Abstract
Introduction
Osteoporosis is an under-recognized problem threatening men. Bisphosphonates are the main treatment but their comparative efficacy is unclear for men with osteoporosis. Therefore, we performed this systematic review with network meta-analyses to summarize the evidence of comparative efficacy of bisphosphonates in men with osteoporosis.
Methods
We completed network meta-analyses with a frequentist model to compare the efficacy of different bisphosphonates. Randomized controlled trials investigating bisphosphonates used in men with osteoporosis were included. The primary outcome was the rate of patients with a new vertebral fracture. The secondary outcome was the rate of patients with a non-vertebral fracture, which was defined as any fractures reported other than vertebral fractures. Pairwise meta-analyses were performed to compare bisphosphonates with placebo. We included open-label studies in the analyses as a sensitivity analysis.
Results
Ten trials were included, using alendronate, ibandronate, risedronate, and zoledronic acid. No significant difference was found between any pairs of alendronate, ibandronate, risedronate, and zoledronic acid for both vertebral and non-vertebral fractures. Zoledronic acid ranked as the most effective in preventing vertebral fracture in primary osteoporosis. Risedronate ranked best in preventing non-vertebral fracture in both primary osteoporosis and corticosteroid-induced osteoporosis. In the sensitivity analyses with the open-label studies, the ranking order did not change.
Conclusion
The current evidence for bisphosphonates used in men with osteoporosis is inadequate. On the basis of the current evidence, zoledronic acid is most effective at preventing vertebral fractures, while risedronate has the highest possibility to rank the first in preventing non-vertebral fracture in men with primary osteoporosis and corticosteroid-induced osteoporosis. More well-designed studies are needed to test our findings and to better know the comparative efficacy of bisphosphonate to prevent vertebral fracture in men with osteoporosis.
Electronic supplementary material
The online version of this article (doi:10.1007/s40744-016-0030-6) contains supplementary material, which is available to authorized users.
Keywords: Bisphosphonate, Fracture, Men, Network meta-analysis, Osteoporosis
Introduction
Osteoporosis is still an under-recognized problem in men [1]. In a recent updated systematic review summarizing the evidence of pharmacologic treatments to prevent fractures in primary osteoporosis, the author only found one randomized trial of men with osteoporosis designed with a primary fracture reduction outcome [2]. Although the incidence of osteoporosis in men is less frequent compared to that in women, a large number of men have osteoporosis and their health is threatened by this condition. Approximately 20% of all clinical vertebral fractures and 30% of all hip fractures occur in men, but mortality in men with vertebral or hip fractures is significantly higher than in women [3–6].
In more than 50% of men with osteoporosis, the disease is the result of an identifiable cause that results in bone loss and bone fragility. The most common causes of secondary osteoporosis in men are glucocorticoid excess, hypogonadism, and excessive alcohol consumption, which are believed to cause bone resorption to outweigh bone formation, resulting in bone loss and fractures in men [3, 7, 8]. Men also suffer bone loss naturally, which is more common with deficient testosterone or estradiol level and is accelerated after the age of 70 years [1].
Three bisphosphonates, namely alendronate, risedronate, and zoledronic acid, are recommended for treating men with osteoporosis in the 2012 clinical practice guideline of the Endocrine Society [9]. Although bisphosphonates cannot remove the secondary causes, they may prevent bone loss and fractures by inhibiting bone resorption [10]. Bisphosphonates have positive effects on bone mass density and bone biomarkers, and reduce vertebral fractures in men with osteoporosis [11].
In order to clarify the comparative efficacy of different bisphosphonates in preventing fractures, several network meta-analyses and multiple treatment analyses were carried out [12–14]. However, none of them specifically addressed men with osteoporosis and these studies primarily focused on the use of bisphosphonates in treating postmenopausal osteoporosis. Therefore, we performed this systematic review with network meta-analyses to evaluate the comparative efficacy of bisphosphonates in men with osteoporosis. We report the outcomes for osteoporosis with different causes separately, since patients with different types of osteoporosis may respond to bisphosphonates differently.
