Abstract
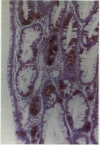
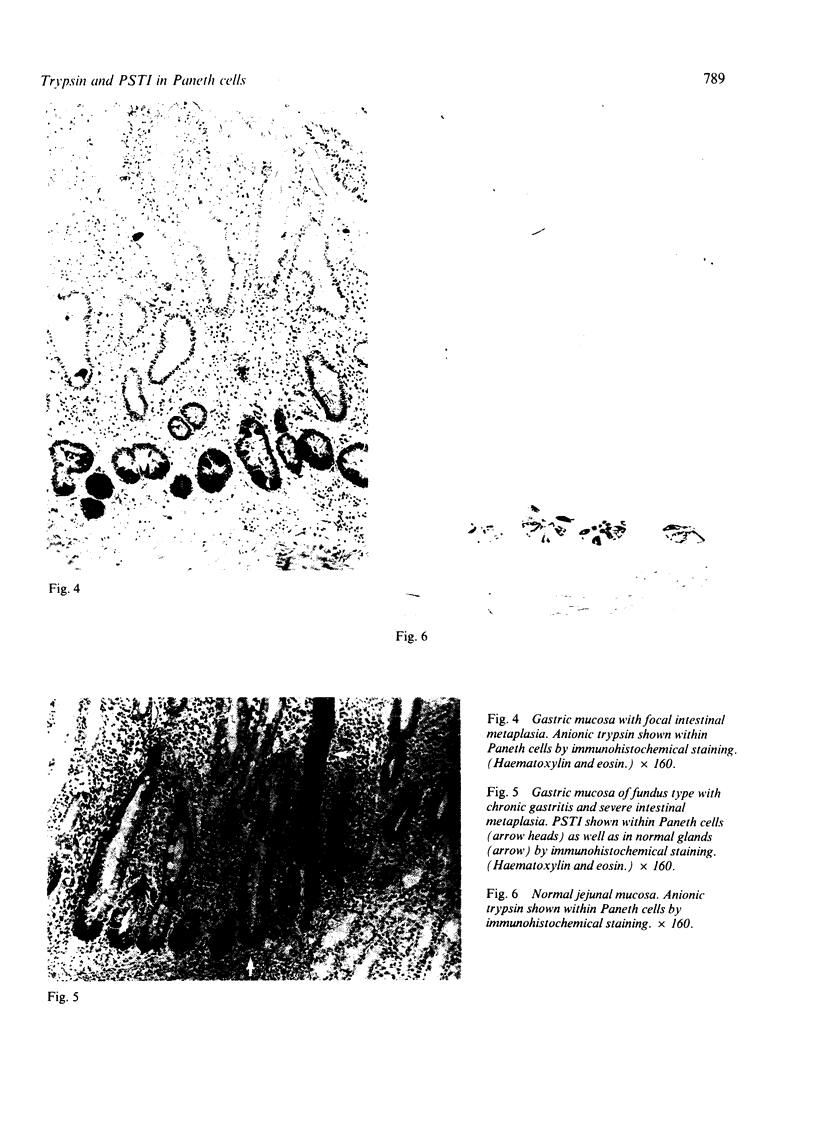
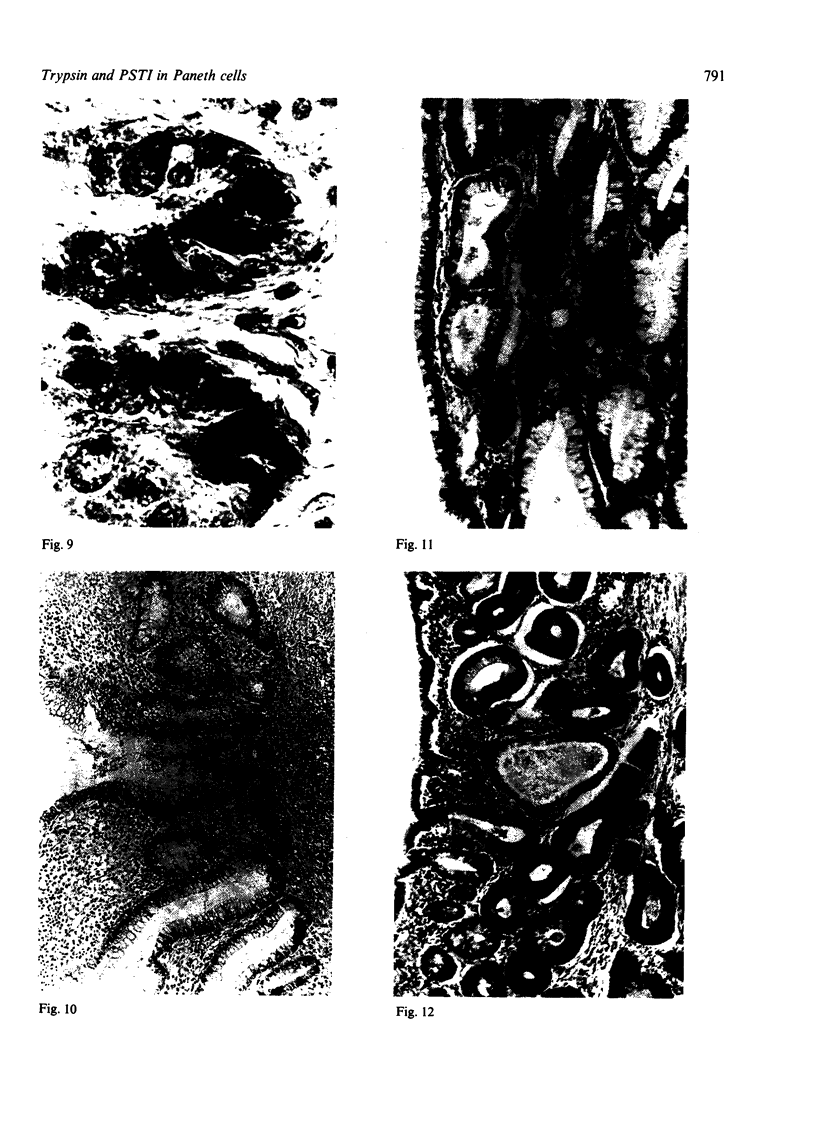
Normal and metaplastic gastrointestinal mucosa obtained at surgical resection were studied by light microscopy, using the unlabelled antibody enzyme method for immunohistochemical staining of lysozyme, pancreatic endoproteases, and pancreatic secretory trypsin inhibitor (PSTI). Paneth cells in the mucosa of normal small intestine, gastric mucosa with intestinal metaplasia, and colonic metaplastic mucosa were found to contain anionic trypsin, cationic trypsin, lysozyme, and PSTI immunoreactivity, but not chymotrypsin and elastase immunoreactivity. Normal gastric and colonic mucosa and some goblet cells in the small intestine showed positive PSTI immunoreactivity but no endoprotease immunoreactivity. The presence of immunoreactive trypsin and immunoreactive PSTI in the Paneth cells, which are of secretory type, probably indicates an important extrapancreatic source of these proteins rather than a storage of endocytosed material.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonen A., Penttilä A. Effect of Trasylol on Paneth cells of the mouse. Experientia. 1975 May 15;31(5):577–578. doi: 10.1007/BF01932467. [DOI] [PubMed] [Google Scholar]
- Balas D., Senegas-Balas F., Bertrand C., Frexinos J., Ribet A. Effects of pancreatic duct ligation on the hamster intestinal mucosa. Histological findings. Digestion. 1980;20(3):157–167. doi: 10.1159/000198435. [DOI] [PubMed] [Google Scholar]
- Bohe M., Borgström A., Genell S., Ohlsson K. Determination of immunoreactive trypsin, pancreatic elastase and chymotrypsin in extracts of human feces and ileostomy drainage. Digestion. 1983;27(1):8–15. doi: 10.1159/000198913. [DOI] [PubMed] [Google Scholar]
