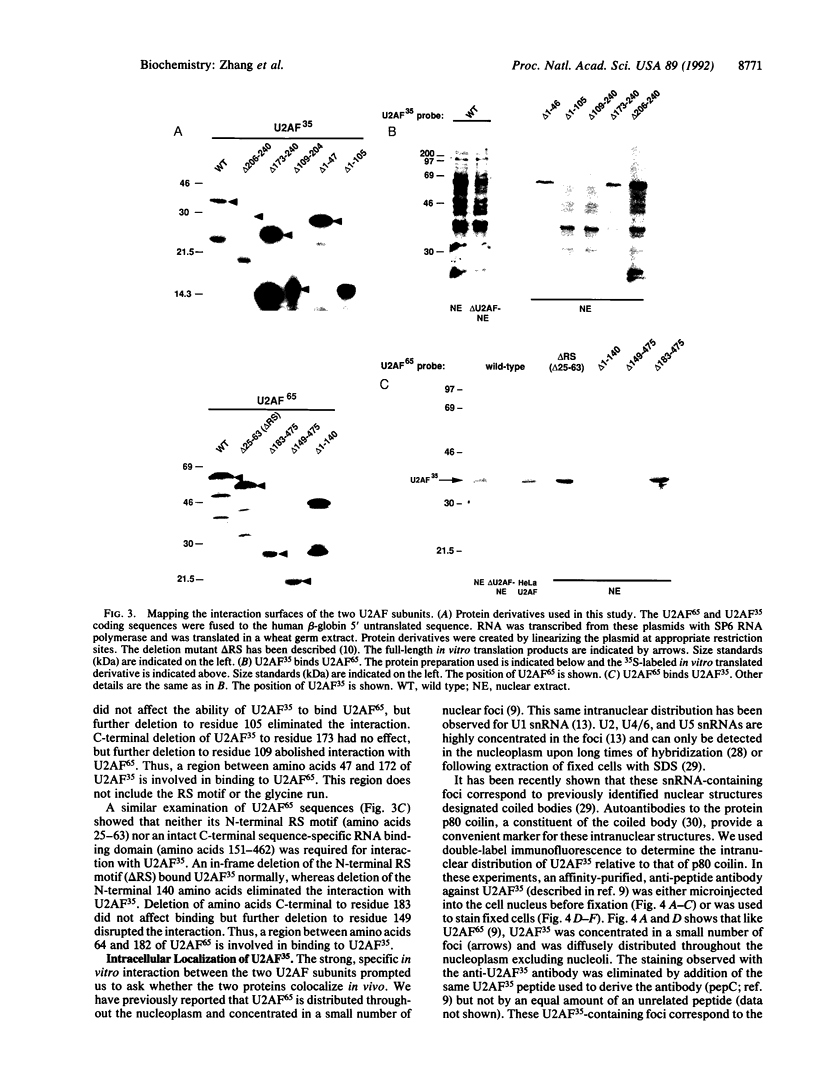
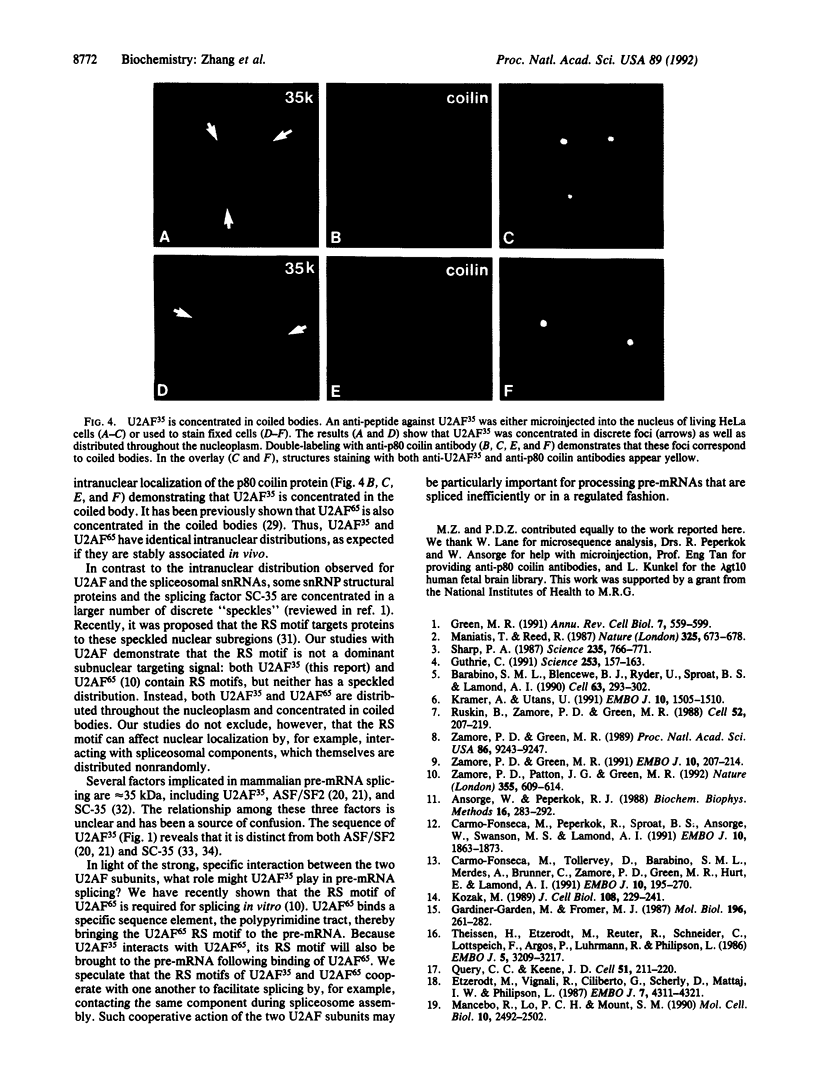
Abstract
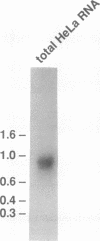
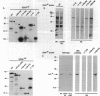
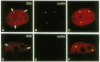
U2 small nuclear ribonucleoprotein auxiliary factor (U2AF), an essential mammalian splicing factor, is composed of two subunits: a 65-kDa protein (U2AF65), which binds the pre-mRNA polypyrimidine tract and is required for in vitro splicing, and an associated 35-kDa protein (U2AF35). Here we report the isolation of a cDNA encoding U2AF35. U2AF35 contains sequence motifs found in several mammalian pre-mRNA splicing factors. We show directly that U2AF65 and U2AF35 interact with each other and delineate the regions of both proteins that mediate this interaction. Using anti-peptide antibodies against U2AF35, we show that the protein has the intracellular distribution characteristic of U2AF65. Both U2AF65 and U2AF35 are concentrated in a small number of nuclear foci corresponding to coiled bodies, subnuclear organelles first identified by light microscopy in 1903.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein H., Gorman M., Nöthiger R. The sex-determining gene tra-2 of Drosophila encodes a putative RNA binding protein. Cell. 1988 Dec 23;55(6):1025–1035. doi: 10.1016/0092-8674(88)90247-4. [DOI] [PubMed] [Google Scholar]
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge W., Pepperkok R. Performance of an automated system for capillary microinjection into living cells. J Biochem Biophys Methods. 1988 Aug;16(4):283–292. doi: 10.1016/0165-022x(88)90062-0. [DOI] [PubMed] [Google Scholar]
- Barabino S. M., Blencowe B. J., Ryder U., Sproat B. S., Lamond A. I. Targeted snRNP depletion reveals an additional role for mammalian U1 snRNP in spliceosome assembly. Cell. 1990 Oct 19;63(2):293–302. doi: 10.1016/0092-8674(90)90162-8. [DOI] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Sproat B. S., Ansorge W., Swanson M. S., Lamond A. I. In vivo detection of snRNP-rich organelles in the nuclei of mammalian cells. EMBO J. 1991 Jul;10(7):1863–1873. doi: 10.1002/j.1460-2075.1991.tb07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Tollervey D., Pepperkok R., Barabino S. M., Merdes A., Brunner C., Zamore P. D., Green M. R., Hurt E., Lamond A. I. Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J. 1991 Jan;10(1):195–206. doi: 10.1002/j.1460-2075.1991.tb07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. B., Zachar Z., Bingham P. M. Developmental expression of a regulatory gene is programmed at the level of splicing. EMBO J. 1987 Dec 20;6(13):4095–4104. doi: 10.1002/j.1460-2075.1987.tb02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzerodt M., Vignali R., Ciliberto G., Scherly D., Mattaj I. W., Philipson L. Structure and expression of a Xenopus gene encoding an snRNP protein (U1 70K). EMBO J. 1988 Dec 20;7(13):4311–4321. doi: 10.1002/j.1460-2075.1988.tb03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990 Feb 1;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Isolation of a complementary DNA that encodes the mammalian splicing factor SC35. Science. 1992 Apr 24;256(5056):535–538. doi: 10.1126/science.1373910. [DOI] [PubMed] [Google Scholar]
