Abstract
Background
Cardiovascular disease (CVD) death rates are much higher in blacks than whites in the United States (US). It is unclear how CVD risk and events are distributed among blacks vs. whites and how interventions reduce racial disparities.
Methods
We developed risk models for fatal and for fatal-and-nonfatal CVD using 8 cohorts in the US. We used 6,154 adults aged 50–69 years in the National Health and Nutrition Examination Survey 1999–2012 to estimate the distributions of risk and events in blacks and whites. We estimated the total as well as disparity impacts of a range of population-wide, targeted and risk-based interventions on 10-year CVD risks and event rates.
Results
25% (95% confidence interval 22–28) of black men and 12% (10–14) of black women were at ≥ 6.67% risk of fatal CVD (almost equivalent to 20% risk of fatal or nonfatal CVD), compared with 10% (8–12) of white men and 3% (2–4) of white women. These high-risk individuals accounted for 55% (49–59) of CVD deaths among black men and 42% (35–46) in black women, compared with 30% (24–35) in white men and 18% (13–22) in white women. We estimated that an intervention that treated multiple risk factors in high-risk individuals could reduce black-white difference in CVD death rate from 1,659 to 1,244 per 100,000 in men and from 1,320 to 897 in women. Rates of fatal-and-nonfatal CVD were generally similar between black and white men. In women, a larger proportion of women were at ≥ 7.5% risk of CVD (30% versus 19% in whites) and an intervention that targeted multiple risk factors among this group was estimated to reduce black-white differences in CVD rates from 1,688 to 1,197 per 100,000.
Conclusions
A substantially larger proportion of blacks have a high risk of fatal CVD and bear a large share of CVD deaths. A risk-based intervention that reduces multiple risk factors could substantially reduce overall CVD rates and racial disparities in CVD death rates.
Keywords: coronary heart disease risk, disparities, prevention, risk factor
Introduction
Cardiovascular diseases (CVD) are the leading causes of death in the United States (US), with substantially higher death rates among blacks than whites.1, 2 Previous research has shown that up to three quarters of absolute disparities between blacks and whites in CVD mortality may be due to differences in classic risk factors (i.e. raised blood pressure and serum cholesterol, diabetes, obesity, and smoking).3, 4 Therefore, interventions that reduce these risk factors are expected to reduce disparities in CVD mortality between blacks and whites but it is not clear which types of interventions, population-wide or targeted, can reduce racial disparities. Population-wide interventions can have large impacts on overall disease burden,5 but their impact on disparities depend on how they change risk factors in different subgroups of the population. For example, health education may reduce or widen disparities depending on how it is delivered.6–8 The disparity impact of interventions that target high-risk individuals (identified using a single risk factor or a combination of risk factors) will depend on whether the worse-off group has more or less high-risk individuals. Therefore, it is essential to have information on not only the average CVD risk and events, but also how CVD risk and events are distributed in better-off and worse-off subgroups of the population.
Some studies have qualitatively or quantitatively assessed the impacts of current risk factor exposures or scenarios of reducing risk factors on disparities in CVD or total mortality.3, 4, 9–14 Most of these studies have considered hypothetical risk factor reductions as opposed to interventions that could be implemented in practice. Other studies have used inconsistent or incomparable data and methods for calculating mortality effects across different risk factors, therefore reducing comparability. Furthermore, no study has assessed the disparity impact of risk-based prevention that is recommended by recent clinical guidelines,15, 16 because information on distributions of absolute CVD risk by race was not available. In this paper, we analyzed the total as well as disparity impacts of a range of population-wide, targeted and risk-based interventions on 10-year CVD risks and rates using consistent methods and data. We hypothesized that a much larger proportion of blacks are at high risk of CVD than whites, and hence the disparity in high-risk subgroup is responsible for a large part of disparity in event rates between races.
Methods
Overview
We estimated the effects of three types of interventions on CVD risk and events, as well as their disparities between blacks and whites: (1) population-wide interventions (alone or in combination); (2) interventions to lower risk factor level among individuals with high levels for a single risk factor; and (3) a risk-based intervention that targeted individuals with high predicted 10-year CVD risk and treated several risk factors simultaneously (Table 1). We first estimated the 10-year risk and events of both fatal and fatal-and-nonfatal coronary heart disease (CHD) or stroke for a representative sample of blacks and whites in the US. Risks were predicted based on systolic blood pressure (SBP), serum total cholesterol (TC), diabetes and smoking, using risk prediction equations that were recalibrated for each age-sex-race group.29 We then assessed how each intervention changed the predicted risk as well as events for each age-sex-race group.
Table 1.
Selected risk factors, their exposure metrics, and examples of population-wide, single raised risk factor, and risk-based interventions.