Methods
Search Strategy
We searched for randomized controlled trials in the Cochrane Library, Embase, PubMed, and ClinicalTrials.gov. Our search terms, combining osteoporosis and bisphosphonates, consisted of medical subject headings and text keywords of “osteoporosis”, “alendronate”, “clodronate”, “etidronate”, “ibandronate”, “minodronate”, “neridronate”, “olpadronate”, “pamidronate”, “risedronate”, “tiludronic acid”, and “zoledronic acid”. This search strategy was amended for each database. We searched each database from inception until December 27, 2015 (date of final search). We also manually searched the references of cited articles. Supplement 1 includes the systematic search strategy.
Selection Criteria
Studies meeting all the following criteria were included: (1) randomized controlled trials that enrolled men with osteoporosis; (2) reported fracture events; (3) a comparison of bisphosphonates against other bisphosphonates or placebo; (4) full-text publication or clinical trials that reported results. Trials that enrolled men with osteoporosis related to cancer were excluded, as fracture in cancer-associated bone disease may result from causes other than osteoporosis [15]. Two independent reviewers (JZ and XZ) worked together to screen the titles and abstracts of all initially identified studies according to the selection criteria.
Outcome Measurement
The primary outcome was new vertebral fracture. Vertebral fracture outcomes assessed by quantitative morphometric (QM) or semiquantitative (SQ) measurements were collected. For the QM measurents, vertebral fracture was defined by using ratios derived from direct vertebral body height measurements. For the SQ measurements, vertebral fracture was defined according to height and area reduction with the help of visual grading [16]. If both measurements were used in the assessment of vertebral fracture, we preferred the data with the higher rate of reported fractures. The secondary outcome was non-vertebral fracture. Non-vertebral fracture included all fractures reported other than vertebral fracture. Hypogonadism-induced osteoporosis and primary osteoporosis were considered the same type of osteoporosis here.
Data Extraction and Quality Assessment
Two reviewers (JZ, XZ) independently extracted baseline data and assessed the studies’ methodological quality using the risk of quality assessment tool recommended by the Cochrane Handbook for randomized controlled trials [17]. The authors considered random sequence generation, allocation concealment, blinding of participants, caregivers, fracture outcome assessors, incomplete information, selective reporting, and other bias. The criteria were all classified into low, high, or unclear risk of bias on the basis of guidance from The Cochrane Collaboration [17].
Data Synthesis and Analysis
We excluded comparisons with zero events in both groups from the relevant analysis since such comparisons provided no information on the magnitude of the treatment effect [18].
We conducted network meta-analyses with a frequentist model [19–21] using STATA release 13.1 [22]. We based direct probability statements on 50,000-simulation iterations to identify the best and most representative data, assuming comparable interstudy variances for all treatment effects for the same outcomes. The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter (τ 2) estimated from the network meta-analysis models [23]. Inconsistency was checked if a comparison loop existed [24–26]. We included the randomized but open-label studies in the network meta-analyses for sensitivity analyses. We also performed a sensitivity analyses with a Bayesian model [27] to check on the robustness of the network meta-analyses.
Pairwise meta-analyses were performed in Review Manager 5.2 using the random effect model for each outcome comparing each bisphosphonate with placebo. For outcomes in which studies reported zero events in one treatment arm, we added 0.5 to the numerator and 1 to the denominator. Results were expressed as an odds ratio (OR) with 95% confidence intervals (CI). We assessed and quantified heterogeneity using Chi2 test and I 2 statistic computed in this software.
