Abstract
Background Green infrastructure is a strategic network of green spaces designed to deliver ecosystem services to human communities. Green infrastructure is a convenient concept for urban policy makers, but the term is used too generically and with limited understanding of the relative values or benefits of different types of green space and how these complement one another. At a finer scale/more practical level, little consideration is given to the composition of the plant communities, yet this is what ultimately defines the extent of service provision. This paper calls for greater attention to be paid to urban plantings with respect to ecosystem service delivery and for plant science to engage more fully in identifying those plants that promote various services.
Scope Many urban plantings are designed based on aesthetics alone, with limited thought on how plant choice/composition provides other ecosystem services. Research is beginning to demonstrate, however, that landscape plants provide a range of important services, such as helping mitigate floods and alleviating heat islands, but that not all species are equally effective. The paper reviews a number of important services and demonstrates how genotype choice radically affects service delivery.
Conclusions Although research is in its infancy, data are being generated that relate plant traits to specific services, thereby helping identify genotypes that optimize service delivery. The urban environment, however, will become exceedingly bland if future planting is simply restricted to monocultures of a few ‘functional’ genotypes. Therefore, further information is required on how to design plant communities where the plants identified (1) provide more than a single benefit (multifunctionality), (B) complement each other in maximizing the range of benefits that can be delivered in one location, and (3) continue to maintain public acceptance through diversity. The identification/development of functional landscape plants is an exciting and potentially high-impact arena for plant science.
Keywords: Alien plants, biodiversity, building energy efficiency, carbon sequestration, ecosystem services, green infrastructure, human health and well-being, policy, pollution, storm-water management, temperature regulation, urban.
INTRODUCTION
What is green infrastructure?
Green infrastructure (GI) is a term that was coined to provide an antonym to grey infrastructure (Benedict and McMahon, 2012). Grey infrastructure comprises the built components of cities, including buildings, roads, pavements, sewers and other structural utilities. Green infrastructure is meant to spatially complement grey infrastructure and at the same time counterbalance some of the negative effects associated with grey infrastructure. Natural England, UK (2009) in the UK defines green infrastructure as:
A strategically planned and delivered network comprising the broadest range of high quality green spaces and other environmental features. It should be designed and managed as a multifunctional resource capable of delivering those ecological services and quality of life benefits required by the communities it serves and needed to underpin sustainability. Its design and management should also respect and enhance the character and distinctiveness of an area with regard to habitats and landscape types.
Green infrastructure is composed of a range of green landscape typologies, including parks, nature reserves, street trees, gardens, river corridors, ponds, green roofs and walls, farmed land and allotments, as well as linking elements such as the ‘green corridors’ found alongside roadways and railway lines (note that water features themselves are sometimes referred to as components of ‘blue infrastructure’). Although ‘green infrastructure’ is a very convenient concept to describe an urban green network, the term is used by policy makers all too generically with little understanding of the balance and interlinking of the different typologies [or indeed their different ‘values’ in terms of ecosystem service (ES) delivery]. Mell (2008) raised concerns that any underestimation of the complexity of green spaces within the urban–rural matrix could undermine the value of these spaces and hinder their function. In practical terms, GI is now seen by most city planners as a necessary requirement, but what actually populates those green spaces that are ‘blocked out’ between the buildings is often given inadequate attention (Matthews et al., 2015). Furthermore, implementing new or improving existing green space is hampered by financial constraints, limited expertise and a lack of tools to value the different green space types as well as a lack of comprehension of how landscape typology affects service provision (Sandström et al., 2006; De Groot et al., 2010; Hunter and Luck, 2015). At a finer scale, i.e. at a plant or plant-community level, frequently little consideration is given to the composition of these spaces, and it is rarely in terms of the benefits that might be conferred other than the purely aesthetic. Even in relatively detailed policy documents such as the European Commission’s ‘Building a green infrastructure for Europe’ (EU, 2013), only one plant species is mentioned in relation to its ES delivery: namely, Cymodocea nodosa in relation to the value of this seagrass in supporting fishing stocks. Likewise, in a comprehensive review on ‘The multifunctionality of green infrastructure’ (EU, 2012), there is no mention of any specific plant species within the 37-page report, although both these reports begin to acknowledge that different typologies (habitats) provide distinctive ESs.
As stated in the definition above, GI should be designed and managed to accentuate the ESs and quality of life benefits to human society. These ecosystem ‘services’ are normally defined as (1) supporting (e.g. soil formation, photosynthesis, primary production, nutrient and water cycling); (2) provisioning (e.g. food, fibre, fuel, fresh water, genetic resources, natural pharmaceuticals and chemicals); (3) regulating (ecosystem processes including regulation of air and water quality, climate, pests and disease) and (4) cultural (including cognitive development, spiritual enrichment, recreation and aesthetic experiences) (Millennium Ecosystem Assessment, 2005). There are a number of instances where these services are well understood, and illustrate obvious links between a given service and certain genotypes, for example in the case of plants those members of the Gramineae who represent the major food crops of the world (wheat, rice, sorghum etc.). There are numerous additional cases, however, where optimum service provision is difficult to articulate in terms of plant genotypes. This is where the plant scientist can play an important role, both in defining more precisely the benefits of GI and in determining how these are dictated by genotype choice, the level of service delivery being determined by the selection of appropriate plant species, but also, within a horticultural context, by cultivar choice within a species.
The urban context
In an urban GI context, plant genotype choice is very much determined historically by aesthetics (private gardens), cultural symbolism (civic squares), ecological suitability and niche opportunities (wasteland or ‘brownfield’ sites) and functionality in relation to food (allotments, vegetable plots and orchards). We have some notion of what plant genotypes inhabit or could be used to populate these ‘spaces’ in terms of their suitability for certain environmental conditions and soil types. The issue of plant selection becomes much more difficult, however, when GI is designed around wider human needs, e.g. to:
regulate urban air temperature, noise and atmospheric pollution;
intercept rainfall, reduce storm water run-off and mitigate flash flooding;
maximize the thermal insulation of buildings and thus reduce energy consumption.
Even when a role for plants per se has been recognized in such situations, the choice of genotype has been seen as largely irrelevant up to now. But should this be the case? Do all plants respond in a similar way, have comparable functional traits or provide broadly the same level of ecosystem service?
THE RESEARCH AGENDA
Research largely driven by these questions has been carried out over the last few years. These research programmes have attempted not only to better quantify the extent to which plants contribute to certain ‘urban’ ESs (e.g. Tzoulas et al., 2007; Cameron et al., 2012; Gómez-Baggethun and Barton, 2013) but also to begin to identify genotypes that optimize the desired service provision (e.g. Freer-Smith et al., 2005; Blanuša et al., 2013). However, only a small fraction of the potentially useful genotypes have been studied so far, and further evaluations are required to furnish policy makers with more comprehensive and accurate lists of beneficial plants. The urban environment, however, will become a bland place indeed if planting is limited to simply a few ‘functional’ genotypes placed strategically at relevant locations. Information is thus required on how to design entire plant communities where the plants identified (1) provide more than a single benefit (multifunctionality) and (2) complement each other in terms of maximizing the range of benefits that can be delivered in the one locale. Such concepts are not new in urban horticulture. Plants have been chosen to provide a range of complementary flower colours to appeal to human aesthetics, e.g. pastel blues harmonizing with pale pink in an Edwardian flower border (Bisgrove, 2013). The difference now is that these plant communities should not only be visually appealing, but also enhance the functionality of the site. As a case in point, a city-centre roadside planting may need to be designed in future to provide nectar and pollen for native invertebrates, act as a filter to remove particulate matter emitted by passing vehicles, provide localized cooling through shading and evapotranspiration, and help relieve the psychophysiological stress experienced by pedestrians as they walk along the road, as well as be deemed aesthetically pleasing in its own right. Not only this, but this plant community may need to be resilient enough to tolerate periods of suboptimal irrigation, high aerial temperatures in summer and the effects of de-icing salts applied in winter. To date, little information exists to provide the appropriate plant palette.
Research in this context is not solely focused on identifying plants for future use. It is also important in understanding the extent to which existing popular cultivars and their ES delivery are vulnerable to abiotic, biotic and even societal change. For example, for a cultivar that is currently dominant in the landscape and which provides a specific positive service, a change in popularity either to a different species or even just a different clonal form, may alter the delivery of that service. As an illustration, the replacement of golden/light green foliage conifers commonly placed in garden hedges (e.g. × Cuprocyparis leylandii ‘Castlewellan Gold’ or Cupressus macrocarpa ‘Goldcrest’) with cultivars possessing darker foliage is likely to reduce the albedo of the hedges and increase the amount of solar energy absorbed in that location/neighbourhood. Even subtle changes in cultivar abundance due to fashion, for example, might change the service delivery level that a given species confers.
Urban services – old and new
For certain services, differences in genotype have been evident over many decades, largely through anecdotal observations. The identification of plants specifically to aid wildlife conservation falls into this category. Only since the concept of ES became mainstream, however, has the full service potential of urban plants been more widely investigated (Cameron and Hitchmough, 2016). For a number of these more recently defined services, evidence is now also beginning to build about the extent to which plant choice matters. A range of service areas are highlighted below, with evidence of how genotype choice can affect the level of service delivery.
HOW MUCH DOES GENOTYPE CHOICE MATTER?
