14.0, Stata Institute, TX) and R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
Among 1334 detected patients, a total of 1115 pancreatic adenocarcinoma patients met the eligibility criteria patients and 219 were excluded because they had an inappropriate histologic subtype (N = 188, other than pancreatic adenocarcinoma) and loss of follow-up (N = 31, visited only a single time on outpatient clinic). Among 1115 eligible patients, 675 (60.5%) were men and 572 (51.3%) were 65 years of age or older. The median age of overall patients was 64.6 with range between 27.5 and 86.8. More than half of patients (54.5%) had metastatic pancreatic adenocarcinoma and nearly half of them (27.1%) had multiple metastatic lesions. A total of 209 (18.7%) patients underwent major abdominal surgery with the aim of curative treatment and 852 (77.3%) received chemotherapy with adjuvant or palliative aims (Table 1).
TABLE 1.
Patients Characteristics
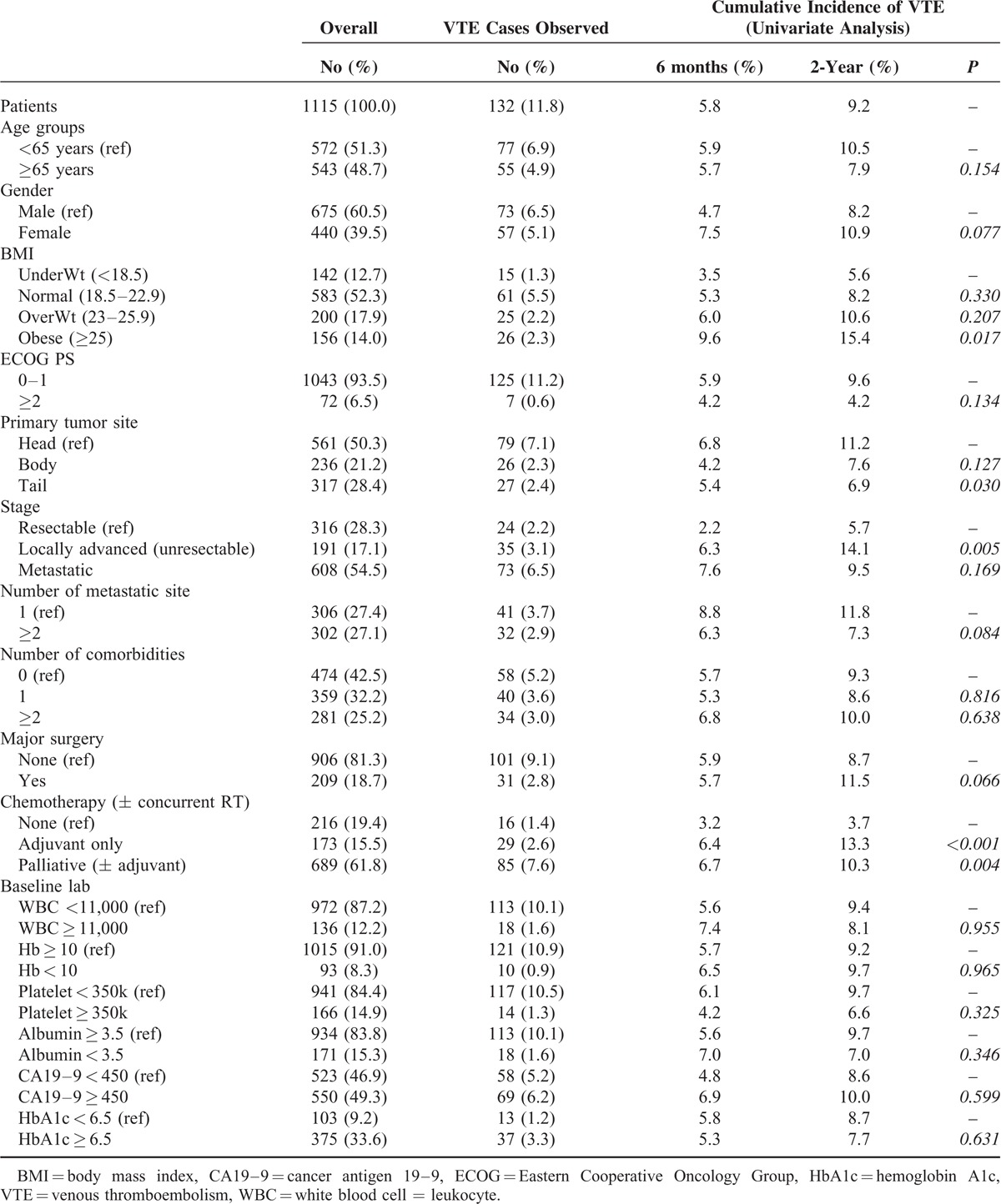
Anatomical Distribution of VTE
Divided overall 132 patients as anatomical site, 55 (41.7%) showed DVT/PTE and 77 (58.3%) showed other IVT. According to the clinical symptom, the symptomatic VTE was 37 (28.8) and incidental VTE was 95 (72.0%). In the DVT/PTE group, more than half of patients were symptomatic whereas only 4 patients were symptomatic in IVT group. The 4 symptomatic IVT patients included 3 ascites and 1 mesenteric ischemia. Table 2 shows a 2 × 2 table according to symptom and anatomical site.
TABLE 2.
Anatomical Distribution of VTE
Risk Factors for VTE Development