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
After screening 2653 citations and 683 clinical trials (Fig. 1), we included 10 studies [28–37], ten of which reported vertebral fractures and five reported non-vertebral fractures. Patients who were included were treated with alendronate, ibandronate, risedronate, zoledronic acid, or placebo. They were classified into patients with primary osteoporosis, corticosteroid-induced osteoporosis, and osteoporosis with Parkinson disease. Two studies were head-to-head comparison trials: One compared zoledronic acid with risedronate [30] and the other compared zoledronic acid with alendronate [36]. Characteristics of the included studies are summarized in Table 1. Two randomized but open-label studies [38, 39] were included in the sensitivity analyses.
Fig. 1.
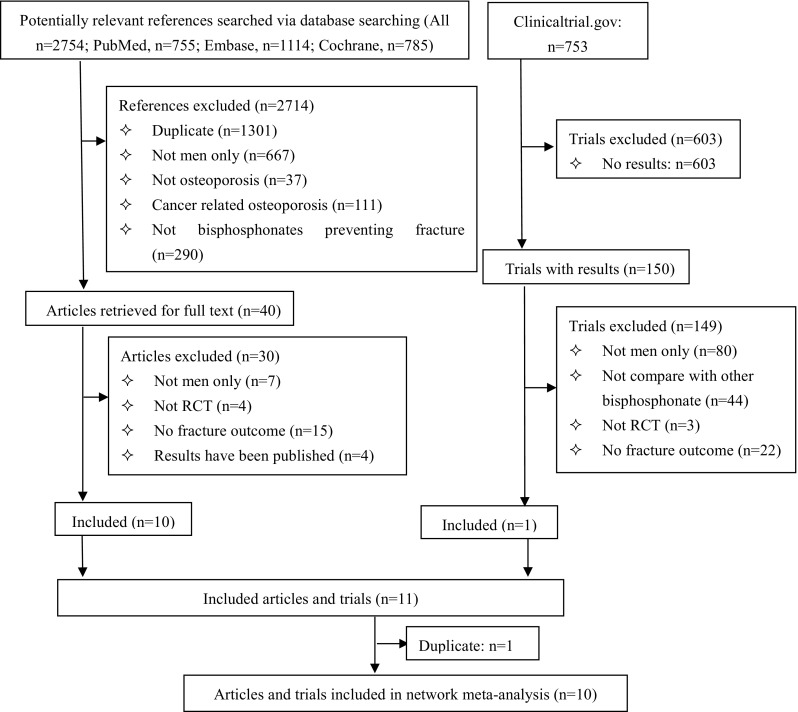
Review profile
Table 1.
Key features of included studies
| Study/year | Osteoporosis | Treatment | Dosage | Follow-up | Number of patients | Mean age (years) | T score (LA-BMD) | T score (FN-BMD) | Prevalent fracturea | Background treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Orwoll 2000 [28] | Primary | Alendronate | 10 mg daily | 24 | 146 | 63 | −2.1 | −2.3 | 51% | 500 mg calcium, 400–450 IU vitamin D |
| Boonen 2009 [29] | Primary | Risedronate | 35 mg weekly | 24 | 191 | 61 | −3.2 | −2.0 | 35% | 1000 mg calcium, 400–500 IU vitamin D |
| Orwoll 2010 [30] | Primary | Zoledronic acid | 5 mg yearly | 24 | 154 | 64 | −2.5 | NA | 67%b | 1000 mg calcium, 800–1000 IU vitamin D |
| Alendronate | 70 mg weekly | 148 | ||||||||
| Orwoll 2010 [31] | Primary | Ibandronate | 150 mg monthly | 12 | 87 | 64 | −2.1 | −2.3 | 40%c | 1000 mg calcium, 400 IU vitamin D3 |
| Boonen 2012 [32] | Primary | Zolendronic acid | 5 mg yearly | 24 | 588 | 66 | NA | −2.2 | 32% | 1000–1500 mg calcium, 800–1200 IU vitamin D |
| Saag 1998 [33] | Corticosteroid-induced | Alendronate | 5−10 mg daily | 12 | 89 | 55 | (−2, −1) | NA | 16% | 800–1000 mg calcium, 250–500 IU vitamin D |
| Cohen 1999 [34] | Corticosteroid-induced | Risedronate | 2.5–5 mg daily | 12 | 25 | 59 | −0.5 | NA | 30% | 500 mg calcium. up to 500 IU vitamin D |
| Reid 2000 [35] | Corticosteroid-induced | Risedronate | 2.5–5 mg daily | 12 | 36 | 57 | −1.7 | NA | 40% | 1000 mg calcium, 400 IU vitamin D |
| Sambrook 2012 [36] | Corticosteroid-induced | Zoledronic acid | 5 mg yearly | 12 | 131 | 56 | −1.1 | NA | 42%b | 1000 mg calcium, 400–1200 IU vitamin D |
| Risedronate | 5 mg daily | 134 | ||||||||
| Sato 2007 [37] | Osteoporosis with Parkinson | Risedronate | 2.5 mg daily | 24 | 121 | 71 | <−2.5 | NA | 37%d | 1000 IU ergocalciferol |