Urban biodiversity
Plant choice is often determined by a genotype’s ability to support certain faunal taxa or guilds. Paradoxically, this does not result in simply recommending native plant species, but potentially also utilizing non-native (alien) species to support native fauna, a point that has caused much debate around the relative merits/risks associated with planting non-native species in an urban environment (Shackelford et al., 2013; Standish et al., 2013). As an example, the Asiatic shrub Buddleia davidii has long been valued by UK gardeners for its ability to provide nectar to native Lepidoptera species (Hardy and Dennis, 2008), being considered more effective in this respect than any native shrub during mid to late summer (as a consequence of this the species’ common name is ‘butterfly bush’). Recent systematic studies support the notion that native is not always best. Helden et al. (2012) ranked both UK native and non-native tree species for their value in supporting phytophagous invertebrates and their associated avian predators. The results demonstrated that not all native trees are necessarily superior in providing habitat/food resource compared with non-natives. For example, natives such as Corylus avellana and Sorbus aucuparia host fewer Hemiptera (true bugs) than non-native Sorbus intermedia or Quercus rubra. Although there are genuine concerns that some non-native plants are invasive and cause radical reductions in native flora and fauna (Alpert et al., 2000), there are situations where introduced alien species have restored services that were previously lost after the elimination of the dominant native species. In California, for example, stands of non-native Eucalyptus globulus provide habitat that is as rich in understorey plants, leaf litter invertebrates, amphibians and birds as the native Quercus agrifolia/Umbellularia californica-dominated forests (Sax, 2002). Species composition varies between the two woodland types, but overall richness does not. Other conservation-based services that non-native plants provide include nesting/feeding habitat for birds (Chen, 2001; Berens et al., 2008; Sogge et al., 2008; Bajema et al., 2009), refuge habitat for rare invertebrates (Chiba, 2010) and acting as ‘nurse crops’ to allow more effective establishment of native vegetation (Lugo, 2004; Sullivan et al., 2007).
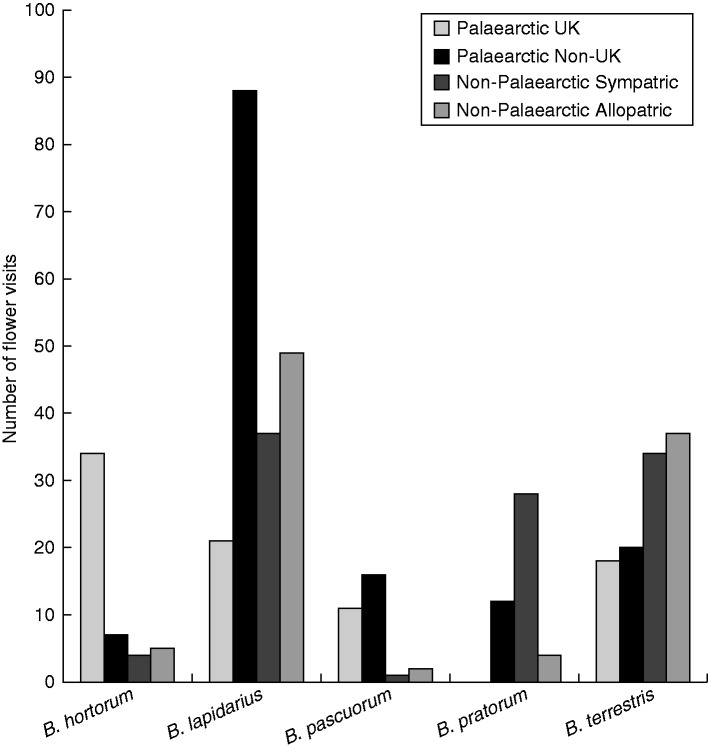
As with Buddleia, many non-native plant species are encouraged because they supply nectar and pollen to pollinating insects, such bees and hoverflies, although concerns have been raised about how this impacts on pollination rates within native plant species due to increased competition and cross pollination (Bjerknes et al., 2007). Certain key factors determine the value of non-native plants to native invertebrates, including the ability to provide access to nectar or pollen and the volume of nectar available (Potts et al., 2003; Carvalheiro et al., 2014). Inter-relationships are often prevalent if the plant is from the same biogeographical region, albeit not the same country (so-called ‘near natives’), as there may have been some co-evolution in the past with the native insects, or closely related species. For example, in UK gardens plants native to nearby European countries, North America and northern Asia (i.e. the Holarctic ecozone) may be particularly beneficial to the native insects, as their evolutionary histories have overlap (Goulson et al., 2008; Salisbury et al., 2015). So Salvia nemorosa with a natural distribution within central Europe, when planted in the UK offers a similar service to pollinators as the native Salvia pratensis (Carreck et al., 1997; Anon, 2013). Another factor affecting the value of non-native plants as a food source relates to the feeding behaviour of the insects themselves. In bumblebees, species with catholic (polylectic) diets, such as Bombus pratorum and Bombus terrestris, actually favour plants outwith their biogeographical range, whereas those that are more specialist feeders, e.g. Bombus hortorum and Bombus pascuorum, are more reliant on native or near-native plants (i.e. from the Palaearctic ecozone) (Fig. 1) (Hanley et al., 2014).
Fig. 1.
Total number of flower visits by UK bumblebee (Bombus) species recorded in urban gardens on flowering plants derived from the Palaearctic region (UK native and non-native) and from outwith this region, including locations where bumblebees naturally occur (sympatric) or outside their natural evolutionary range (allopatric). Overall, B. hortorum and B. pascuorum showed a preference for Palaearctic plants (P < 0·01), whereas B. terrestris showed a preference for non-Palaearctic plants (P < 0·01). Bombus pratorum did not visit any native UK plant species. Modified from Hanley et al. (2014).
Irrespective of these factors around evolutionary overlap or feeding strategies, there can be remarkable differences in flower attractiveness based on cultivated forms even within the same plant species. Garbuzov and Ratnieks (2015) recorded that, within the aster Aster novi-belgii, the cultivars ‘Alice Haslam’ and ‘Dandy’ had 15.2 and 10.1 insect visits per square metre of plant cover respectively, compared with no visits in the morphologically similar cultivars ‘Sheena’ and ‘White Wings’. Similar large variations were recorded across cultivars of Lavandula (Garbuzov and Ratnieks, 2014). In essence, either relatively small morphological differences or more fundamental (but less obvious) physiological differences (e.g. carbohydrate form and concentration in the nectar) are determining whether a genotype is a useful service provider or not.
Local temperature regulation
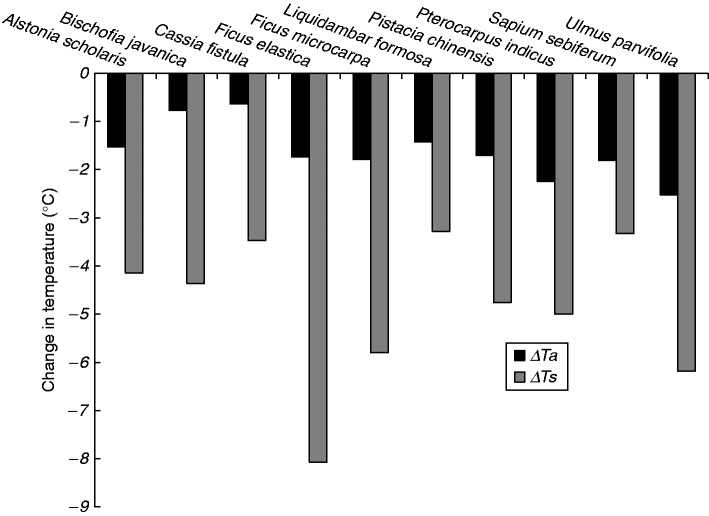
Vegetation provides a localized cooling service through (1) shading land and built surfaces from solar irradiance, (2) evapotranspiration, with solar energy being converted to latent heat (thus avoiding a rise in leaf and surrounding air temperatures), (3) transforming a small proportion of thermal energy to chemical energy via photosynthesis, and (4) an albedo effect, reflecting incoming solar energy back to the atmosphere and thereby reducing the potential for short-wave irradiance to be converted to long wave infra-red wavelengths (i.e. heat) at ground level. Species vary in their ability to interact with solar irradiance, and hence the capacity to cool their immediate locality. Street and park trees are highly valued, especially in the tropics, for their ability to cool ground surfaces and the surrounding air, although the extent of cooling is radically altered by the characteristics of the chosen tree (Fig. 2). Lin and Lin (2010) demonstrated that those species typified by dense canopies [high leaf area index (LAI)] and thick leaves (e.g. Ficus elastica) were effective at cooling the soil surface, whereas species with lightly coloured foliage, such as Ulmus parvifolia and Pterocarpus indicus, were better placed to directly cool the air below the canopy. Within parks, correlations between tree canopy cover and reductions in air temperature have been noted in Ethiopia (0.2 °C cooler for every 10 % increment in cover). Groves of trees dominated by Eucalyptus (E. grandis, E. camaldulensis and E. globulus) showed greatest temperature reductions, followed by stands of Olea (O. europaea and O. capensis), while populations of Grevillea robusta and Cupressus lusitanica were less effective at cooling (Feyisa et al., 2014). In warm, arid climates, where stomatal conductance (gs) is frequently suppressed during the hottest periods of the day as an adaptation to conserve water, shade cooling may be a more critical factor in aiding human thermal comfort (Shashua-Bar et al., 2010; Feyisa et al., 2014).
Fig. 2.
The effect of subtropical tree species on changing air (ΔTa) and land surface (ΔTs) temperature under their canopies. Data refer to mean temperature differences measured during July and August 2007 in Taiwan using a replicate of ten specimens per species. Ulmus parvifolia and Pterocarpus indicus provided significantly greater air cooling, and Ficus elastica significantly greater surface cooling than other species (P < 0·001). Modified from Lin and Lin (2010).
Even in temperate climates, such as that of the UK, greater attention is being paid to understanding and adopting those tree species that provide greater street cooling. Investigations into the influence of evapotranspirational cooling (Rahman et al., 2015) showed that the more rapidly growing species tended to have greatest gs and thus enhanced cooling capacity (Table 1). Indeed, genotypes that combined high gs with wide canopies and high LAI, such as Pyrus calleryana and Crataegus laevigata, provided 3- to 4-fold more cooling than alternatives such as Sorbus arnoldiana and Prunus ‘Umineko’. These latter genotypes showed some susceptibility to urban stress, a possible cause of relatively low gs values. The red-leaved Malus ‘Rudolph’ was tolerant of urban conditions, but also provided low cooling potential. This may relate to colour affecting the energy balance of the leaf, or the fact that leaves with an atypical colour often correspond to lower gs; Vaz Monteiro et al. (2016) found this to be so in Heuchera cultivars, the red/purple leaved Heuchera ‘Obsidian’ having lower gs and higher leaf temperatures than cultivars with green or gold/yellow foliage.
Table 1.