The 2-year cumulative incidence of VTE after cancer diagnosis was 9.2% and 6-month cumulative incidence was 5.8%. In the univariate analysis, the cancer stage, major surgery, chemotherapy were associated with higher incidence of VTE. The BMI level did not show statistically significant association with VTE incidence, but overweight and obesity showed an increasing trend with high VTE (Table 1).
The 7 patients had VTE and PC simultaneously in the first imaging study. Two of them had previous CT imaging in the EMR system, and both had VTE before PC diagnosis. Other five patients did not have previous imaging data before taking the 1st CT imaging with PC diagnosis, and we could not evaluate which diseased between VTE and PC had preceded another one. Because all the 7 patients did not have evidence of having newly detected VTE, they were not included to VTE group. Also, because of too small sample size, we did not consider the 7 patients in modifying statistical analysis.
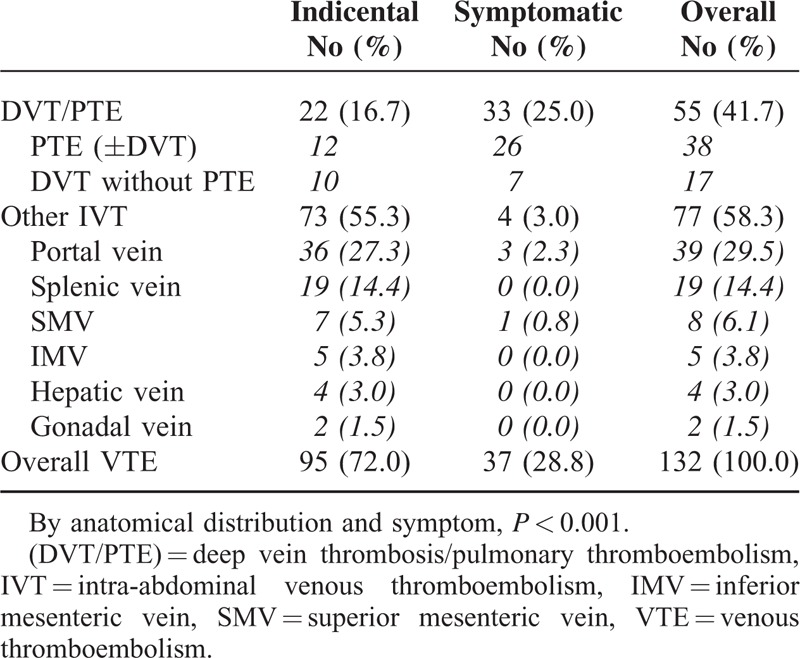
The results of a multivariable analysis of potential risk factors associated with developing the VTE are presented in Table 3. Advanced cancer stage at the time of diagnosis was the strongest risk factor, with a 3.6-fold higher risk of VTE in metastatic cancer (hazard ratio (HR) = 3.62; 95% CI: 1.91, 6.84; P < 0.001) and 2.9-fold higher in locally advanced cancer (HR = 2.96; 95% CI: 1.58, 5.57; P = 0.001) compared with resectable PC. Major surgery was another risk factor (HR = 2.18; 95% CI: 1.23, 3.85; P = 0.007) and