- Bohe M., Borgström A., Lindström C., Ohlsson K. Trypsin-like immunoreactivity in human Paneth cells. Digestion. 1984;30(4):271–275. doi: 10.1159/000199119. [DOI] [PubMed] [Google Scholar]
- Eddeland A., Ohlsson K. Purification and immunochemical quantitation of human pancreatic secretory trypsin inhibitor. Scand J Clin Lab Invest. 1978 May;38(3):261–267. doi: 10.1080/00365517809108421. [DOI] [PubMed] [Google Scholar]
- Feinstein G., Hofstein R., Koifmann J., Sokolovsky M. Human pancreatic proteolytic enzymes and protein inhibitors. Isolation and molecular properties. Eur J Biochem. 1974 Apr 16;43(3):569–581. doi: 10.1111/j.1432-1033.1974.tb03444.x. [DOI] [PubMed] [Google Scholar]
- Fryksmark U., Ohlsson K., Polling A., Tegner H. Distribution of antileukoprotease in upper respiratory mucosa. Ann Otol Rhinol Laryngol. 1982 May-Jun;91(3 Pt 1):268–271. doi: 10.1177/000348948209100308. [DOI] [PubMed] [Google Scholar]
- Lewin K. The Paneth cell in disease. Gut. 1969 Oct;10(10):804–811. doi: 10.1136/gut.10.10.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Skude G. Demonstration and semiquantitative determination of complexes between various proteases and human alpha2-macroglobulin. Clin Chim Acta. 1976 Jan 2;66(1):1–7. doi: 10.1016/0009-8981(76)90365-x. [DOI] [PubMed] [Google Scholar]
- Sandow M. J., Whitehead R. The Paneth cell. Gut. 1979 May;20(5):420–431. doi: 10.1136/gut.20.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senegas-Balas F., Bastie M. J., Balas D., Escourrou J., Bommelaer G., Bertrand C., Arany Y., Ribet A. Histological variations of the duodenal mucosa in chronic human pancreatitis. Dig Dis Sci. 1982 Oct;27(10):917–922. doi: 10.1007/BF01316576. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]