Evapotranspirational cooling in street tree genotypes based on energy loss and typical stomatal conductance (gs) as measured in the UK during the month of July. Data are mean ± standard error. Modified from Rahman et al. (2015)
| Species | Energy loss per unit leaf area (W m−2) | Energy loss per tree (W tree−1) | gs (mmol m−2 s−1) |
|---|---|---|---|
| Crataegus laevigata | 240 ± 12 | 1720 ± 250 | 220 ± 12 |
| Sorbus arnoldiana | 180 ± 5 | 585 ± 55 | 185 ± 6 |
| Prunus ‘Umineko’ | 190 ± 6 | 490 ± 18 | 212 ± 6 |
| Pyrus calleryana | 445 ± 14 | 2210 ± 280 | 395 ± 10 |
| Malus ‘Rudolph’ | 168 ± 10 | 1030 ± 80 | 200 ± 8 |
Smaller scales of ‘green intervention’ are also used to promote local cooling, particularly on or around buildings. Most green roof systems utilize Sedum spp. due to their tolerance of shallow substrates and drought stress. Recent research, however, showed that these are not the best species to employ if cooling is the overarching priority (Blanuša et al., 2013; Vaz Monteiro et al., 2016). Rather, species with light-coloured, non-succulent leaf canopies were superior in cooling capacity to Sedum, due to a combination of higher values for transpiration, LAI and latent heat loss and lower values for both sensible and soil heat transfer. Indeed, species choice alone could result in 2-, 3- and 5-fold differences in latent, sensible and soil heat fluxes, respectively. As with trees, short-stature shrubs (e.g. Salvia officinalis and Stachys byzantina) that possess traits including high LAI, high evapotranspiration rate and light-coloured, silvery or hirsute leaves appear most effective at cooling.
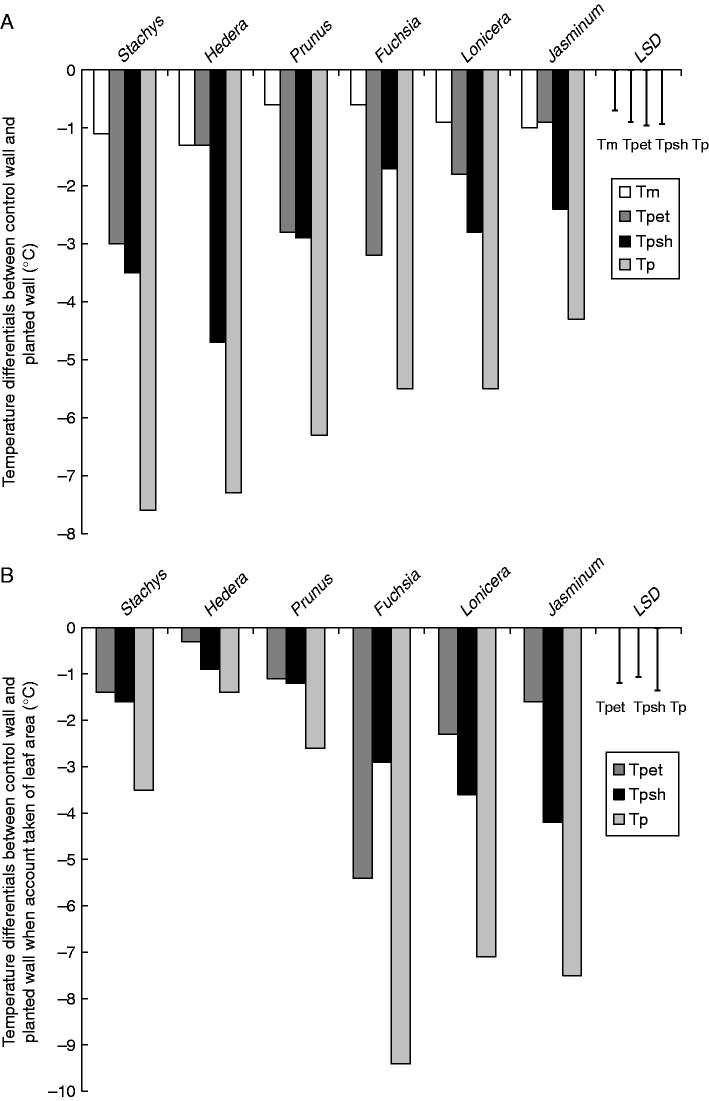
Stachys was also shown to provide a >7·0 °C cooling effect on a green wall system (comparable to the common evergreen climber Hedera helix). By blocking stomatal and cuticular water loss with poly(1-acetyloxyethylene) sealant in a proportion of the plants, the relative cooling effects of shading and evapotranspiration could be calculated. The data indicated that Hedera reduced temperature largely through shading, whereas both shading and evapotranspiration were key factors in the cooling conferred by Stachys (Fig. 3A). When evaluated on a leaf area basis, these species were outperformed, however, by Fuchsia, Jasminum and Lonicera (Fig. 3B). Again, the mechanisms by which the cooling was conferred varied markedly between species (Cameron et al., 2014). Fuchsia promoted evapotranspirational cooling, whereas shade cooling was more important in Jasminum and Lonicera. This variation in mechanism is important to recognize, because specific manipulation of a given species can further enhance the desired traits; for example, the effectiveness of individual leaves can be influenced through careful training of the stems. In species that confer cooling via shade, attaining multiple layers of leaves that maximize light interception is the objective. Conversely, species that cool via evapotranspiration and provide a single layer of evenly spaced leaves may be the priority in an attempt to optimize moisture transfer from the leaves to the surrounding atmosphere.
Fig. 3.
(A) Comparison of mean cooling (temperature differential, °C) for walls screened with different species and bare control walls (Tp), and derived values for cooling due to shade (Tpsh), evapotranspiration (Tpet) and evaporation from medium (Tm). Bars = Least significant difference (LSD) (P=0·05); d.f. = 32. Modified from Cameron et al. (2014). (B) Comparison of mean cooling (temperature differential, °C) for walls screened with different species and bare control walls, based on leaf area index (Tp), and derived values for cooling due to shade (Tpsh), evapotranspiration (Tpet) and evaporation from medium (Tm). Bars = LSD (P=0·05); d.f. = 32. Modified from Cameron et al. (2014).
Energy conservation
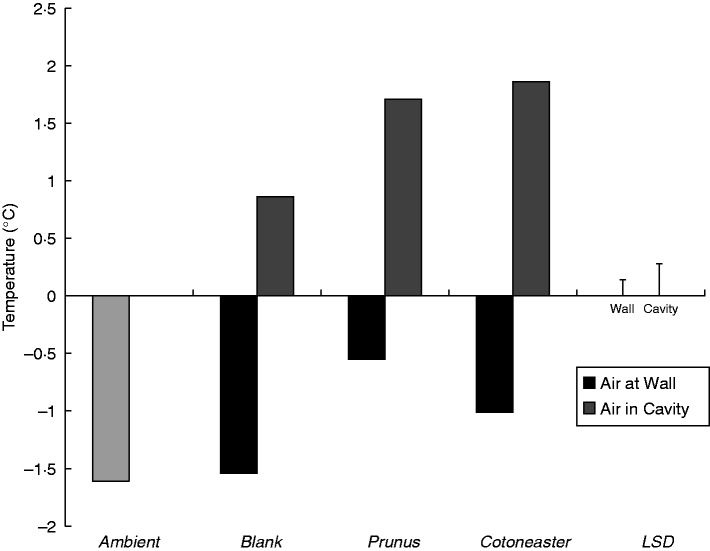
The cooling ability of plants in summer and their ability to insulate buildings in winter impact not only on human thermal comfort, but also on energy conservation and economics. Akbari et al. (2001) estimated that increasing the urban forest within the USA would reduce national energy use by 20 % and save over $10 billion a year through reduced reliance on artificial air conditioning and improvements in air quality. Using tree belts to protect buildings from cold wind helps entrap warm air around the building fabric and reduces energy loss by conduction and convection of heat from the interior of the building. Models indicated that shelter from trees reduced winter energy consumption in Scottish buildings by 17 % (Liu and Harris, 2008). An empirical study using replicated heated brick cuboids showed that placing a green façade around the structures reduced mean winter energy use by 38 % (and under some severe weather conditions improved savings by up to 45 %) (Cameron et al., 2015). Subsequent studies indicated that thicker-leaved Prunus laurocerasus improved air temperature at a wall surface during cold nights, compared with the smaller-leaved, less densely foliated Cotoneaster franchetti. Yet the latter species was overall the more beneficial as it enhanced the temperature within the wall cavity, due to it allowing more solar heat gain onto the wall during the daylight hours than the Prunus, whilst also conferring some insulation effect at night (Fig. 4).
Fig. 4.
The effect of vegetated façades (thick-leaved, high LAI, Prunus laurocerasus versus small-leaved, low LAI, Cotoneaster franchetti) and non-vegetated façades on mean air temperatures at a wall surface and within the cavity space of the wall. Data were recorded at 21·00 h during subzero conditions on 11 separate days between January and March 2011. Each treatment was represented by three replicate walls, and values for 21·00 h meaned from readings recorded at 20:50, 21:00 and 21:10 h, respectively, on each occasion. Bars = LSD (P = 0·05, d.f. 107). Reproduced with permission from Taylor (2012).