chemotherapy also affected higher VTE incidence (HR = 2.56; 95% CI: 1.23, 5.33; P = 0.012). Age, abnormal laboratory value, and number of comorbidities were not significantly associated with increased risk of developing VTE. Obesity showed a trend toward higher VTE incidence but not a significant difference. Kaplan–Meier plots of the incidence of VTE, stratified by stage, showed a strong relationship between cancer stage and VTE incidence (Figure 1).
TABLE 3.
Multivariate Analysis of Risk for VTE
FIGURE 1.
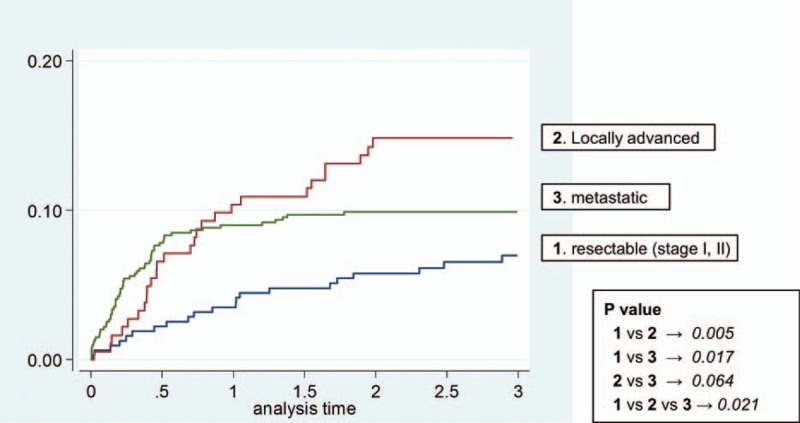
Kaplan–Meier curves of incidence of venous thromboembolism, by stage.
Risk Factors for Death After TE events
Risk factors predicting death after PC diagnosis, based on univariate and multivariate analyses, are presented in Table 4. Overall median survival was 9.4 month. In multivariable analysis, significant risk factors include metastatic cancer stage (HR = 3.40; 95% CI: 2.88, 4.03; P < 0.001), worse ECOG performance status (HR = 1.75; 95% CI: 1.34, 2.30; P < 0.001), leukocytosis (HR = 1.51; 95% CI: 1.24, 1.84; P < 0.001), and lower albumin level (HR = 1.60; 95% CI: 1.31, 1.95; P < 0.001) at the time of cancer diagnosis. Neither overall VTE nor each subgroup of VTE was predictive factors for death. Advanced age (HR = 1.15; 95% CI: 1.01, 1.31; P = 0.036), high CA19–9 level (HR = 1.30; 95% CI: 1.14, 1.49; P < 0.001) were associated with higher death risk. Unlike VTE risk, higher BMI showed an inverse trend of death risk, which means that lower BMI was associated with lower survival rate.
TABLE 4.