- Gardiner-Garden M., Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987 Jul 20;196(2):261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Ge H., Zuo P., Manley J. L. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991 Jul 26;66(2):373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- Goralski T. J., Edström J. E., Baker B. S. The sex determination locus transformer-2 of Drosophila encodes a polypeptide with similarity to RNA binding proteins. Cell. 1989 Mar 24;56(6):1011–1018. doi: 10.1016/0092-8674(89)90634-x. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Guthrie C. Messenger RNA splicing in yeast: clues to why the spliceosome is a ribonucleoprotein. Science. 1991 Jul 12;253(5016):157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):305–308. doi: 10.1073/pnas.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. R., Mayeda A., Kozak D., Binns G. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell. 1991 Jul 26;66(2):383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- Li H., Bingham P. M. Arginine/serine-rich domains of the su(wa) and tra RNA processing regulators target proteins to a subnuclear compartment implicated in splicing. Cell. 1991 Oct 18;67(2):335–342. doi: 10.1016/0092-8674(91)90185-2. [DOI] [PubMed] [Google Scholar]
- Mancebo R., Lo P. C., Mount S. M. Structure and expression of the Drosophila melanogaster gene for the U1 small nuclear ribonucleoprotein particle 70K protein. Mol Cell Biol. 1990 Jun;10(6):2492–2502. doi: 10.1128/mcb.10.6.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Zamore P. D., Green M. R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988 Jan 29;52(2):207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Theissen H., Etzerodt M., Reuter R., Schneider C., Lottspeich F., Argos P., Lührmann R., Philipson L. Cloning of the human cDNA for the U1 RNA-associated 70K protein. EMBO J. 1986 Dec 1;5(12):3209–3217. doi: 10.1002/j.1460-2075.1986.tb04631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellard M., Sureau A., Soret J., Martinerie C., Perbal B. A potential splicing factor is encoded by the opposite strand of the trans-spliced c-myb exon. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2511–2515. doi: 10.1073/pnas.89.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A., Gibson W., Graves J. P., Sterling J. F., Eisenberg M. T. The Drosophila suppressor of sable gene encodes a polypeptide with regions similar to those of RNA-binding proteins. Mol Cell Biol. 1991 Feb;11(2):894–905. doi: 10.1128/mcb.11.2.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991 Jan;10(1):207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]