Storm-water retention
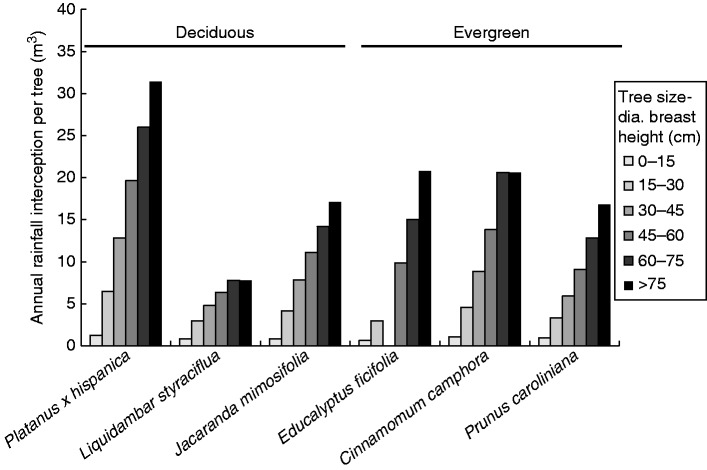
Vegetation plays a key role in capturing, retaining and detaining precipitation, and if placed/designed in harmony with hydrological flow pathways mitigates storm-water flooding. Selection and the age of park/street trees affect the ability to capture rainfall and store it on leaves, stems and bark (Xiao and McPherson, 2002). Due to its greater canopy and leaf size and more extensive branch structure, Platanus × hispanica retains a greater volume of rainwater than e.g. Liquidambar styraciflua (Fig. 5). ‘Fine-textured’ canopies, as promoted by the numerous needles in evergreen conifer species, e.g. Pinus strobus, are very effective at holding moisture within the tree canopy too. Similarly, species possessing rough bark with many grooves and fissures (e.g. Quercus rubra) hold more water than equivalent smooth-barked species (e.g. Betula lenta). For a 300-mm diameter tree, normative bark water storage capacities ranged from approximately 100 L for B. lenta to 250 L for Q. rubra (Levia and Herwitz, 2005).
Fig. 5.
Tree size and character (leaf/branch habit and duration of leaf retention) affect rainfall interception. Larger trees (increasing diameter at breast height) capture more rainfall, but also note differences between deciduous species. Modified from Xiao and McPherson (2002).
For green roofs, storm water management too is influenced by plant selection (Lundholm et al., 2010; Schroll et al., 2011). Nagase and Dunnett (2012) indicated that water retention was improved by using grasses and forbs rather than succulents, largely due to structural differences. As much of the water retained on a green roof is held within the pore structure of the substrate (VanWoert et al., 2005), however, the ability to remove this existing water via evapotranspiration (i.e. recharging the storage capacity) before the next storm event is also critical. Plant choice is also important here, as by deploying genotypes with high transpiration rates the substrate can be dried quickly and the storage capacity restored over a relatively short timeframe (S. Kemp pers. comm., University of Reading, UK).
Carbon sequestration
Using biomass as a predictor of carbon levels within urban trees, McPherson and Simpson (1999) indicated that species could be ranked based on their ability to absorb carbon dioxide, e.g. after 40 years of growth: Quercus ilex > Ceratonia siliqua > Eucalyptus globulus > Cinnamomum camphora > Pinus radiata > Pinus strobus > Cupressus macrocarpa. These data, however, do not indicate how much carbon might be transported to the soil via leaf litter, root dieback or root exudates. Other studies suggest that this could be significant, with 75 % of terrestrial carbon held within soils (Edmondson et al., 2014). Again, such studies indicate that genotype influences the soil carbon pool. Soil organic carbon stocks within urban parks were enhanced under Fraxinus excelsior (26 kg m−2 of land area) and mixed stands of Acer pseudoplatanus/Acer platanoides (19 kg m−2) compared with stands of Quercus robur or even other mixed woodland types (both 14 kg m−2) (Edmondson et al., 2014).
Mitigating the effects of soil, aerial and water pollutants
The beneficial services provided by certain plants are acknowledged within parts of the land remediation sector. Tolerance of heavy metal elements and thus the ability to phytoremediate contaminated soils varies markedly within tree genera such as Salix (Punshon and Dickinson, 1999), tolerance in this genus being clone- or hybrid-specific rather than species-specific. This is due to either different selection pressures based on population provenance or active breeding between tolerant genotypes.
Leaf structure affects the extent to which plants are able to capture particulate matter from the air (Beckett et al., 2000; Freer-Smith et al., 2005; Kardel et al., 2011; Blanuša et al., 2015). Consequently, the choice of street tree impacts on the potential to remove particle-based pollution along roadways. Particulate matter emitted from diesel engines is a specific health concern, with particulate matter <10 μm in diameter able to enter human airways, particulate matter <2·5 μm accessing pulmonary air sacs and particulate matter <0·1 μm entering the blood system. In a study evaluating the pollution capture potential of Italian street trees, it was evident that Tilia cordata and Platanus × hispanica were the favoured choices for capturing particles <10 μm, whereas Quercus cerris and Q. ilex were more effective at capturing particulate matter >10 μm (Blanuša et al., 2015). UK studies also differentiated species effects; Pinus nigra var. maritima and Sorbus aria were superior to other trees for trapping particulate matter >10 μm, and Pinus was also effective at accumulating particulate matter <10 μm (Beckett et al., 2000). Speak et al. (2012) found that grasses (Agrostis stolonifera and Festuca rubra) were more effective at particulate matter <10 μm capture than either broad-leaved Plantago lanceolata or succulent Sedum album when investigating typical green roof vegetation. This was attributed to grass species having complex canopy structures which reduce near-surface air flow and increase deposition rates, as well as possessing parallel grooves on their leaves which trap particles and prevent their re-suspension. Although large areas of green space are required to reduce air pollutants across the entire urban matrix, discrete interventions using vegetation along roadways or to target point sources of pollution, such as industrial complexes, may have a place in mitigating problems at a local scale.
Plants also act as biofilters to remove pollutants from storm-water run-off. Read et al. (2008) showed that the volume of total suspended solids, concentrations of organic and inorganic N and P, and concentrations of Cu varied 2- to 4-fold among the species tested. Moreover, for pollutants such as NOx, , Mn, Pb and Fe, differences between species could be as much as 20-fold. When root mass was taken into account, remediation potential was even more marked, Carex appressa, Melaleuca ericifolia, Juncus flavidus and Juncus amabilis being significantly more effective at retaining/absorbing N and P than Leucophyta brownii, Microlaena stipoides and Acacia suaveolens.
Human health and well-being
The value of plants in providing therapeutic landscapes has been under intensive study for the last two decades (e.g. Tzoulas et al., 2007; Sandifer et al., 2015). The key attribute of green space in this context is an ability to alleviate stress in humans (attention restoration) (Kaplan, 1995) and to encourage physical activity in a consistent and sustained manner (e.g. gardening or hill walking) (Cameron, 2014). Others consider that plant-derived volatile chemicals such as α-pinene and β-pinene have a direct role by enhancing the body’s immunological function and/or providing anti-cancer properties (Li et al., 2007), although the evidence for this is more circumspect. Again, the assumption has been that all green spaces have equal merit in stress alleviation, but there is some evidence to suggest that the quality of the landscape influences restoration potential. Plant choice may be one of the subfactors affecting quality of landscape. Although working with a relatively small population, Li et al. (2012) indicated that plants noted for their green foliage or predominately purple–blue flower hues (Lavandula angustifolia) resulted in more positive psychological responses in participants than species that exhibited red (Papaver rhoeas), yellow (Brassica napus) or white (Leucanthemum vulgare) flower colours. This was supported by lower ratings of irritability, fatigue and anxiety and higher scores for vigour. In contrast, exposure to all flower communities irrespective of predominant colour induced physiological improvements in the participants compared with exposure to non-natural scenes. This included decreases in systolic and diastolic blood pressure, heart rate, electrocardiogram readings and fingertip pulse rates and increased galvanic skin response. There is also a limited amount of evidence that humans have a preference for particular plant forms (Heerwagen and Orians, 1993; Lohr and Pearson-Mims, 2006; Lee et al., 2014), e.g. flat-topped specimen trees (e.g. Acer palmatum, Cornus controversa, Pinus sylvestris var. scotica) reminiscent of Vachellia tortilis (umbrella thorn acacia), a key landscape icon that was present during human evolution on the African savannahs. Whether this sort of visual preference actually translates into a health benefit remains to be determined.
Noise mitigation
Noise is considered a stress-inducing factor, and plants are used to absorb, diffuse and deflect noise (noise attenuation). Shelter belts of vegetation are now used to protect residents from road, rail and industrial sources of noise. Dense plantings of shrubs, where plants are a few metres higher than the receiver of the noise, are considered optimal for noise attenuation. Species such as Bambusa dolichoclada and Garcinia subelliptica, characterized by dense foliage and low forking branches, correlate with greater noise reduction compared with Nageia nagi, which has broader-spaced branches and leaves (Fang and Ling, 2003). As with other phenomena discussed previously, plant traits also interact with sound waves. Greater density, height, length and width of shelter belts help diffuse noise more effectively, whereas increasing leaf size and branching characteristics aid absorption of sound waves. Even in situations where vegetation does not alter the decibel level per se, the presence of plants seems to provide a psychological benefit and recipients perceive the noise to be lower than it actually is (Irvine et al., 2009).
Crime and security
Parks and other green spaces are often associated with crime and antisocial behaviour (James et al., 2009), and although crimes do undoubtedly occur in parks the perceptions of crime are often greater than the reality. Indeed there is evidence that green space can mitigate against criminal activity, or at least certain types of activity. Kuo and Sullivan (2001), for example, correlated a loss of green views from apartment blocks with increased incidences of domestic violence, the mechanism being that the green vistas were providing a beneficial restoration effect from physio-psychological stress, but on the removal of this therapeutic influence (trees within sight of the apartment blocks had been cut down) this stress could manifest itself as aggression. Other research has suggested that re-greening vacant urban spaces also inhibits criminal activity. In Philadelphia (USA) between 1999 and 2008 approximately 4400 open derelict spaces were cleaned up, planted with trees, grass and other landscape plants, and surrounded by wooden fences, thus providing an impression of greater care and maintenance being conferred on each of the sites (Branas et al., 2011). Regression analyses associated re-greening with consistent reductions in gun assaults across the four different sections of the city studied (P <0·001) and consistent reductions in vandalism in one of these sections (P <0·001). The extent to which these positive responses are linked to more/better quality vegetation per se or simply to a perception that the sites were more effectively managed is difficult to prove. The fact that residents also reported less stress around the re-vegetated plots, however, may indicate there was at least some restorative effect being activated. Increasing tree cover has also been linked with reductions in robbery, burglary, theft and shooting; modelling by Troy et al. (2012) suggested that a 10 % increase in tree canopy cover across the urban matrix correlates with a 12 % reduction in crime rates, even when confounding factors such as socio-economic considerations are accounted for. These aspects need further exploration and we are not at the point where specific genotypes can be advocated to stop homicides! Nevertheless, trees are better than other forms of vegetation, and that those with broad crowns or high trunk‐crown ratios are preferable to other forms (Lohr and Pearson‐Mims, 2006; Gerstenberg and Hofmann, 2016), may be the plants to consider when promoting a relaxed ambience (see examples above, but also, at a larger, landscape scale, selections such as Acer cappadocicum, Quercus robur, Morus alba, Catalpa bignonioides and Prunus yedoensis).