NA not available, LS-BMD lumbar spine bone mineral density, FN-BMD femoral neck bone mineral density
aVertebral fracture
bAny fracture
cNon-vertebral fracture
dFall
Overall, low risk of bias was identified (Supplement 2). Two studies [34, 35] were at high risk of bias for incomplete outcome data because of high rate of loss to follow-up. One study [35] was at unclear risk of bias in outcome assessment because of not mentioning the blinding and another three [28, 29, 35] in other bias because of the sponsorship by manufacturers in the studies. Others were at low risk of bias.
Table 2 summarizes the comparative efficacy of bisphosphonates versus placebo and the ranking of different bisphosphonates. Only one pairwise meta-analysis comparing risedronate and placebo for corticosteroid-induced osteoporosis was performed, since this is the only comparison including two trials contributing data. Network meta-analyses were performed for vertebral and non-vertebral fractures in primary osteoporosis, and for vertebral fracture in corticosteroid-induced osteoporosis (Table 3). Forest plots for network meta-analyses are provided in Supplements 3 and 4.
Table 2.
Summary of the outcomes
| No. of studies contributing data | Size | Pairwise (OR) | Network (OR) | Ranking | |
|---|---|---|---|---|---|
| Primary osteoporosis VF | |||||
| Zoledronic acid | 1 | 1199 | 0.32 (0.15, 0.69) | 0.23 (0.05, 1.06) | 1 |
| Risedronate | 1 | 284 | 2.47 (0.12, 51.91) | 2.47 (0.09, 69.00) | 4 |
| Alendronate | 1 | 241 | 0.09 (0.01, 0.72) | 0.22 (0.03, 1.55) | 2 |
| Ibandronate | 1 | 133 | 0.26 (0.02, 3.00) | 0.26 (0.02, 4.25) | 3 |
| Primary osteoporosis NVF | |||||
| Zoledronic acid | 1 | 1199 | 0.65 (0.21, 1.99) | 0.62 (0.11, 3.37)a | 2 |
| Risedronate | 1 | 284 | 0.55 (0.18, 1.69) | 0.53 (0.10, 2.99)a | 1 |
| Alendronate | 1 | 241 | 0.77 (0.23, 2.60) | 0.78 (0.13, 4.65)a | 3 |
| Ibandronate | 1 | 133 | 2.81 (0.13, 59.77) | NAb | 4 |
| Corticosteriod-induced osteoporosis VF | |||||
| Zoledronic acid | 0 | 0 | NA | 0.34 (0.02, 5.34) | 2 |
| Risedronate | 2 | 188 | 0.15 (0.04, 0.57) | 0.15 (0.04, 0.58) | 1 |
| Alendronate | 1 | 130 | 0.60 (0.04, 9.82) | 0.60 (0.04, 9.82) | 3 |
| Osteoporosis with PD NVF | |||||
| Risedronate | 1 | 242 | 0.32 (0.08, 1.20) | NA | NA |
VF vertebral fracture, NVF non-vertebral fracture, PD Parkinson disease, NA not available
aResults from ADDIS with Bayesian model because the included studies were not adequate enough to perform the network meta-analysis using STATA with meta-regression model
bUnstable data with 0 events happen when performing network meta-analysis using Bayesian model
Table 3.