Prognostic Factors Influencing OS—Univariate and Multivariate Analysis
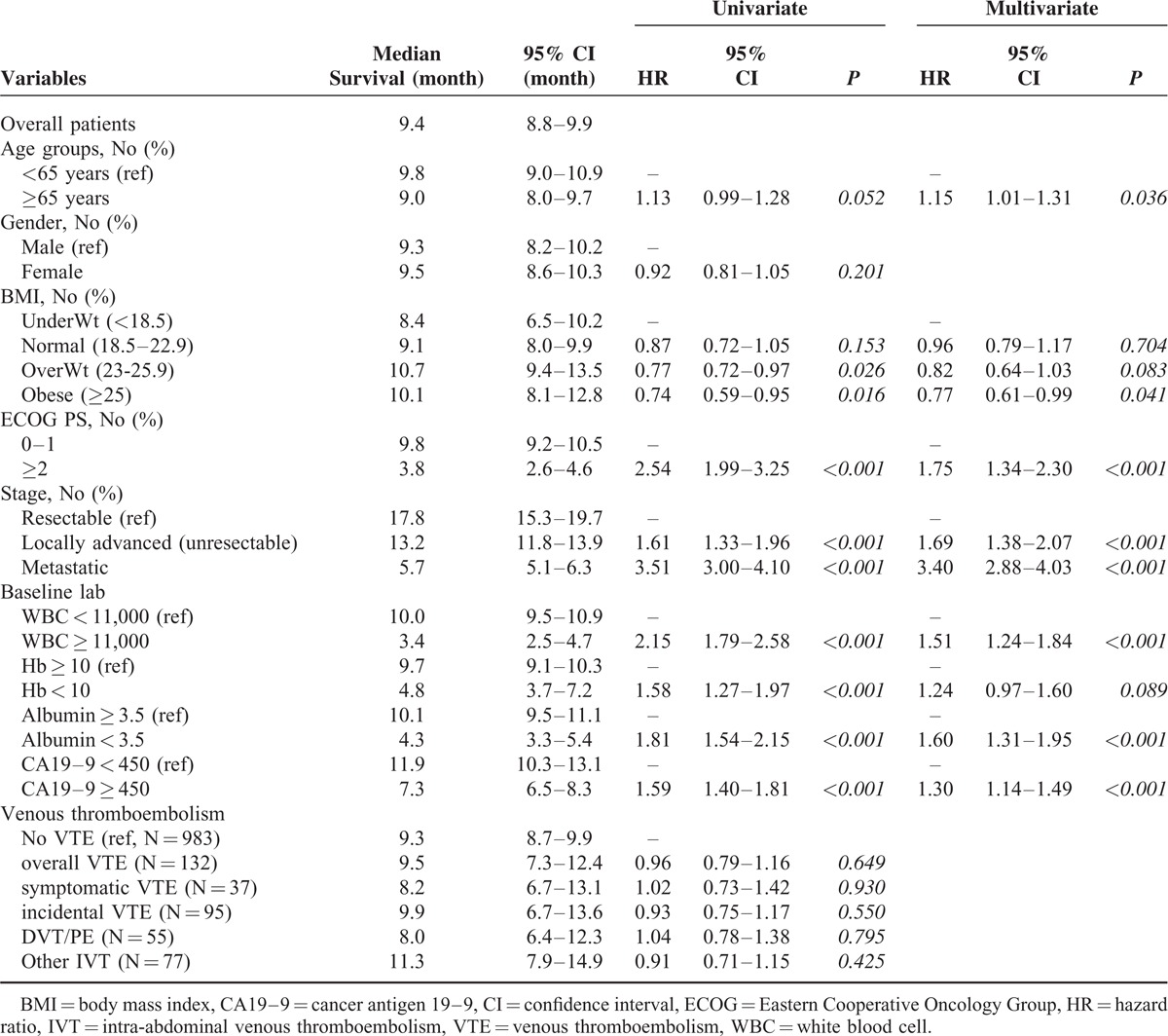
Two Kaplan–Meier survival plots show survival after PC diagnosis stratified by stage and VTE development (Figure 2). VTE development did not showed survival difference, whereas cancer stage affected the patients’ survival definitely.