In contrast to trees, shrubs, or at least dense belts of shrubs, are positively associated with crime or perceptions of crime (Troy et al., 2012). Such plantings can conceal criminals before an attack or afford criminals a place to hide their activities or their stolen goods. To counteract this, shrubs/small trees that either have bare stems at the base or can be readily pruned to remove basal foliage (e.g. Prunus laurocerasus, Corylus avellana, Cotoneaster cornubia and Cercis siliquastrum) are advocated for those park sites where retaining sight lines through the vegetation reduces the risk of crime. Here, however, is an example of a trade-off between two different ecosystem services – the precise opposite of this vegetation design/shrub form being required to attenuate noise problems. Around the home, however, other shrub species provide a distinct security service, notably those with thorns or sharp serrations to the leaves. Species/cultivars within genera such as Pyracantha, Berberis, Rosa, Ilex, Rubus and Ulex are commonly utilized to protect domestic property from intruders.
Educational and cultural opportunities around engagement with nature
In a society that is rapidly becoming urbanized, urban green space is a vital component enabling citizens – children in particular – to engage with nature. Engagement in the natural world has been linked to health benefits (see above), but also personal and social development, positive attitudes and values, greater resilience to stressful life events, opportunities for self-discovery and unstructured play and improved cognitive functioning, as well as acting as a catalyst for social interactions that themselves promote an aptitude for learning (Wells and Evans, 2003; Charles and Louv, 2009; Gundersen et al., 2016). Indeed, such engagement enhances ecological literacy with corresponding ‘life chances’, including opportunities for careers in the natural environment, not least in the environmental and biological sciences. A report from the Royal Society for the Protection of Birds (RSPB, 2013), however, suggested that up to 80 % of children in the UK have ‘insufficient connection to nature’. This has implications for educational opportunities and well-being for the children, but also for the future conservation of species, as a lack of knowledge often relates to a lack of care (Charles and Louv, 2009). This is one reason why conservation bodies such as the RSPB and the UK Wildlife Trusts are now investing in research and promotional campaigns around this issue. Interestingly, the RSPB report indicates that engagement with nature can be highest in urban situations, suggesting that urban green spaces are playing a significant role in allowing wildlife to be present, appreciated and relatively easy for people to access. Highly visual animal taxa encourage interactions with the natural world, but so too do ‘iconic’ or cultural-linked plant species, e.g. for children in Western cultures Helianthus annuus (sunflower), Antirrhinum majus (snapdragon), Papaver rhoeas (corn poppy), Aesculus hippocastanum (horse chestnut), Taraxacum officinale (dandelion) and Narcissus cultivars (daffodils), amongst many others. In addition, being involved with growing food in home gardens, allotments and community gardens educates children about natural processes and enhances awareness about food. Moreover, such activities improve social capital, within and outwith the family (Thompson et al., 2007) and introduce children to healthier eating habits (Carney, 2012).
Disservices
Despite the range of services provided by landscape plants, some species also present drawbacks (disservices). Landscape architects are under pressure to avoid plants that drop fruit onto pavements, so male plants are the preferred choice rather than the female equivalent where this is a problem, e.g. either only the male form of Ginkgo biloba or the seedless form ‘Fastigiata’ are recommended as roadside plantings. As well as being unsightly, over-ripe fruit, as with domestic plum (Prunus domestica), for example, attract nuisance insect species, notably wasps and flies. Trees that are associated with large amounts of leaf litter (e.g. Aesculus, Juglans, Populus, Salix spp.) are best avoided in pedestrian precincts and those linked with high honeydew secretions (sugary exudates from aphids and scale insects), e.g. Tilia × europaea, are not appropriate for planting within car parks, due to the risk of marking the paintwork of cars. Poor choice of species also correlates with problems associated with trapping litter (Cotoneaster spp.), excessive pollen production (Betula), the release of biovolatile organic compounds (which elicit ozone formation and reduce air quality, e.g. Pinus and Eucalyptus spp.), or irritant hairs (Fremondodendron californica), or even direct toxic effects if ingested (Laburnum × watereri ‘Vossii’). Other species are notorious for the level of maintenance they require to keep them in shape and within their designated boundaries (e.g. Cupressus × leylandii). Again, the choice of species becomes paramount in minimizing the potential for disservices and promoting positive traits.
Multiple benefits
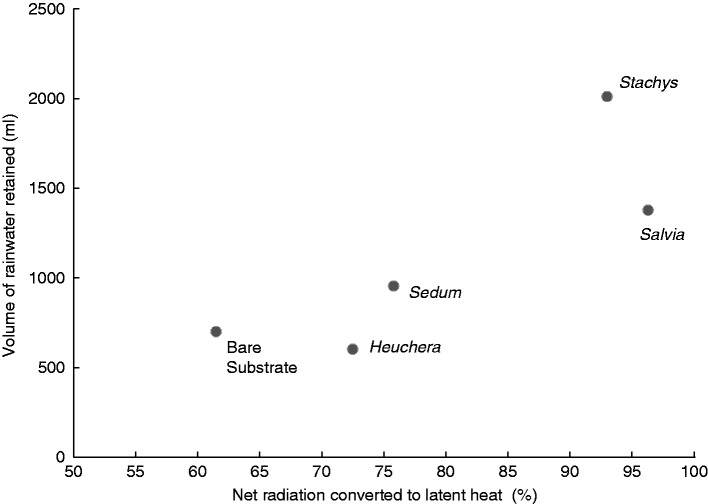
Research within the authors’ teams has started to investigate the multiple services offered by green roof plants. Certain leaf/canopy traits identified as contributing to localized cooling (Vaz Monteiro et al., 2016) are also closely correlated with a species’ capacity to mitigate flooding by reducing surface run-off (S. Kemp pers. comm., University of Reading, UK). In these latest experiments, Salvia officinalis and Stachys byzantina (previously identified as the species with greatest cooling capacity from our small selection of model species) demonstrated the greatest ability to retain water in the canopy (∼5 % of that applied) and to increase the ‘recharge potential’ of the substrate (they increased water holding capacity by 50 %, compared with only 30 % with Sedum) before a subsequent rainfall event. In this case, the common trait of high evapotranspiration rate has a positive influence on both the cooling service and the ability to recharge the water-holding capacity of the substrate (Fig. 6).
Fig. 6.
The relationship between the volume of rainfall captured in a set volume of substrate (i.e. water storage capacity after 3 d of previous evaporation/transpiration activity) and the amount of net radiation converted to latent heat (i.e. energy consumed in the process of transpiration, thus leading to temperature reduction) as affected by different plant species, with bare substrate used in comparison. High evapotranspiration rates associated with Stachys allow this species to dry out green roof substrates quickly, thereby increasing the ability of the roof to retain rainwater after any subsequent rainfall events, thus reducing run-off to drains and sewers. The conversion of liquid water to the gaseous phase though evapotranspiration also uses energy as latent heat, and thus there is less increase in local air temperatures compared with other species or bare substrate. (S. Kemp, University of Reading, UK, unpubl. res.)
Identifying single functional traits that have potential to provide multiple services has been an objective in other ecosystem management approaches (e.g. de Bello et al., 2010). In addition to our studies highlighting the benefits of Salvia and Stachys for urban temperature and water management, these two species are identified with wider service provision [the hairs of Stachys leaves provide nesting material for wool-carder bees, Anthidium manicatum (Garbuzov and Ratnieks, 2014) and are effective at trapping aerial pollutants (Shackleton et al., 2013); Salvia provides nectar (Mačukanović-Jocić et al., 2011) and pollen (Bozek, 2002) for honey bees, Apis mellifera, and its foliage is a fundamental ingredient in Mediterranean cuisine and a source of essential oils (Carrubba and Catalano, 2014)].
Other studies show too that certain species are better than others in promoting multiple services. In woodland systems, for example, both Picea and Betula forests increase timber resources, dead wood occurrence (important habitat provision) and soil carbon accumulation compared with woodland stands of alternative species (Gamfeldt et al., 2013). Pinus, on the other hand, has less influence on soil carbon, but provides timber, deadwood and a more open canopy that facilitates groundcover with Vaccinium, the berries of which are used as a local food source by both humans and wildlife. So different woodland types may offer multiple services, but also a different suite of services based on the community composition (Isbell et al., 2011), an important point to consider when designing plant communities in the urban landscape.
What next?
The concept that plants provide a range of ESs to the urban environment has become gradually recognized over the last two decades, but the notion that the choice of plants and their community structure and dynamics may be important components in this service delivery remains to be universally acknowledged. From the point of view of most practitioners, plant choice within urban GI still tends to be determined by what survives and what is aesthetic (and in some circumstances, e.g. urban nature reserves and the geographical origin of the plants). This paper highlights, though, that genotype selection can make a radical difference to the level of ES delivery, and further research is required to help populate a more comprehensive database relating plant selection to key benefit(s). This will allow practitioners to select appropriate genotypes to meet specific situations and scenarios. This will inevitably involve developing inventories, and allied publications to disseminate information and advice to end users. In very practical terms, it would be useful to see this new information added to the labels of commercially retailed plants, such that these not only state the plants’ aesthetic qualities, e.g. ‘good autumn colour’, but also add information about their service provision, e.g. ‘helps cool the patio’ or ‘improves wall insulation’. To date, this service provision has only been documented with respect to wildlife conservation value (‘fruit attracts birds’, ‘perfect for pollinators’ etc.), but this should go further. At a more strategic level, information on ‘model’ functional plant communities and case studies of where these have been put into practice should be made available to policy makers and other stakeholders. As outlined above, many policy makers now understand the ‘whys’ for GI, but focus now needs to shift to the ‘hows’ and ‘wheres’ to help ensure effective implementation.
