Abstract
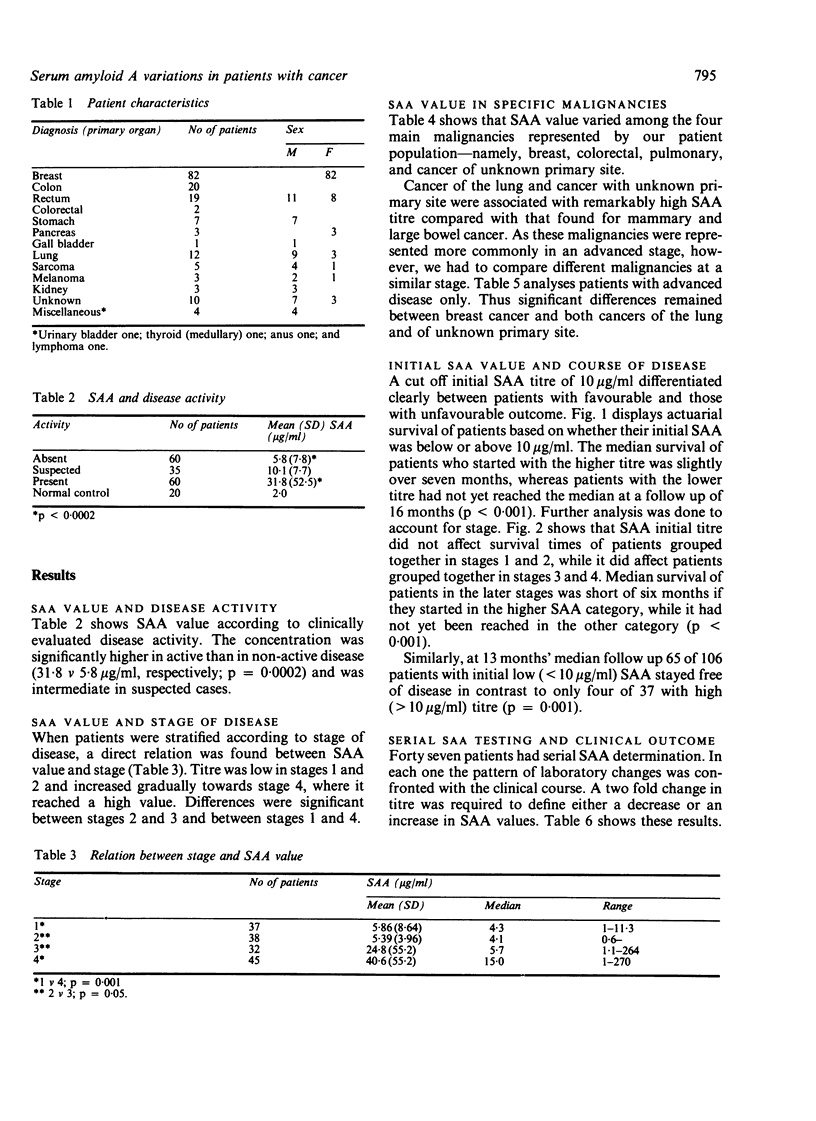
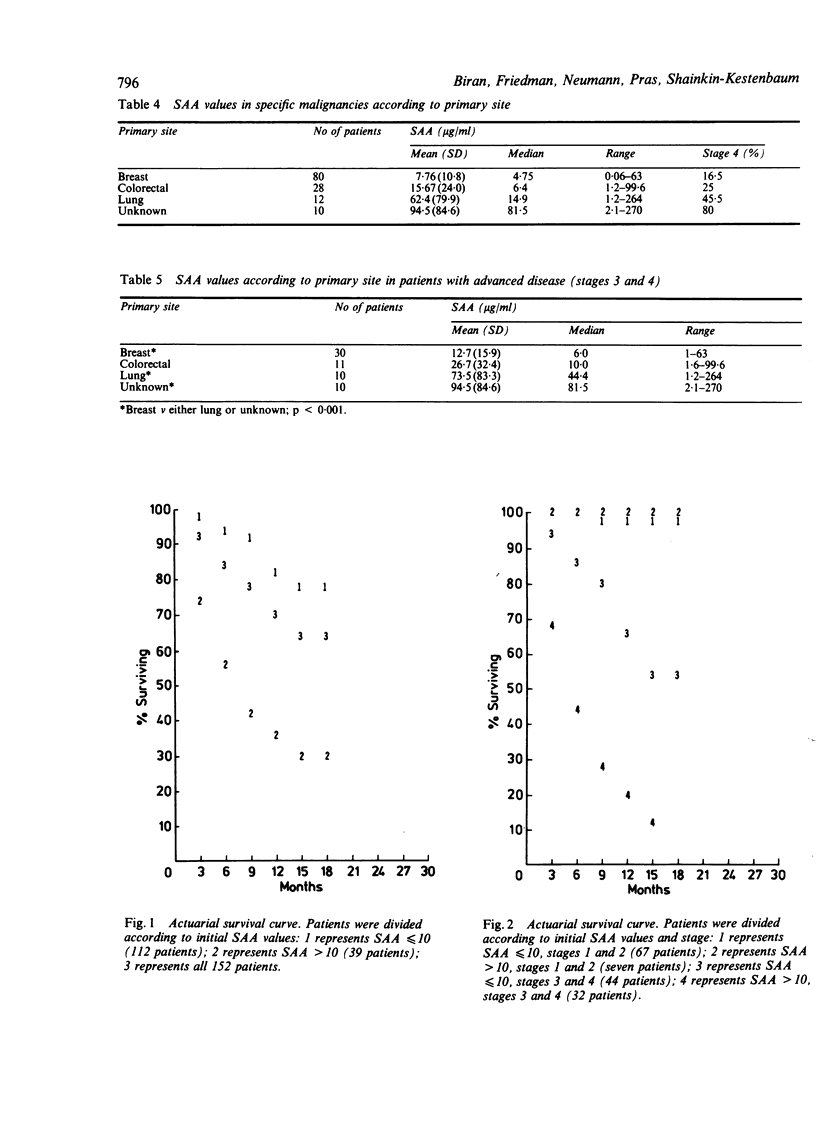
Serum amyloid A (SAA) was determined in 160 patients with cancer. Active disease was associated with high titre compared with the titre in non-active condition (31.8 v 5.8 micrograms/ml, respectively; p = 0.0002). SAA value showed a direct correlation with the stage of the disease: it was lowest at stages 1 and 2 and highest at the metastatic stage 4 (stage 1 v 4, p = 0.001; stage 2 v 3, p = 0.05). Cancers of the lung and unknown primary site were characterised by highly increased SAA concentration. Initial SAA value had prognostic significance: a value below 10 micrograms/ml correlated with survival advantage, whereas a higher initial value indicated a greater likelihood of a poor outcome (actuarial survival analysis p less than 0.001). When stage was accounted for, initial SAA value had significant prognostic bearing on survival of patients with advanced disease (stages 3 and 4) but not on that of patients with limited disease (stages 1 and 2). Serial testing showed good concordance between changes in SAA titre and clinical course.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneti J., Winikoff Y., Zimlichman S., Shainkin-Kestenbaum R. Importance of serum amyloid A (SAA) level in monitoring disease activity and response to therapy in patients with prostate cancer. Urol Res. 1984;12(5):239–241. doi: 10.1007/BF00256147. [DOI] [PubMed] [Google Scholar]