Results of network meta-analyses
| Placebo | Zoledronic acid | Risedronate | Alendronate | Ibandronate | |
|---|---|---|---|---|---|
| Vertebral fracture in men with primary osteoporosis | |||||
| Ibandronate | 0.26 (0.02, 4.25) | 1.16 (0.05, 27.77) | 0.11 (0.00, 8.20) | 1.22 (0.04, 36.48) | – |
| Alendronate | 0.22 (0.03, 1.55) | 0.95 (0.19, 4.68) | 0.09 (0.00, 4.21) | – | – |
| Risedronate | 2.47 (0.09, 69.00) | 10.82 (0.28, 424.62) | – | ||
| Zoledronic acid | 0.23 (0.05, 1.06) | – | |||
| Placebo | – | ||||
| Non-vertebral fracture in men with primary osteoporosisa | |||||
| Ibandronate | NAb | NAb | NAb | NAb | – |
| Alendronate | 0.78 (0.13, 4.65) | 1.25 (0.12, 14.67) | 1.40 (0.13, 15.86) | – | – |
| Risedronate | 0.53 (0.10, 2.99) | 0.85 (0.08, 8.98) | – | ||
| Zoledronic acid | 0.63 (0.11, 3.37) | – | |||
| Placebo | – | ||||
| Vertebral fracture in men with corticosteroid-induced osteoporosis | |||||
| Alendronate | 0.60 (0.04, 9.82) | 1.79 (0.04, 91.17) | 4.00 (0.18, 89.08) | – | – |
| Risedronate | 0.15 (0.04, 0.58)* | 0.45 (0.04, 5.00) | – | ||
| Zoledronic acid | 0.34 (0.02, 5.34) | – | |||
| Placebo | – | ||||
* Statistically significant result
aResults from ADDIS with Bayesian model because the included studies were not adequate enough to perform the network meta-analysis using STATA with meta-regression model
bUnstable data with 0 events happen when performing network meta-analysis using Bayesian model
Primary Osteoporosis
In the network meta-analyses for vertebral fracture, no significant difference between any pairs of bisphosphonates was found. Compared with placebo, zoledronic acid, alendronate, and ibandronate prevented vertebral fracture [OR with 95% CI 0.23 (0.05, 1.06), 0.22 (0.03, 1.55), and 0.26 (0.02, 4.25), respectively], but all with insignificant difference. Significant heterogeneity was found in the network meta-analysis (τ 2 = 0.68). One loop existed in the network meta-analysis for vertebral fracture and no significant inconsistency was found (P = 0.17). In the probability ranking order, zoledronic acid ranked as the most effective agent in preventing vertebral fracture. On the basis of one single trial, we found that zoledronic acid and alendronate prevented vertebral fractures in primary osteoporosis significantly [0.32 (0.15, 0.69) and 0.09 (0.01, 0.72), respectively].
In the network meta-analysis for non-vertebral fracture, no significant difference between any pairs of bisphosphonates was found. Compared with placebo, zoledronic acid, risedronate, and alendronate prevented non-vertebral fracture [0.62 (0.11, 3.37), 0.53 (0.10, 2.99), and 0.78 (0.13, 4.65), respectively], but all with insignificant difference. Heterogeneity and inconsistency were not checked, since no more than two trials for the same comparison were included and no loop existed. In the probability ranking order, risedronate ranked the highest in preventing non-vertebral fracture.