FIGURE 2.
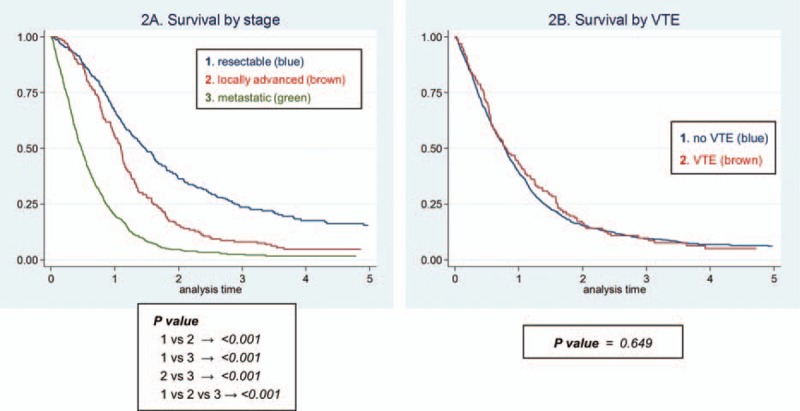
Survival after diagnosis of PC, by staging (A) and venous thromboembolism (B).
Subgroup Analysis in VTE Patients
The subgroup analysis was performed by symptom and site (Figure 3). Symptomatic VTE showed worse prognosis after VTE event compared with incidental VTE (HR 1.87; 95% CI 1.26, 2.78; P = 0.002). DVT/PTE also showed worse prognosis after VTE event compared with other IVT group (HR 1.53; 95% CI 1.02, 2.20; P = 0.022). These differences were mainly shown in resectable PC, whereas advanced stage showed less survival difference by symptom and anatomical sites. (Supplementary data 4).
FIGURE 3.
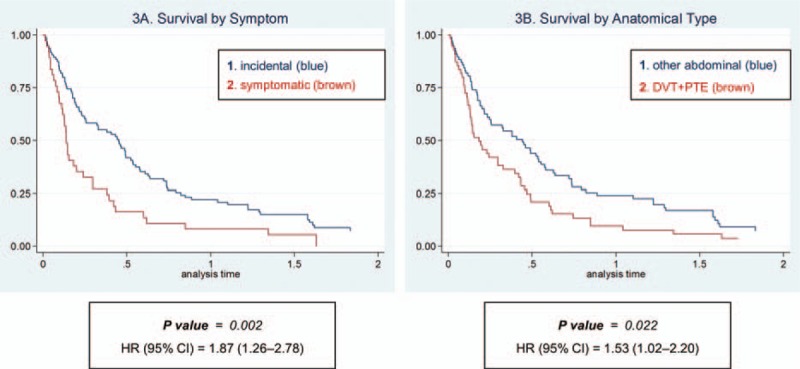
Survival after venous thromboembolic event by symptom (A) and cancer type (B).
DISCUSSION
This retrospective study is the first attempt to provide a large population-based analysis of the epidemiology of VTE among patients with newly diagnosed pancreatic adenocarcinoma in an East Asian ethnic population. According to this study, the 2-year cumulative incidence of total VTE events associated with pancreatic adenocarcinoma in Korean patients is 9.2%, which is lower than that of Western groups in recently reported studies.24–26
Apart from overall incidence, our data show several different characteristics in East Asian ethnics compared with Western populations. The most important point is, contrary to previous studies, the VTE event did not affect the overall survival after cancer diagnosis in patients with PC regardless of symptom or anatomical site. Although the causative mechanism is not clear, we hypothesized that the balance between aggressiveness of cancer and that of VTE would explain this difference. Because PC itself is the most aggressive cancer, relatively less aggressive feature of VTE—the possible characteristics of East Asian population—would not affect the overall mortality. From the colorectal cancer data of Chew et al,7 we can infer that the effect of VTE would be less in the advanced stage. In this study, differences in death incidence were largest among patients with loco-regional cancer; no significant differences were found among patients with metastatic cancer, which can be explained by our hypothesis that metastatic cancer's aggressiveness can dominate the clinical meaning of VTE. Shaib et al41 also showed that there was no survival difference between patients with VTE compared with those without VTE in metastatic PC patients. The similar findings are shown in our data as Figure 3 and supplementary data 4. These data implicate that VTE would have larger influence on survival in earlier stage of PC, whereas it would be masked in the more advanced cancer stage. From these findings, we can hypothesize that the aggressive cases such as PC or metastatic stage of other cancers would dominate the VTE aggressiveness and if Asian populations’ VTE is less aggressive, this imbalance would be accelerated and conceal the VTE influence.