Plants that optimize ES provision need to be embedded in the urban fabric more effectively. This means providing them with the appropriate space and necessary resources. Indeed, the body of data collected to date challenges a number of current paradigms about urban GI. Rather than trying to get robust, stress-adapted species to just survive on green roofs and walls, placement of appropriate infrastructure (e.g. deeper substrates and artificial irrigation) could allow much more functional species to be employed. The advantages gained potentially significantly outweigh any additional costs associated with the enhanced infrastructure.
Site and management limitations currently threaten plant survival and hence functionality in many urban situations. Even larger and more expensive plants, such as standard trees, may fail due to site limitations. These may relate to issues such as compaction, leading to poor soil structure with inadequate aeration or drainage properties, pollutants, excessively high or low pH (influenced by residual building materials) and phytotoxicity through de-icing salts and other ‘urban’ contaminants, as well as direct physical damage to roots and trunks, e.g. from trenching associated with utility provision and maintenance within streetscapes (Jim, 1998; Cameron and Hitchmough, 2016). Insufficient irrigation remains a problem for many landscape plants, most notably during their establishment phase. These factors will need to be addressed, especially if the philosophy moves away from simply ‘what will survive’ to ‘what provides function’, i.e. potentially a greater use of less resilient but perhaps more functional plants in future. Despite the financial implications, however, of dealing with these issues effectively, increasing attention is already being paid to ensuring greater plant longevity, at least from the more technically advanced landscape companies. For example, many street trees are now planted into ‘structured’ soils, where the aggregate is designed to withstand compaction and remain well aerated over time (Buhler et al., 2007). Likewise, better integrated approaches to urban design exploit rainfall run-off and recycled waste water more effectively, thereby meeting plants’ irrigation requirements, the more sophisticated systems being automated to save on human labour too. The encouraging aspect here is that as the cost–benefit analyses move in favour of the benefits (i.e. society fully understands the value of the plantings), higher costs can be, and often are, justified. Relatively expensive ‘micro’ green walls (so-called air pollution units) are being introduced to bus stops and other locations along roadsides, with air actively passed through the rhizosphere in an attempt to remove aerial contaminates such as nitrous oxides (Henry, 2015). If these prove to be effective, they are likely to be adopted as local authorities become more concerned about urban air pollution. Economic models and traditional views on who are the custodians of urban landscapes may also change in line with ES provision. If trees provide greater thermal comfort in and around shopping precincts (Taleghani et al., 2016; Sanusi et al., 2016), then tree plantings may be implemented and maintained by the retailers rather than the local authority, as greater comfort of employees and customers correlates with potentially greater sales returns (Kolb et al., 2012); see also the point below about the role of residents/volunteers.
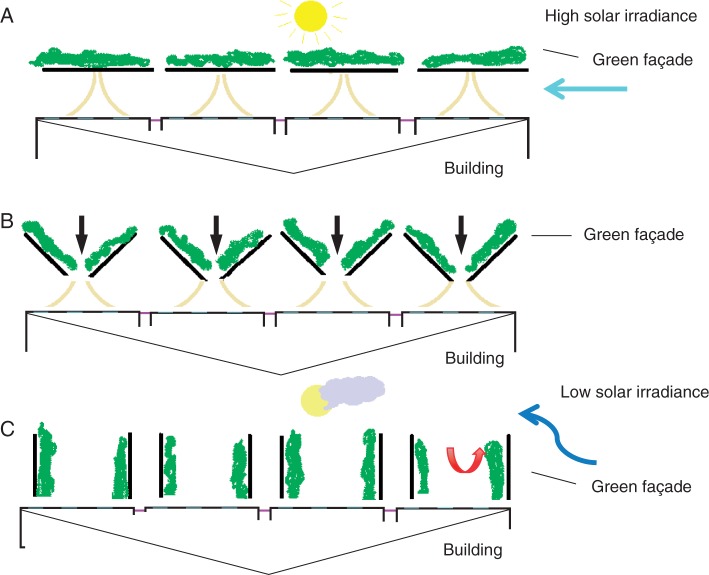
Urban design also needs to be more imaginative and should aim to exploit plant traits to the full. Planted living walls could be mobile and their position altered to reflect the needs of the building during different seasons; in a northern hemisphere scenario, for example, façades would be placed on a building’s southern aspect and used to provide direct shade to the building during hot, sunny summer days, but their orientation could be altered on the cooler, duller days of winter to maximize natural daylight from the south to illuminate the building (Figs 7 and 8). Further research is warranted to investigate the feasibility and cost–effectiveness of such approaches.
Fig. 7.
‘Mobile’ green façade system used to alter temperature on the southern wall (northern hemisphere) of a single-storey building (diagram shows wall and façade from above). (A) During summer the façade is positioned to directly intercept solar irradiance and cool the building through shade and evapotranspiration. Prevailing summer breezes are funnelled between the building and the façade, thereby providing further cooling. (B) The green façade comprises plants grown in troughs, each section of façade being dividable in the middle and orientated around 90° (e.g. using castors and guiding ground rail); façades push in towards the building, like doors. The position of the façades can be altered, e.g. in autumn and spring, to change the amount of irradiance hitting the building wall. (C) During winter the façades enable solar irradiance to reach the building wall, thereby maximizing natural light entering the building and creating a warmer environment around the building wall. Now the façades act as baffles, deviating any cold wind away from the wall, trapping pockets of warm air and further elevating the temperature of the building envelope on the southern aspect.
Fig. 8.

Some green façades are designed to be mobile and used as temporary walls to create wind, solar irradiance and sound baffles. Photo courtesy of Sean Farrell, University of Staffordshire, UK.
Advances should not be limited to those of a technical nature, however. Existing paradigms around the social/societal context may need to be challenged too, particularly with respect to the management of urban green landscapes. There may be movement away from central civic control to those situations where residents, volunteers and more locally organized groups take on greater responsibility for the management of the new plant communities. This may not just be solely due to financial constraints on local authorities, but also the fact that some of the service delivery (e.g. health and well-being) depends on citizens taking a more active role within, and indeed actively advocating for, their green spaces.
Detailed information is required before an individual genotype can be fully assessed for its functional merits. This limits the number of species/cultivars that can be evaluated within a given time and could lead to a situation where only a small proportion of the useful plant genotypes are identified and actively endorsed for their ES provision, at least initially. The urban environment is going to be poorly served, however, if only ‘monocultures’ of a few ‘functional keystone’ genotypes are slavishly promoted and used. At the same time it is not feasible to evaluate in depth the ES potential of every one of the 400 000 genotypes available to the landscape sector. Thus, we argue that an initial step forward is to deepen our understanding about how particular structural/physiological traits (e.g. colour, hairiness, size of canopy and/or root system, inherent evapotranspiration rate) correlate with the provision of specific ESs. By providing an extended choice of plant genotypes which offer good service in various categories (cooling, rainfall mitigation, pollutant trapping etc.), the diversity of urban planting will be increased whilst also improving overall ES delivery. The extent to which the possession of ‘generic’ structural traits, however, actually relates to the magnitude of service delivery remains to be tested in full. For example, do all grey-leaved plants provide a similar albedo? Obviously too, where space is limited, it is desirable to identify genotypes that deliver more than a single service.
Additionally, although some species have a greater number of functional traits than others, designing more diverse plant communities is likely to provide greater resilience in the long term (Isbell et al., 2011; Lundholm, 2015), as well as being intrinsically more interesting per se. So the urban plant communities of the future should incorporate a range of genotypes that are targeted at a particular service (e.g. atmospheric cooling) but also include others that cover different ES provision requirements (e.g. noise abatement), as well as adding yet further genotypes, simply to help ensure variety and diversity. Similarly, a more complete understanding of the desired goals of these communities will in itself help drive their design and management. For example, plant communities that are designed to attract Lepidoptera, for example, may need to provide a source of nectar for a longer period than is possible for any one (transiently flowering) plant species alone. Similarly, the community should include plant species that act as hosts to the larval stages, as well as simply feeding the adults, thus allowing the insect to complete its entire lifecycle within a fairly small geographical range. Unlike the rural environment, where emphasis should remain on native species and natural/semi-natural ecosystems, the urban environment provides more opportunity to experiment with how vegetation can be used more effectively and with greater innovation (with appropriate safeguards) to optimize ES delivery. The concept of GI is now in place in many of our towns and cities, but its true potential will only be realized when we elaborate and enrich the details and develop a diverse matrix of functional green spaces.
CONCLUSIONS
This paper calls for greater attention to be paid to individual plants when considering ecosystem service provision in an urban context. The arguments presented here indicate, quite simply, that plant choice matters! It is no longer appropriate for urban green space to be populated with plant species in an ad hoc manner relating to a vague notion of aesthetics, or even simply on a ‘what can survive’ basis. As urbanization increases and there is greater pressure on land for development, green spaces need to be able to justify their inclusion within the urban matrix, based on effective and wide-ranging service delivery. More research is required to understand better how plant traits impact on that service delivery, and how functional communities of plants can be designed to address specific problems or provide a range of important services with a limited space. Our knowledge base in selecting appropriate plants/communities is in its infancy, but as the value of and requirement for urban GI become more apparent, there will be increasing pressure to ensure that plant choice is optimized and that this choice is based on a strong scientific rationale. Consequently, this is an exciting and important new area for plant research. Moreover, the opportunity for scientists and practitioners to transform the urban matrix through more effective and wider used of plants (in some cases quite literally turning our grey cities green) has significant and notable implications for plant science globally. This arena of research provides opportunities to link plants directly to the key issues of the day, including human health and well-being, climate change adaptation, crime reduction and social cohesion, and, through better habitat provision for wildlife, to allow us to still engage with nature irrespective of where we live. In a human society now largely urbanized (Hall and Pfeiffer, 2013), implementing more effective GI will have substantial benefits, perhaps in due time becoming the second most important aspect of plant cultivation globally after that aligned to commercial food production.