Secondary Osteoporosis
For corticosteroid-induced osteoporosis, no significant difference between any pairs of bisphosphonates was found for vertebral fracture in the network meta-analysis. Compared with placebo, risedronate significantly prevented vertebral fracture [0.15 (0.04, 0.58)], and zoledronic acid and alendronate insignificantly prevented vertebral fracture [0.34 (0.02, 5.34) and 0.60 (0.04, 9.82), respectively]. No significant heterogeneity was found (τ 2 = 0.00). One loop existed in this network meta-analysis and no significant inconsistency was found (P = 0.51). In the probability ranking order, risedronate ranked the highest in preventing vertebral fracture in corticosteroid-induced osteoporosis. On the basis of the meta-analysis, we also found that risedronate significantly prevented vertebral fracture in corticosteroid-induced osteoporosis [0.15 (0.04, 0.57), P = 0.68, I 2 = 0].
For osteoporosis with Parkinson disease, only one trial comparing risedronate with placebo could be included. It found that risedronate prevented non-vertebral fracture with statistically insignificant efficacy [0.32 (0.08, 1.20)].
In the sensitivity analyses including the open-label studies, results were consistent in showing that zoledronic acid, risedronate, and alendronate significantly prevented vertebral fracture in primary osteoporosis [0.29 (0.15, 0.57), 0.37 (0.20, 0.72), and 0.33 (0.15, 0.70), respectively] and risedronate significantly prevented non-vertebral fracture in corticosteroid-induced osteoporosis [0.50 (0.29, 0.86)]. In the sensitivity analyses using Bayesian model, we found that alendronate instead of zoledronic acid ranked best in preventing vertebral fracture in primary osteoporosis (Supplement 5).
Discussion
In this systematic review, we summarized the comparative efficacy of preventing fracture with bisphosphonates in men with osteoporosis by integrating all available direct and indirect evidence. We found that zoledronic acid had the highest probability to rank best in preventing vertebral fracture in primary osteoporosis, and risedronate had the highest probability to rank best in preventing non-vertebral fracture in both primary osteoporosis and corticosteroid-induced osteoporosis. Our summary of the results also shows that the available eligible studies were inadequate to have high confidence in results. More well-designed studies focusing on bisphosphonates treating men with osteoporosis are needed.
Studies focused on osteoporosis with different causes were not combined in the network meta-analyses to minimize heterogeneity. Except for evaluating primary osteoporosis and corticosteroid-induced osteoporosis, we also found one study showing that risedronate significantly prevents non-vertebral fracture in men with osteoporosis and Parkinson disease [37]. Although cancer-related osteoporosis was not considered in our analysis, as fracture in cancer-associated bone disease may result from causes other than osteoporosis [15], other meta-analyses [40, 41] found that zoledronic acid was effective in preventing fractures for patients under androgen deprivation therapy for prostate cancer and nonmetastatic prostate cancer. No other randomized controlled study investigating the efficacy of bisphosphonates in men with osteoporosis was found.
Network meta-analyses focusing on the use of bisphosphonates in treating postmenopausal women should be considered when treating men with osteoporosis using bisphosphonates. Compared with our study, these network meta-analyses have a larger sample size, therefore the estimates could be more precise to show comparative efficacy among different bisphosphonates. The study by Jansen et al. [13] found that zoledronic acid and risedronate ranked the highest in preventing vertebral fracture and non-vertebral fracture, respectively, in postmenopausal women, which is consistent with our systematic review regarding their efficacy in men. Until adequate evidence is available to better evaluate the comparative efficacy of preventing fracture with bisphosphonates in men, we can also refer to the available studies for postmenopausal women.
Our study has a few improvements compared with the similar network meta-analysis [42] comparing eight drugs in treating men with osteoporosis. Different from the cited analysis, our study exclusively focused on bisphosphonates, excluding strontium ranelate, teriparatide, and parathyroid hormone, which have different mechanisms of preventing fracture than bisphosphonates. In addition, our study separated osteoporosis into primary osteoporosis, corticosteroid-induced osteoporosis, and osteoporosis with Parkinson disease. Also, we separated fracture into vertebral fracture and non-vertebral fracture. With these approaches, our analysis has lower heterogeneity and fewer confounding factors.