Our data suggest that the significant risk factors associated with the development of VTE after PC diagnosis are primary site, stage, major surgery, and chemotherapy. Unlike other factors (stage, major surgery, and chemotherapy) which have been already shown in previous studies,25–27 the tumor site (pancreatic head) as a risk factor for VTE seems to be unfamiliar. We hypothesized that pancreatic head cancer might be frequently associated with early detection at relatively early stage because of symptoms and signs such as pain, jaundice, or Courvoisier's sign. The tumor site, stage, and chemotherapy showed minimal degree of multicollinearity, which means they did not highly affected each other in multivariate analysis.
In regards of symptom and the anatomical site, our data showed higher proportion of incidental and intra-abdominal VTE than previous studies. Because incidental and IVT showed less aggressiveness than symptomatic or DVT/PTE, the proportion would affect the overall prognosis after VTE event. In the same manner, the symptomatic VTE showed worse progress than incidental VTE, however all these VTE showed no significant difference than non-VTE group. This finding is different from the results of Khorana et al.27 We cannot assure this difference is the East Asian ethnic's characteristics, but can suppose that higher proportion of other IVT is the characteristics of East Asian (Table 2) and that would attribute the “less aggressiveness” of East Asian ethnics. In summary, VTE event did not affect survival after PC diagnosis, but anatomical site and symptom appearance of VTE affected survival after VTE diagnosis.
About BMI and prognosis in patients with PC, the most recent two systematic review and meta-analyses showed interesting results. Majumder et al42 showed positive correlation between obesity and mortality in PC patients in Western but not Asia-Pacific populations. Ramsey and Martin43 reported that BMI increases the complexity in resection of PC, but aggressive para-operative care could mitigate obesity-associated morbidity and mortality. These two results imply that there is no “universally applicable” relationship between BMI and prognosis in PC and the relationship has to be individually evaluated according to clinical or demographical setting. Our data suggest that obesity BMI is associated with higher incidence of VTE but underweight is associated with higher mortality in East Asian's PC patients.
The reason why there are several differences between our data and Western's data is still unclear. However, we cautiously hypothesize that some genetic, biological, somatometric factor would majorly affect this result, not clinical practice pattern such as less screening tests. That is because in Korean practice, PC patients take imaging test regularly, and moreover, the study is performed in one of the largest center with public confidence in South Korea.
The first limitation of this study is that it was conducted at a single institution. However, Severance Hospital is one of the biggest volume hospitals in Korea and admits patients from across the country and we recognize that we can generalize the entire Korean population. The second limitation is the absence of detailed information about the use of prophylactic anticoagulation. However, in Severance Hospital, there was no routine prophylactic anticoagulation for chemotherapy or operation. So, anticoagulant prophylaxis does not have a chance to be a hidden confounding factor. Third, the impact of anatomical site and symptom of VTE on survival after VTE event was not accessed in multivariate analysis because lack of data in the timing of VTE event. Instead, we only showed Kaplan–Meier curves in Figure 3. Fourth, because chest CT is not routinely checked during follow-up period of PC patients, the incidence of incidental DVT/PTE might be underestimated. However, there is the same possibility in Western population, because the follow-up practice is not different between Korean and Western hospitals. Because the aim of this study is to compare the VTE incidence between Korean and other population, the possibility to underestimate the incidental DVT/PTE could not be a critical pitfall.
