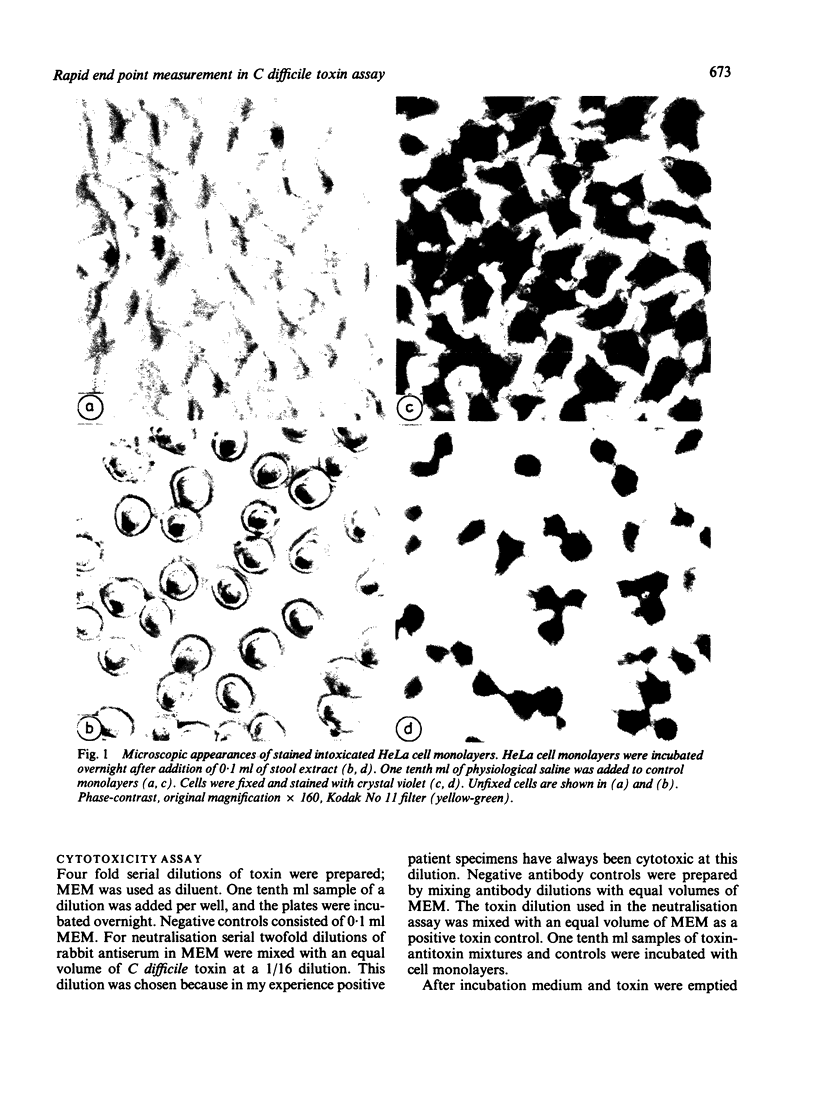
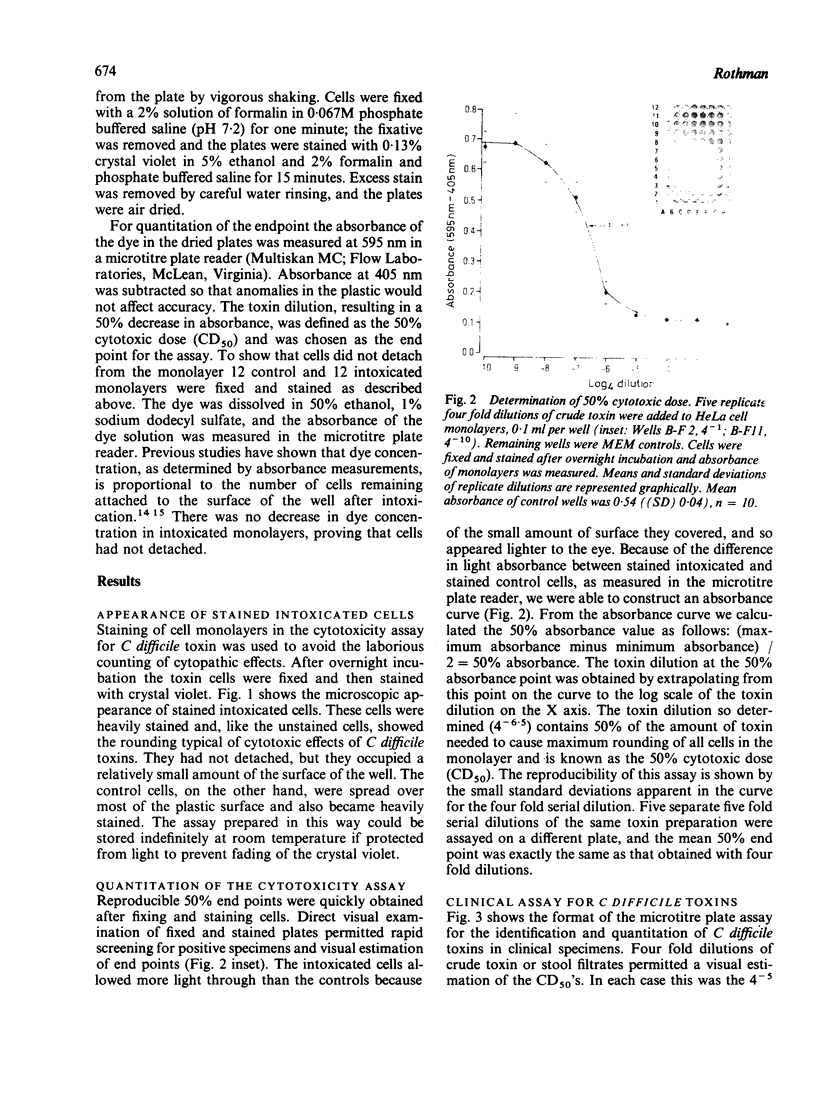
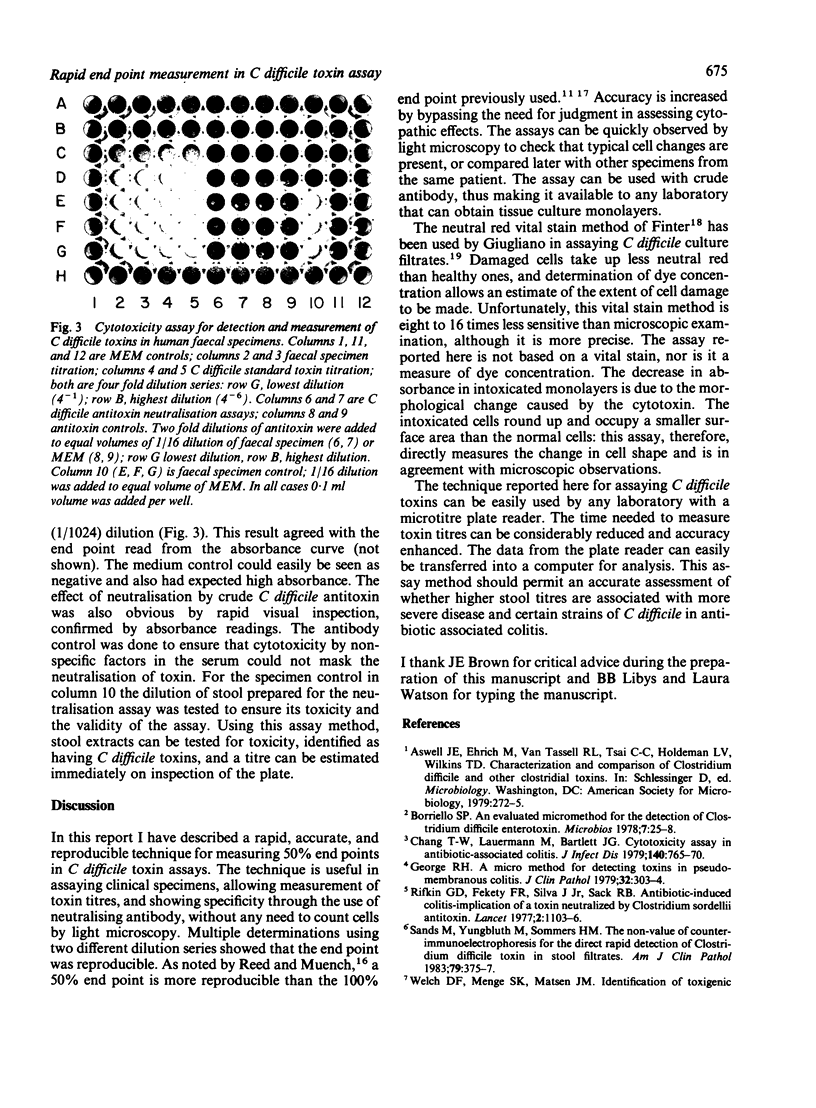
Abstract
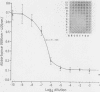
Serial dilutions of Clostridium difficile culture filtrates were incubated overnight with HeLa cell monolayers. Cells were fixed in formalin, stained with crystal violet, rinsed, and drained. Cell rounding could be observed microscopically in the stained monolayers. Absorbance of the retained dye on monolayers in the drained wells was measured at 595 nm-405 nm. End points could also be estimated visually. The dilution at which dye absorbance was reduced by 50% agreed with that determined by microscopic observations. Five replicate dilution series showed high reproducibility. Specificity was verified by neutralisation with crude rabbit antibody to C difficile toxins. Cytotoxicity in faecal specimens was assayed in the same way, allowing reporting of titres, comparison with standard toxin preparations, and determination of the extent of neutralisation to be made. This novel assay technique has proved effective and reliable in a clinical setting and should allow the gathering of more information on the epidemiology of antibiotic associated colitis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Griffin D. E., Rothman S. W., Doctor B. P. Purification and biological characterization of shiga toxin from Shigella dysenteriae 1. Infect Immun. 