Limitations exist in this study. Firstly, our estimates have uncertainty. The evidence for using bisphosphonates to treat men with osteoporosis is inadequate, and the sample sizes of the eligible studies are mostly small. Secondly, we did not include some studies of bisphosphonates preventing fracture in men [43, 44], because women were also included in these studies and we could not extract the data of men only. Thirdly, somewhat different assessment criteria of vertebral fracture were applied in included studies. Semiquantitative methods and quantitative morphometric methods [16] were assumed equally sensitive in our meta-analysis. Last, heterogeneity from the study design may exist as only two studies considered the outcome of fracture as their primary outcome, while others considered the outcome of fracture as a secondary outcome.
Conclusion
On the basis of the current evidence, zoledronic acid is most effective at preventing vertebral fractures, while risedronate has the highest possibility to rank the first in preventing non-vertebral fracture in men with primary osteoporosis and corticosteroid-induced osteoporosis. More well-designed studies are needed to support our findings and to better know the comparative efficacy of bisphosphonate to prevent vertebral fracture in men with osteoporosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All the named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Junwen Zhou, Tiansheng Wang, Xilan Zhao, Donald R. Miller, and Suodi Zhai have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhance content for this article go to www.medengine.com/Redeem/3B84F06077C3C989.
Electronic supplementary material
The online version of this article (doi:10.1007/s40744-016-0030-6) contains supplementary material, which is available to authorized users.
References
- 1.Ebeling PR. Osteoporosis in men. Curr Opin Rheumatol. 2013;25(4):542–552. doi: 10.1097/BOR.0b013e328362164d. [DOI] [PubMed] [Google Scholar]
- 2.Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fracture: an updated systematic review. Ann Intern Med. 2014;161(10):711–723. doi: 10.7326/M14-0317. [DOI] [PubMed] [Google Scholar]
- 3.Gielen E, Vanderschueren D, Callewaert F, et al. Osteoporosis in men. Best Pract Res Clin Endocrinol Metab. 2011;25:321–335. doi: 10.1016/j.beem.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Atkinsson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 5.Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 6.Cree M, Soskolne CL, Belseck E, et al. Mortality and institutionalization following hip fracture. J Am Geriatr Soc. 2000;48:283–288. doi: 10.1111/j.1532-5415.2000.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 7.Walsh JS, Eastell R. Osteoporosis in men. Nat Rev Endocrinol. 2013;9:637–645. doi: 10.1038/nrendo.2013.171. [DOI] [PubMed] [Google Scholar]
- 8.Drake MT, Murad MH, Mauck KF, et al. Risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1861–1870. doi: 10.1210/jc.2011-3058. [DOI] [PubMed] [Google Scholar]
- 9.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–1822. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman JM, Lapauw B, Goemaere S. Current and future treatments of osteoporosis in men. Best Pract Res Clin Endocrinol Metab. 2014;28:871–884. doi: 10.1016/j.beem.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Giusti A, Bianchi G. Treatment of primary osteoporosis in men. Clin Interv Aging. 2015;10:105–115. doi: 10.2147/CIA.S44057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen JP, Bergman GJ, Huels J, Olson M. Prevention of vertebral fractures in osteoporosis: mixed treatment comparison of bisphosphonate therapies. Curr Med Res Opin. 2009;25(8):1861–1868. doi: 10.1185/03007990903035281. [DOI] [PubMed] [Google Scholar]
- 13.Jansen JP, Bergman GJ, Huels J, Olson M. The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Semin Arthritis Rheum. 2011;40(4):275–284.e2. doi: 10.1016/j.semarthrit.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Migliore A, Broccoli S, Massafra U, Cassol M, Frediani B. Ranking antireabsorptive agents to prevent vertebral fractures in postmenopausal osteoporosis by mixed treatment comparison meta-analysis. Eur Rev Med Pharmacol Sci. 2013;17(5):658–667. [PubMed] [Google Scholar]
- 15.Rizzoli R, Body J, Brandi L, et al. Cancer-associated bone disease. Osteoporos Int. 2013;24:2929–2953. doi: 10.1007/s00198-013-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oei L, Rivadeneira F, Ly F, et al. Review of radiological scoring methods of osteoporotic vertebral fractures for clinical and research settings. Eur Radiol. 2013;23(2):476–486. doi: 10.1007/s00330-012-2622-z. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Sterne JAC, editors. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration. 2011; http://www.cochrane-handbook.org/. Accessed 27 March 2015.