In conclusion, the overall incidence of VTE events in Korean PC patients was lower than that of Western groups. The most significant risk factor associated with a new VTE event and higher mortality was advanced cancer stage. Unlike Western population, VTE event did not affect overall prognosis. However, DVT/PTE and symptomatic VTE showed higher mortality after VTE events than their counterparts, which were dominant at earlier cancer stage. Future studies are necessary that explore the link between biological characteristics of East Asians and lower VTE aggressiveness, and ultimately, the goal of this study is to be a clue to update of current “blanket” guidelines into more detailed, multi-dimensional, and real-world guideline.
Supplementary Material
Acknowledgment
The authors thank the “E-world Editing” for English proofreading.
Footnotes
Abbreviations: ACCP = American college of chest physicians, AJCC = American Joint Committee on Cancer, ASCO = American society of clinical oncology, BMI = body mass index, CA19–9 = cancer antigen 19–9, CI = confidence interval, DVT = deep vein thrombosis, ECOG = Eastern Cooperative Oncology Group, ESMO = European society for medical oncology, HR = hazard ratio, interAD = duration between cancer diagnosis and death (or censoring), interAK = duration between cancer diagnosis and VTE event, interKD = duration between VTE event and death (or censoring), IVC = inferior vena cava, IVT = intra-abdominal venous thromboembolism, KSSO = Korean Society for the Study of Obesity, NCCN = national comprehensive cancer network, PTE = pulmonary thromboembolism, SMV = superior mesenteric vein, VTE = venous thromboembolism
We certify that we do not have any funding or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript (eg, employment, consultancy, stock ownership, and honoraria).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Trousseau A. Phlegmasia alba dolens. Clin Med Hotel-Dieu Paris 1865; 3:654–712. [Google Scholar]
- 2.Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006; 166:458–464. [DOI] [PubMed] [Google Scholar]
- 3.Stein PD, Beemath A, Meyers FA, et al. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med 2006; 119:60–68. [DOI] [PubMed] [Google Scholar]
- 4.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016; 41:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5:632–634. [DOI] [PubMed] [Google Scholar]
- 7.Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 2007; 25:70–76. [DOI] [PubMed] [Google Scholar]
- 8.Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol 2006; 24:1112–1118. [DOI] [PubMed] [Google Scholar]
- 9.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol 2009; 27:4902–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000; 343:1846–1850. [DOI] [PubMed] [Google Scholar]
- 11.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013; 31:2189–2204. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network (NCCN) Guideline. Cancer-Associated Venous Thromboembolic Disease. (2015) Available at: http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf Accessed March 31, 2016. [Google Scholar]
- 14.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 2 suppl:e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandala M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO clinical practice guidelines. Ann Oncol 2011; 22 suppl 6:vi85–vi92. [DOI] [PubMed] [Google Scholar]
- 16.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol 2009; 10:943–949. [DOI] [PubMed] [Google Scholar]
- 17.Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012; 366:601–609. [DOI] [PubMed] [Google Scholar]
- 18.Pelzer U, Opitz B, Deutschinoff G, et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol 2015; 33:2028–2034. [DOI] [PubMed] [Google Scholar]
- 19.Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012; 48:1283–1292. [DOI] [PubMed] [Google Scholar]