1982 Jun;36(3):996–1005. doi: 10.1128/iai.36.3.996-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Rothman S. W., Doctor B. P. Inhibition of protein synthesis in intact HeLa cells by Shigella dysenteriae 1 toxin. Infect Immun. 1980 Jul;29(1):98–107. doi: 10.1128/iai.29.1.98-107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Lauermann M., Bartlett J. G. Cytotoxicity assay in antibiotic-associated colitis. J Infect Dis. 1979 Nov;140(5):765–770. doi: 10.1093/infdis/140.5.765. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H. A micro-method for detecting toxins in pseudomembranous colitis. J Clin Pathol. 1979 Mar;32(3):303–304. doi: 10.1136/jcp.32.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Viscidi R. P., Gdovin S. L., Yolken R. H., Bartlett J. G. Enzyme immunoassays for detection of Clostridium difficile toxins A and B in fecal specimens. J Infect Dis. 1984 May;149(5):781–788. doi: 10.1093/infdis/149.5.781. [DOI] [PubMed] [Google Scholar]
- Rifkin G. D., Fekety F. R., Silva J., Jr Antibiotic-induced colitis implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977 Nov 26;2(8048):1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- Rothman S. W., Brown J. E., Diecidue A., Foret D. A. Differential cytotoxic effects of toxins A and B isolated from Clostridium difficile. Infect Immun. 1984 Nov;46(2):324–331. doi: 10.1128/iai.46.2.324-331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands M., Yungbluth M., Sommers H. M. The non-value of counterimmunoelectrophoresis for the direct rapid detection of Clostridium difficile toxin in stool filtrates. Am J Clin Pathol. 1983 Mar;79(3):375–377. doi: 10.1093/ajcp/79.3.375. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Bryan L. E., Gaffney D., Coderre S. E., Gordon R., Pai C. H. Latex agglutination test for detection of Clostridium difficile toxin in stool samples. J Clin Microbiol. 1984 Sep;20(3):339–341. doi: 10.1128/jcm.20.3.339-341.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D. F., Menge S. K., Matsen J. M. Identification of toxigenic Clostridium difficile by counterimmunoelectrophoresis. J Clin Microbiol. 1980 May;11(5):470–473. doi: 10.1128/jcm.11.5.470-473.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Wilkins T. D. Problems associated with counterimmunoelectrophoresis assays for detecting Clostridium difficile toxin. J Clin Microbiol. 1982 Feb;15(2):347–349. doi: 10.1128/jcm.15.2.347-349.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]