- 18.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 19.White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analsyis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3:111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White IR. Multivariate random-effects meta-regression: updates to mvmeta. STATA J. 2011;11:255–270. [Google Scholar]
- 22.Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41(3):818–827. doi: 10.1093/ije/dys041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in metaanalysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 25.Salanti G, Marinho V, Higgins JP. A case study of multipletreatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol. 2009;62(8):857–864. doi: 10.1016/j.jclinepi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network metaanalysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ADDIS 1.16.6. http://drugis.org/software/addis1/index. Accessed 14 Oct 2015.
- 28.Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 29.Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res. 2009;24(4):719–725. doi: 10.1359/jbmr.081214. [DOI] [PubMed] [Google Scholar]
- 30.Orwoll ES, Miller PD, Adachi JD, et al. Efficacy and safety of a once-yearly iv. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res. 2010;25(10):2239–2250. doi: 10.1002/jbmr.119. [DOI] [PubMed] [Google Scholar]
- 31.Orwoll ES, Binkley NC, Lewiecki EM, et al. Efficacy and safety of monthly ibandronate in men with low bone density. Bone. 2010;46:970–976. doi: 10.1016/j.bone.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367(18):1714–1723. doi: 10.1056/NEJMoa1204061. [DOI] [PubMed] [Google Scholar]
- 33.Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. New Eng J Med. 1998;339(5):292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Levy RM, Keller M, et al. Risedronate therapy prevents corticosteroid-induced bone loss. Arthritis Rheum. 1999;42(11):2309–2318. doi: 10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. J Bone Miner Res. 2000;15(6):1006–1013. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook PN, Roux C, Devogelaer JP, et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone. 2012;50:289–295. doi: 10.1016/j.bone.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Sato Y, Honda Y, Iwamoto J. Risedronate and ergocalciferol prevent hip fracture in elderly men with Parkinson disease. Neurology. 2007;68(12):911–915. doi: 10.1212/01.wnl.0000257089.50476.92. [DOI] [PubMed] [Google Scholar]
- 38.Ringe JD, Farahmand P, Faber H, et al. Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int. 2009;29(3):311–315. doi: 10.1007/s00296-008-0689-2. [DOI] [PubMed] [Google Scholar]
- 39.Ringe JD, Farahmand P, Faber H, et al. Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int. 2009;29(3):311–315. doi: 10.1007/s00296-008-0689-2. [DOI] [PubMed] [Google Scholar]
- 40.Neto AS, Tobias-Machado M, Esteves MAP, et al. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2012;15:36–44. doi: 10.1038/pcan.2011.4. [DOI] [PubMed] [Google Scholar]
- 41.Ding H, Yang L, Du W, et al. Bisphosphonates for osteoporosis in nonmetastatic prostate cancer patients receiving androgen-deprivation therapy: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2013;14:3337–3343. doi: 10.7314/APJCP.2013.14.5.3337. [DOI] [PubMed] [Google Scholar]
- 42.Chen LX, Zhou ZR, Li YL, et al. Comparison of bone mineral density in lumbar spine and fracture rate among eight drugs in treatments of osteoporosis in men: a network meta-analysis. PLoS One. 2015;10(5):e0128032. doi: 10.1371/journal.pone.0128032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura T, Nakano T, Ito M, et al. Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int. 2013;93:137–146. doi: 10.1007/s00223-013-9734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