- 20.Marks MA, Engels EA. Venous thromboembolism and cancer risk among elderly adults in the United States. Cancer Epidemiol Biomarkers Prev 2014; 23:774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khorana AA, Dala M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013; 119:648–655. [DOI] [PubMed] [Google Scholar]
- 22.Lyman GH, Eckert L, Wang Y, et al. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist 2013; 18:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med 2012; 9:e1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menapace LA, Peterson DR, Berry A, et al. Symptomatic and incidental thromboembolism are both associated with mortality in pancreatic cancer. Thromb Haemost 2011; 106:371–378. [DOI] [PubMed] [Google Scholar]
- 25.Epstein AS, Soff GA, Capanu M, et al. Analysis of incidence and clinical outcomes in patients with thromboembolic events and invasive exocrine pancreatic cancer. Cancer 2012; 118:3053–3061. [DOI] [PubMed] [Google Scholar]
- 26.Munoz Martin AJ, Garcia Alfonso P, Ruperez Blanco AB, et al. Incidence of venous thromboembolism (VTE) in ambulatory pancreatic cancer patients receiving chemotherapy and analysis of Khorana's predictive model. Clin Trans Oncol 2014; 16:927–930. [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh SY, Kim JH, Lee KW, et al. Venous thromboembolism in patients with pancreatic adenocarcinoma: lower incidence in Asian ethnicity. Thromb Res 2008; 122:485–490. [DOI] [PubMed] [Google Scholar]
- 29.Amer MH. Cancer-associated thrombosis: clinical presentation and survival. Cancer Manag Res 2013; 5:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keenan CR, White RH. The effects of race/ethnicity and sex on the risk of venous thromboembolism. Curr Opin Pulm Med 2007; 13:377–383. [DOI] [PubMed] [Google Scholar]
- 31.Kearon C. Epidemiology of venous thromboembolism. Semin Vasc Med 2001; 1:7–26. [DOI] [PubMed] [Google Scholar]
- 32.Margaglione M, Grandone E. Population genetics of venous thromboembolism. A narrative review. Thromb Haemost 2011; 105:221–231. [DOI] [PubMed] [Google Scholar]
- 33.Tang L, Hu Y. Ethnic diversity in the genetics of venous thromboembolism. Thromb Haemost 2015; 114:901–909. [DOI] [PubMed] [Google Scholar]
- 34.Chan SL, Goh BC, Chia KS, et al. Effects of CYP4F2 and GGCX genetic variants on maintenance warfarin dose in a multi-ethnic Asian population. Thromb Haemost 2011; 105:1100–1102. [DOI] [PubMed] [Google Scholar]
- 35.American Joint Committee on Cancer (AJCC). Pancreatic Cancer Staging. 7th ed.2010; Available at: https://cancerstaging.org/Pages/default.aspx. Accessed March 31, 2016. [Google Scholar]
- 36.Rush University. Normal Range for Common Laboratory Tests. 2013; Available at: https://web.archive.org/web/20130114222140/http://www.rush.edu/webapps/rml/RMLRangesCMP.jsp. Accessed March 31, 2016. [Google Scholar]
- 37.American Diabetes Association. Diabetes Management Guidelines. 2015; Available at: http://www.ndei.org/ADA-diabetes-management-guidelines-diagnosis-A1C-testing.aspx. Accessed March 31, 2016. [Google Scholar]
- 38.World Health Organization (WHO). The Asia-Pacific Perspective: Redefining Obesity and its Treatment. 2000; Available at: http://www.wpro.who.int/nutrition/documents/Redefining_obesity/en/. Accessed March 31, 2016. [Google Scholar]
- 39.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med 2006; 355:779–787. [DOI] [PubMed] [Google Scholar]
- 40.Kim MK, Lee WY, Kang JH, et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab 2014; 29:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaib W, Deng Y, Zilterman D, et al. Assessing risk and mortality of venous thromboembolism in pancreatic cancer patients. Anticancer Res 2010; 30:4261–4264. [PubMed] [Google Scholar]
- 42.Majumder K, Gupta A, Arora N, et al. Premorbid obesity and mortality in patients with pancreatic cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsey AM, Martin RC. Body mass index and outcomes from pancreatic resection: a review and meta-analysis. J Gastrointest Surg 2011; 15:1633–1642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


