Abstract
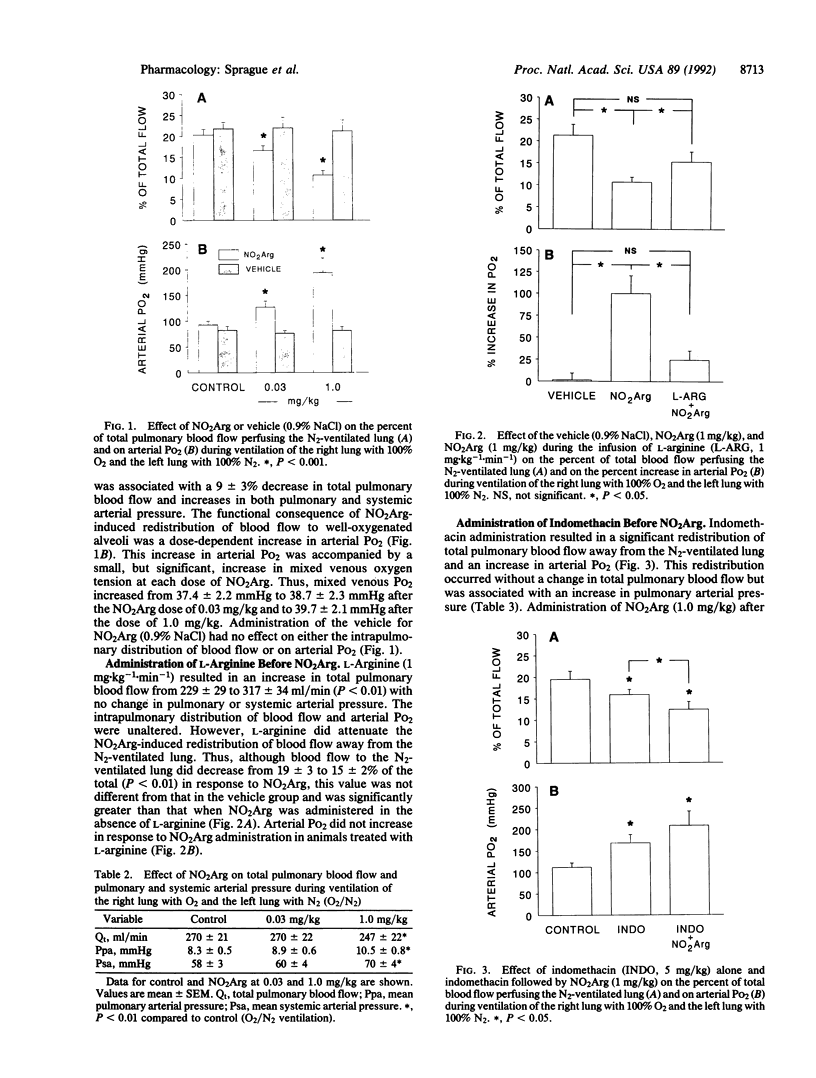
Agents that inhibit nitric oxide synthesis augment hypoxic pulmonary vasoconstriction. In an animal model of unilateral alveolar hypoxia, we investigated the hypothesis that endogenous endothelium-derived relaxing factor/nitric oxide opposes hypoxic pulmonary vasoconstriction and supports blood flow to hypoxic alveoli, resulting in a reduction in arterial oxygen tension (PO2). In pentobarbital-anesthetized rabbits, unilateral alveolar hypoxia was produced by ventilation of one lung with 100% oxygen and the other with 100% nitrogen (O2/N2). NG-Nitro-L-arginine methyl ester (0.03 followed by 1.0 mg/kg i.v.) resulted in dose-dependent decreases in the percent of pulmonary blood flow to the N2-ventilated lung and increases in arterial PO2. L-Arginine (1 mg.kg-1.min-1 i.v.) prevented the NG-nitro-L-arginine methyl ester-induced redistribution of blood flow away from hypoxic alveoli and improvement in arterial PO2. Indomethacin (5 mg/kg i.v.) administered during O2/N2 ventilation resulted in a reduction in the percentage of total blood flow to the hypoxic lung and an increase in arterial PO2. However, NG-nitro-L-arginine methyl ester administered in the presence of indomethacin caused additional diversion of blood flow away from the hypoxic lung. The magnitude of the changes suggests that the endothelium-derived relaxing factor/nitric oxide system has the capacity to make a greater contribution than products of cyclooxygenase-mediated arachidonic acid metabolism in supporting blood flow to hypoxic alveoli in the rabbit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annest S. J., Gottlieb M. E., Rhodes G. R., Paloski W. H., Barie P., Newell J. C., Shah D. M. Nitroprusside and nitroglycerine in patients with posttraumatic pulmonary failure. J Trauma. 1981 Dec;21(12):1029–1031. doi: 10.1097/00005373-198112000-00004. [DOI] [PubMed] [Google Scholar]
- Archer S. L., Rist K., Nelson D. P., DeMaster E. G., Cowan N., Weir E. K. Comparison of the hemodynamic effects of nitric oxide and endothelium-dependent vasodilators in intact lungs. J Appl Physiol (1985) 1990 Feb;68(2):735–747. doi: 10.1152/jappl.1990.68.2.735. [DOI] [PubMed] [Google Scholar]
- Archer S. L., Tolins J. P., Raij L., Weir E. K. Hypoxic pulmonary vasoconstriction is enhanced by inhibition of the synthesis of an endothelium derived relaxing factor. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1198–1205. doi: 10.1016/0006-291x(89)91796-8. [DOI] [PubMed] [Google Scholar]