- Klee G. G., Go V. L. Serum tumor markers. Mayo Clin Proc. 1982 Feb;57(2):129–132. [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes J. G., Cooper E. H. Comparison of serum amyloid A protein and C-reactive protein concentrations in cancer and non-malignant disease. J Clin Pathol. 1983 Jul;36(7):798–803. doi: 10.1136/jcp.36.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975 Apr;55(4):746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Sullivan L. M. Serum amyloid A to monitor cancer dissemination. Ann Intern Med. 1979 Sep;91(3):383–390. doi: 10.7326/0003-4819-91-3-383. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R., Zimlichman S., Winikoff Y., Pras M., Chaimovitz C., Sarov I. Serum amyloid A (SAA) in viral infection: rubella, measles and subacute sclerosing panencephalitis (SSPE). Clin Exp Immunol. 1982 Dec;50(3):503–506. [PMC free article] [PubMed] [Google Scholar]
- Weinstein P. S., Skinner M., Sipe J. D., Lokich J. J., Zamcheck N., Cohen A. S. Acute-phase proteins or tumour markers: the role of SAA, SAP, CRP and CEA as indicators of metastasis in a broad spectrum of neoplastic diseases. Scand J Immunol. 1984 Mar;19(3):193–198. doi: 10.1111/j.1365-3083.1984.tb00919.x. [DOI] [PubMed] [Google Scholar]