LITERATURE CITED
- Akbari H, Pomerantz M, Taha H. 2001. Cool surfaces and shade trees to reduce energy use and improve air quality in urban areas. Solar Energy 70: 295–310. [Google Scholar]
- Alpert P, Bone E, Holzapfel C. 2000. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspectives in Plant Ecology, Evolution and Systematics 3: 52–66. [Google Scholar]
- Anon. 2013. Urban pollinators. http://urbanpollinators.blogspot.co.uk/2013/09/pollinator-friendly-garden-plants-for.html (last accessed 14 September 2015). [Google Scholar]
- Bajema RA, DeVault TL, Scott PE, Lima SL. 2009. Reclaimed coal mine grasslands and their significance for Henslow’s sparrows in the American Midwest. Auk 118: 422–431. [Google Scholar]
- Beckett KP, Freer-Smith PH, Taylor G. 2000. Particulate pollution capture by urban trees: effect of species and windspeed. Global Change Biology 6: 995–1003. [Google Scholar]
- Benedict MA, McMahon ET. 2012. Green infrastructure: linking landscapes and communities. Washington, DC: Island Press. [Google Scholar]
- de Bello F, Lavorel S, Díaz S, et al. 2010. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodiversity and Conservation 19: 2873–2893. [Google Scholar]
- Berens DG, Farwig N, Schaab G, Böhning-Gaese K. 2008. Exotic guavas are foci of forest regeneration in Kenyan farmland. Biotropica 40: 104–112. [Google Scholar]
- Bisgrove R. 2013. The colour of creation: Gertrude Jekyll and the art of flowers. Journal of Experimental Botany 64: 5783–5789. [DOI] [PubMed] [Google Scholar]
- Bjerknes AL, Totland Ø, Hegland SJ, Nielsen A. 2007. Do alien plant invasions really affect pollination success in native plant species? Biological Conservation 138: 1–12. [Google Scholar]
- Blanuša T, Vaz Monteiro MM, Fantozzi F, Vysini E, Li Y, Cameron RWF. 2013. Alternatives to Sedum on green roofs: can broad leaf perennial plants offer better ‘cooling service’? Building and Environment 59: 99–106. [Google Scholar]
- Blanuša T, Fantozzi F, Monaci F, Bargagli R. 2015. Leaf trapping and retention of particles by holm oak and other common tree species in Mediterranean urban environments. Urban Forestry & Urban Greening 14: 1095–1101. [Google Scholar]
- Bozek M. 2002. Flowering biology and pollen flow of three species from genus Salvia L. Annales Universitatis Mariae Curie-Sklodowska. Sectio EEE, Horticultura 10: 51–57. [Google Scholar]
- Branas CC, Cheney RA, MacDonald JM, Tam VW, Jackson TD, Ten Have TR. 2011. A difference-in-differences analysis of health, safety, and greening vacant urban space. American Journal of Epidemiology 174: 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler O, Kristoffersen P, Larsen SU. 2007. Growth of street trees in Copenhagen with emphasis on the effect of different establishment concepts. Arboriculture and Urban Forestry 33: 330. [Google Scholar]
- Cameron RW. 2014. Health and well-being In: Horticulture: plants for people and places. New York, London: Springer, Dordrecht, Heidelberg. [Google Scholar]
- Cameron RWF, Hitchmough J. 2016. Environmental horticulture: science and management of green landscapes. Boston: CAB International. [Google Scholar]
- Cameron RWF, Blanuša T, Taylor JE, et al. 2012. The domestic garden – its contribution to urban green infrastructure. Urban Forestry & Urban Greening 11: 129–137. [Google Scholar]
- Cameron RWF, Taylor JE, Emmett MR. 2014. What's ‘cool’ in the world of green façades? How plant choice influences the cooling properties of green walls. Building and Environment 73: 198–207. [Google Scholar]
- Cameron RWF, Taylor JE, Emmett MR. 2015. A Hedera green façade – energy performance and saving under different maritime-temperate, winter weather conditions. Building and Environment 92: 111–121. [Google Scholar]
- Carney M. 2012. Compounding crises of economic recession and food insecurity: a comparative study of three low-income communities in Santa Barbara County. Agriculture and Human Values 29: 185–201. [Google Scholar]
- Carreck NL, Williams IH, Little DJ. 1997. The movement of honey bee colonies for crop pollination and honey production by beekeepers in Great Britain. Bee World 78: 67–77. [Google Scholar]
- Carrubba A, Catalano C. 2009. Essential oil crops for sustainable agriculture – a review In: Lichtfouse E, ed. Climate change, intercropping, pest control and beneficial microorganisms. Heidelberg, New York, London: Springer, Dordrecht, 137–187. [Google Scholar]
- Carvalheiro LG, Biesmeijer JC, Benadi G, et al. 2014. The potential for indirect effects between co‐flowering plants via shared pollinators depends on resource abundance, accessibility and relatedness. Ecology Letters 17: 1389–1399. [DOI] [PubMed] [Google Scholar]
- Charles C, Louv R. 2009. Children’s nature deficit: what we know and don’t know. Children and Nature Network. http://agnrgroups.umd.edu/sites/default/files/_images/master-naturalist/DNR%20QW%202014%20Teaching%20and%20Interpretation%20CNNEvidenceoftheDeficit.pdf (last accessed 30 June 2016). [Google Scholar]
- Chen LY. 2001. Cost savings from properly managing endangered species habitats. Natural Areas Journal 21: 197–203. [Google Scholar]
- Chiba S. 2010. Invasive non-native species’ provision of refugia for endangered native species. Conservation Biology 24: 1141– 1147. [DOI] [PubMed] [Google Scholar]
- Edmondson JL, O’Sullivan OS, Inger R, et al. 2014. Urban tree effects on soil organic carbon PLoS One 9: e101872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EU. 2012. The multifunctionality of green infrastructure. Science for environment policy – in-depth report http://ec.europa.eu/environment/nature/ecosystems/docs/Green_Infrastructure.pdf (last accessed 6 April 2016).
- EU. 2013. Building a green infrastructure for Europe http://ec.europa.eu/environment/nature/ecosystems/docs/green_infrastructure_broc.pdf (last accessed 6 April 2016).
- Fang CF, Ling DL. 2003. Investigation of the noise reduction provided by tree belts. Landscape and Urban Planning 63: 187–195. [Google Scholar]
- Feyisa GL, Dons K, Meilby H. 2014. Efficiency of parks in mitigating urban heat island effect: an example from Addis Ababa. Landscape and Urban Planning 123: 87–95. [Google Scholar]
- Freer-Smith PH, Beckett KP, Taylor G. 2005. Deposition velocities to Sorbus aria, Acer campestre, Populus deltoides × trichocarpa ‘Beaupré’, Pinus nigra and × Cupressocyparis leylandii for coarse, fine and ultra-fine particles in the urban environment. Environmental Pollution 133: 157–167. [DOI] [PubMed] [Google Scholar]
- Gamfeldt L, Snäll T, Bagchi R, et al. 2013. Higher levels of multiple ecosystem services are found in forests with more tree species. Nature Communications 4: 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzov M, Ratnieks FL. 2014. Quantifying variation among garden plants in attractiveness to bees and other flower‐visiting insects. Functional Ecology 28: 364–374. [Google Scholar]
- Garbuzov M, Ratnieks FL. 2015. Using the British National Collection of asters to compare the attractiveness of 228 varieties to flower-visiting insects. Environmental Entomology 1: 9. [DOI] [PubMed] [Google Scholar]
- Gerstenberg T, Hofmann M. 2016. Perception and preference of trees: a psychological contribution to tree species selection in urban areas. Urban Forestry & Urban Greening 15: 103–111. [Google Scholar]
- Gómez-Baggethun E, Barton DN. 2013. Classifying and valuing ecosystem services for urban planning. Ecological Economics 86: 235–245. [Google Scholar]
- Goulson D, Lye GC, Darvill B. 2008. Decline and conservation of bumble bees. Annual Review of Entomology 53: 191–208. [DOI] [PubMed] [Google Scholar]
- De Groot RS, Alkemade R, Braat L, Hein L, Willemen L. 2010. Challenges in integrating the concept of ecosystem services and values in landscape planning, management and decision making. Ecological Complexity 7: 260–272. [Google Scholar]
- Gundersen V, Skår M, O'Brien L, Wold LC, Follo G. 2016. Children and nearby nature: a nationwide parental survey from Norway. Urban Forestry & Urban Greening 17: 116–125. [Google Scholar]
- Hall P, Pfeiffer U. 2013. Urban future 21: a global agenda for twenty-first century cities. London: Routledge. [Google Scholar]
- Hanley ME, Awbi AJ, Franco M. 2014. Going native? Flower use by bumblebees in English urban gardens. Annals of Botany 113: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy PB, Dennis RL. 2008. Resources for British butterflies (Lepidoptera: Hesperioidea, Papilionoidea). The alien consumer component and its significance for butterfly habitats. European Journal of Entomology 105: 649–657. [Google Scholar]
- Heerwagen JH, Orians GH. 1993. Humans, habitats, and aesthetics In: Kellert SR, Wilson EO, eds. The biophilia hypothesis. Washington, DC: Island Press, 138–172. [Google Scholar]
- Helden AJ, Stamp GC, Leather SR. 2012. Urban biodiversity: comparison of insect assemblages on native and non-native trees. Urban Ecosystems 15: 611–624. [Google Scholar]
- Henry E. 2015. Units offer relief from air pollution. Horticulture Week http://www.hortweek.com/units-offer-relief-air-pollution/landscape/article/1354885 (last accessed 8 April 2016). [Google Scholar]
- Hunter AJ, Luck GW. 2015. Defining and measuring the social-ecological quality of urban greenspace: a semi-systematic review. Urban Ecosystems 18: 1139–1163. [Google Scholar]
- Irvine KN, Devine-Wright P, Payne SR, Fuller RA, Painter B, Gaston KJ. 2009. Green space, soundscape and urban sustainability: an interdisciplinary, empirical study. Local Environment 14: 155–172. [Google Scholar]
- Isbell F, Calcagno V, Hector A, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477: 199–202. [DOI] [PubMed] [Google Scholar]
- James P, Tzoulas K, Adam MD, et al. 2009. Towards an integrated understanding of green space in the European built environment. Urban Forestry & Urban Greening 8: 65–75. [Google Scholar]