- Brashers V. L., Peach M. J., Rose C. E., Jr Augmentation of hypoxic pulmonary vasoconstriction in the isolated perfused rat lung by in vitro antagonists of endothelium-dependent relaxation. J Clin Invest. 1988 Nov;82(5):1495–1502. doi: 10.1172/JCI113757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. B., Sykes M. K., Reyes A. A hypoxic pulmonary vasoconstrictor response in dogs during and after infusion of sodium nitroprusside. Anesthesiology. 1979 Jun;50(6):484–488. doi: 10.1097/00000542-197906000-00002. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Chaudhuri G. EDRF generation and release from perfused bovine pulmonary artery and vein. Eur J Pharmacol. 1988 Apr 27;149(1-2):79–88. doi: 10.1016/0014-2999(88)90045-3. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Johns R. A., Linden J. M., Peach M. J. Endothelium-dependent relaxation and cyclic GMP accumulation in rabbit pulmonary artery are selectively impaired by moderate hypoxia. Circ Res. 1989 Dec;65(6):1508–1515. doi: 10.1161/01.res.65.6.1508. [DOI] [PubMed] [Google Scholar]
- Liu S. F., Crawley D. E., Barnes P. J., Evans T. W. Endothelium-derived relaxing factor inhibits hypoxic pulmonary vasoconstriction in rats. Am Rev Respir Dis. 1991 Jan;143(1):32–37. doi: 10.1164/ajrccm/143.1.32. [DOI] [PubMed] [Google Scholar]
- Mazmanian G. M., Baudet B., Brink C., Cerrina J., Kirkiacharian S., Weiss M. Methylene blue potentiates vascular reactivity in isolated rat lungs. J Appl Physiol (1985) 1989 Mar;66(3):1040–1045. doi: 10.1152/jappl.1989.66.3.1040. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Gustafsson L. E., Wiklund N. P., Moncada S., Hedqvist P. Endogenous nitric oxide as a probable modulator of pulmonary circulation and hypoxic pressor response in vivo. Acta Physiol Scand. 1990 Dec;140(4):449–457. doi: 10.1111/j.1748-1716.1990.tb09021.x. [DOI] [PubMed] [Google Scholar]
- Rodman D. M., Yamaguchi T., Hasunuma K., O'Brien R. F., McMurtry I. F. Effects of hypoxia on endothelium-dependent relaxation of rat pulmonary artery. Am J Physiol. 1990 Apr;258(4 Pt 1):L207–L214. doi: 10.1152/ajplung.1990.258.4.L207. [DOI] [PubMed] [Google Scholar]
- Sprague R. S., Stephenson A. H., Heitmann L. J., Lonigro A. J. Differential response of the pulmonary circulation to prostaglandins E2 and F2 alpha in the presence of unilateral alveolar hypoxia. J Pharmacol Exp Ther. 1984 Apr;229(1):38–43. [PubMed] [Google Scholar]
- Sprague R. S., Stephenson A. H., Lonigro A. J. Prostaglandin I2 supports blood flow to hypoxic alveoli in anesthetized dogs. J Appl Physiol Respir Environ Exerc Physiol. 1984 May;56(5):1246–1251. doi: 10.1152/jappl.1984.56.5.1246. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Maltby N. H., MacCormack D., Barnes P. J. Pulmonary endothelium-derived relaxing factor is impaired in hypoxia. Clin Sci (Lond) 1989 Dec;77(6):671–676. doi: 10.1042/cs0770671. [DOI] [PubMed] [Google Scholar]
- Weir E. K. Does normoxic pulmonary vasodilatation rather than hypoxic vasoconstriction account for the pulmonary pressor response to hypoxia? Lancet. 1978 Mar 4;1(8062):476–477. doi: 10.1016/s0140-6736(78)90138-1. [DOI] [PubMed] [Google Scholar]