- Jim CY. 1998. Urban soil characteristics and limitations for landscape planting in Hong Kong. Landscape and Urban Planning 40: 235–249. [Google Scholar]
- Kaplan S. 1995. The restorative benefits of nature: toward an integrative framework. Journal of Environmental Psychology 15: 169–182. [Google Scholar]
- Kardel F, Wuyts K, Maher BA, Hansard R, Samson R. 2011. Leaf saturation isothermal remanent magnetization (SIRM) as a proxy for particulate matter monitoring: inter-species differences and in-season variation. Atmospheric Environment 45: 5164–5171. [Google Scholar]
- Kolb P, Gockel C, Werth L. 2012. The effects of temperature on service employees’ customer orientation: an experimental approach. Ergonomics 55: 621–635. [DOI] [PubMed] [Google Scholar]
- Kuo FE, Sullivan WC. 2001. Environment and crime in the inner city does vegetation reduce crime? Environment and Behavior 33: 343–367. [Google Scholar]
- Lee KE, Williams KJ, Sargent LD, Farrell C, Williams NS. 2014. Living roof preference is influenced by plant characteristics and diversity. Landscape and Urban Planning 122: 152–159. [Google Scholar]
- Levia DF, Herwitz SR. 2005. Interspecific variation of bark water storage capacity of three deciduous tree species in relation to stemflow yield and solute flux to forest soils. Catena 64: 117–137. [Google Scholar]
- Li Q, Morimoto K, Kobayashi M, et al. 2007. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. International Journal of Immunopathology and Pharmacology 21: 117–127. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Z, Gu M, et al. 2012. Effects of plantscape colours on psycho-physiological responses of university students. Journal of Food, Agriculture and Environment 10: 702–708. [Google Scholar]
- Lin BS, Lin YJ. 2010. Cooling effect of shade trees with different characteristics in a subtropical urban park. HortScience 45: 83–86. [Google Scholar]
- Liu Y, Harris DJ. 2008. Effects of shelterbelt trees on reducing heating-energy consumption of office buildings in Scotland. Applied Energy 85: 115–127. [Google Scholar]
- Lohr VI, Pearson-Mims CH. 2006. Responses to scenes with spreading, rounded, and conical tree forms. Environment and Behavior 38: 667–688. [Google Scholar]
- Lugo AE. 2004. The outcome of alien tree invasions in Puerto Rico. Frontiers in Ecology and the Environment 2: 265–273. [Google Scholar]
- Lundholm JT. 2015. Green roof plant species diversity improves ecosystem multifunctionality. Journal of Applied Ecology 52: 726–734. [Google Scholar]
- Lundholm JT, MacIvor JS, MacDougall Z, Ranalli M. 2010. Plant species and functional group combinations affect green roof ecosystem functions. PloS One 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mačukanović-Jocić M, Stevanović ZD, Mladenović M, Jocić G. 2011. Flower morphophysiology of selected Lamiaceae species in relation to pollinator attraction. Journal of Apicultural Research 50: 89–101. [Google Scholar]
- Millennium Ecosystem Assessment 2005. Ecosystems and human well-being, Vol. 5. Millennium ecosystem assessment. Washington, DC: Island Press. [Google Scholar]
- Matthews T, Lo AY, Byrne JA. 2015. Reconceptualizing green infrastructure for climate change adaptation: barriers to adoption and drivers for uptake by spatial planners. Landscape and Urban Planning 138: 155–163. [Google Scholar]
- Mell I. 2008. Green infrastructure: concepts and planning. FORUM Ejournal 8: 69–80. [Google Scholar]
- McPherson EG, Simpson JR. 1999. Carbon dioxide reduction through urban forestry. General Technical Report PSW-171. Albany, CA: USDA Forest Service, Pacific Southwest Research Station. [Google Scholar]
- Nagase A, Dunnett N. 2012. Amount of water runoff from different vegetation types on extensive green roofs: effects of plant species, diversity and plant structure. Landscape and Urban Planning 104: 356–363. [Google Scholar]
- Natural England. 2009. Natural England's green infrastructure guidance. www.naturalengland.org.uk/publications (last accessed 30 June 2016). [Google Scholar]
- Potts SG, Vulliamy B, Dafni A, Ne'eman G, Willmer P. 2003. Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84: 2628–2642. [Google Scholar]
- Punshon T, Dickinson N. 1999. Heavy metal resistance and accumulation characteristics in willows. International Journal of Phytoremediation 1: 361–385. [Google Scholar]
- Rahman MA, Armson D, Ennos AR. 2015. A comparison of the growth and cooling effectiveness of five commonly planted urban tree species. Urban Ecosystems 18: 371–389. [Google Scholar]
- Read J, Wevill T, Fletcher T, Deletic A. 2008. Variation among plant species in pollutant removal from stormwater in biofiltration systems. Water Research 42: 893–902. [DOI] [PubMed] [Google Scholar]
- RSPB 2013. Connecting to nature–finding out how connected to nature the UK's children are. Royal Society for the Protection of Birds. https://www.rspb.org.uk/Images/connecting-with-nature_tcm9-354603.pdf (last accessed 28 April 2016). [Google Scholar]
- Salisbury A, Armitage J, Bostock H, Perry J, Tatchell M, Thompson K. 2015. Enhancing gardens as habitats for flower‐visiting aerial insects (pollinators): should we plant native or exotic species? Journal of Applied Ecology 52: 1156–1164. [Google Scholar]
- Sandström UG, Angelstam P, Mikusiński G. 2006. Ecological diversity of birds in relation to the structure of urban green space. Landscape and Urban Planning 77:39–53. [Google Scholar]
- Sanusi R, Johnstone D, May P, Livesley SJ. 2016. Street orientation and side of the street greatly influence the microclimatic benefits street trees can provide in summer. Journal of Environmental Quality 45: 167–174. [DOI] [PubMed] [Google Scholar]
- Sandifer PA, Sutton-Grier AE, Ward BP. 2015. Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: opportunities to enhance health and biodiversity conservation. Ecosystem Services 12: 1–15. [Google Scholar]
- Sax DF. 2002. Equal diversity in disparate species assemblages: a comparison of native and exotic woodlands in California. Global Ecology and Biogeography 11: 49–57. [Google Scholar]
- Schroll E, Lambrinos J, Righetti T, Sandrock D. 2011. The role of vegetation in regulating stormwater runoff from green roofs in a winter rainfall climate. Ecological Engineering 37: 595–600. [Google Scholar]
- Shackelford N, Hobbs RJ, Heller NE, Hallett LM, Seastedt TR. 2013. Finding a middle-ground: the native/non-native debate. Biological Conservation 158: 55–62. [Google Scholar]
- Shackleton K, Bell N, Smith H, Davies L. 2013. The role of shrubs and perennials in the capture and mitigation of particulate air pollution in London. http://content.tfl.gov.uk/role-gi-pmpollution.pdf (last accessed 23 October 2015).
- Shashua‐Bar L, Potchter O, Bitan A, Boltansky D, Yaakov Y. 2010. Microclimate modelling of street tree species effects within the varied urban morphology in the Mediterranean city of Tel Aviv, Israel. International Journal of Climatology 30: 44–57. [Google Scholar]
- Sogge M, Sferra S, Paxton E. 2008. Saltcedar as habitat for birds: implications for riparian restoration in the southwestern United States. Restoration Ecology 16:146–154. [Google Scholar]
- Speak AF, Rothwell JJ, Lindley SJ, Smith CL. 2012. Urban particulate pollution reduction by four species of green roof vegetation in a UK city. Atmospheric Environment 61: 283–293. [Google Scholar]
- Standish RJ, Hobbs RJ, Miller JR. 2013. Improving city life: options for ecological restoration in urban landscapes and how these might influence interactions between people and nature. Landscape Ecology 28: 1213–1221. [Google Scholar]
- Sullivan JJ, Williams PA, Timmins SM. 2007. Secondary forest succession differs through naturalised gorse and native kanuka near Wellington and Nelson, New Zealand. Journal of Ecology 31: 22–38. [Google Scholar]
- Taleghani M, Sailor D, Ban-Weiss GA. 2016. Micrometeorological simulations to predict the impacts of heat mitigation strategies on pedestrian thermal comfort in a Los Angeles neighborhood. Environmental Research Letters 11: 024003. [Google Scholar]
- Taylor JE. 2012. Green walls: the thermoregulatory role of plants adjacent to brick walls in a temperate climate. PhD Thesis, University of Reading, UK.
- Thompson S, Corkery L, Judd B. 2007. The role of community gardens in sustaining healthy communities. In: Proceedings of the State of Australian Cities National Conference. University of South Australia. ; http://149.171.158.96/sites/default/files/upload/research/centres/cf/CFpresentations/SOAC07Thompson_Corkery_Judd.pdf (last accessed 30 June 2016). [Google Scholar]
- Troy A, Grove JM, O’Neil-Dunne J. 2012. The relationship between tree canopy and crime rates across an urban–rural gradient in the greater Baltimore region. Landscape and Urban Planning 106: 262–270. [Google Scholar]
- Tzoulas K, Korpela K, Venn S, et al. 2007. Promoting ecosystem and human health in urban areas using green infrastructure: a literature review. Landscape and Urban Planning 81: 167–178. [Google Scholar]
- VanWoert ND, Rowe DB, Andresen JA, Clayton LR, Fernandez RT, Xiao L. 2005. Green roof stormwater retention: effects of roof surface, slope, and media depth. Journal of Environmental Quality 34: 1036–1044. [DOI] [PubMed] [Google Scholar]
- Vaz Monteiro MM, Blanuša T, Verhoef A, Hadley P, Cameron R. 2016. Relative importance of transpiration rate and leaf morphological traits for the regulation of leaf temperature. Australian Journal of Botany 64: 32–44. [Google Scholar]
- Wells N, Evans G. 2003. Nearby nature: a buffer of life stress among rural children. Environment and Behaviour 35: 311–330. [Google Scholar]
- Xiao Q, McPherson EG. 2002. Rainfall interception by Santa Monica's municipal urban forest. Urban Ecosystems 6: 291–302. [Google Scholar]