| Risk factors | Exposure metric (unit) |
Population-wide interventions |
Single raised risk factor interventions |
Multiple population- level interventions |
Risk-based interventions |
|---|---|---|---|---|---|
| High blood pressure |
Systolic blood pressure (SBP, mmHg) |
Reducing salt intake in packaged and prepared food * |
Two antihypertensive drugs at standard dose if diabetic, or SBP ≥ 140 mmHg for non-diabetic adults aged <60, or SBP ≥ 150 mmHg for non-diabetic adults aged ≥ 60‖ |
Multiple risk factor intervention at the population level, including reducing salt intake, dietary improvement, tobacco control, and increasing the price of sugar-sweetened beverages |
Multiple risk factor intervention, including blood pressure and lipid lowering medications, smoking cessation and life-style modification if 10- year risk of fatal CVD≥ 2.5% (or total CVD risk ≥ 7.5%)‡‡ |
| High serum cholesterol |
Serum total cholesterol (TC, mmol/L) |
Community-based dietary improvement to reduce dairy fat and replace saturated with unsaturated fats, and increase vegetable and fruit consumption† |
High-intensity statin if 10-year ASCVD risk ≥ 7.5%, or LDL cholesterol ≥ 4.9 mmol/L, moderate-intensity statin if diabetic aged 40–75 and 10-year ASCVD risk < 7.5%# |
||
| Tobacco smoking |
Current smoker prevalence (percentage) |
Tobacco control package to ban smoking in indoor workplace, offer cessation treatment in general store, put warning on package, ban advertisements, and increase tobacco tax‡ |
Referral to smoking cessation intervention such as group behavioral therapy** |
||
| Diabetes | Diabetes prevalence (percentage) |
Increase in price of sugar- sweetened beverages§ |
Intensive lifestyle modification intervention if diabetic†† |
We assumed a moderate salt reduction of about 1.4 g/day based on the United Kingdom’s successful experience in reducing salt intake at the population level.17 The effects of modest sodium reduction on blood pressure vary significantly by age, race and hypertensive status. We calculated the SBP reduction using the regression equation from a large meta-analysis of more than 100 randomized controlled trials.18
The dietary improvement intervention is based on the success of the North Karelia Project in Finland, which includes substituting vegetable oil margarine for butter, non-fat or low-fat milk for fatty milk, lean meat for meat high in saturated fat, using vegetable oil for cooking, and increasing vegetable and fruit consumption. We assumed such an intervention would reduce the population mean TC by 0.5 mmol/L, the average decline over 5 years in Finland.19 Similar dietary intervention was also found to improve population mean TC level in New Zealand.20
The tobacco control package is based on the WHO MPOWER tobacco control policies, which include banning smoking in all indoor workplaces, providing NRT and bupropion in general store or pharmacy with prescription, putting bold and graphic warning to cover at least 50% of the package, banning all direct advertisements, and increasing 10% in the retail price of cigarette due to tax. We assumed a 11% reduction in smoking prevalence based on the previous policy evaluation reports.21
We modeled a 50% increase in price of sugar-sweetened beverages, which we estimated would reduce consumption by about 50% based on the experience in Mexico where raising prices by 10% decreased consumption by 12%.22 Having consumption in the US would translate into a 0.5 serving/day reduction, as average sugar-sweetened beverage intake in adults is about one serving per day.23 Evidence shows that 1–2 servings/day sugar-sweetened beverage consumption compared to none or <1 serving/month is associated with 26% increased risk of type 2 diabetes.24 Therefore, 50% increase in price of sugar-sweetened beverages is associated with about 12% decreased risk of type 2 diabetes with a linear effect on log scale.
This intervention is based on the clinical guideline from the Eighth Joint National Committee (JNC 8).25 We calculated the SBP reduction by 0.09*P-5.67, where P is the pre-treatment SBP value (mmHg).26
This intervention is based on the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults.15 We used atorvastatin 80 mg/d as high-intensity statin and atorvastatin 20 mg/d for moderate-intensity statin.
Evidence suggested that group behavioral therapy is one of the most effective strategies for smoking cessation with a risk ratio of 2.71, which translates into 13% reduction in smoking prevalence.27
The lifestyle-modification intervention included a healthy low-calorie, low-fat diet and physical activity of moderate intensity, such as brisk walking, for at least 150 minutes per week. The effect of lifestyle intervention is based on the Diabetes Prevention Intervention,28 which reduces the diabetes incidence by 58%.
The multiple risk factor intervention included two antihypertensive drugs at standard dose, statin treatment, referral to smoking cessation intervention and lifestyle-modification intervention if the 10-year risk of fatal CVD≥ 2.5% (or fatal and non-fatal CVD risk ≥ 7.5%).
Data on Risk Factors
We used data on risk factors from 7 rounds of the National Health and Nutrition Examination Survey (NHANES) 1999–2012 to have stable estimates for each age-sex-race subgroup. We included black or white participants who were 50 to 69 years old and did not have a history of CHD or stroke. We excluded participants older than 70 years of age to focus on the age range commonly considered for premature event and mortality.
We accounted for complex survey design to make estimates of risk factor, predicted risk, and events representative of the national population. We used TC as opposed to LDL-cholesterol because LDL-cholesterol was only measured in half of the participants. Diabetes was defined as having a fasting plasma glucose (FPG) ≥126 mg/dL, hemoglobin A1c (HbA1c) ≥ 6.5%, history of diagnosis by a health professional, or use of insulin or oral hypoglycemic agents.
Data on CVD Deaths
In our primary analysis, we used fatal CVD as the primary outcome because data on nonfatal events, which is required for risk equation recalibration, is not available for the national US population (see below on methods to estimate fatal-and-nonfatal rates by race). We used mortality data from the National Center for Health Statistics (NCHS), to calculate death rates in 2011. We defined CVD death as death from CHD (ICD10 codes I20–I25) or stroke (ICD10 codes I60–I69).
Effect Sizes for Interventions
We obtained the effects of interventions on risk factors from meta-analyses of randomized controlled trials, observational studies, or policy evaluation analyses, as detailed in Table 1. We used a larger effect size for the impact of salt reduction on blood pressure among blacks versus whites based,18 but used the same effect size between blacks and whites for all other interventions because proportional effects have been found to be generally similar by race.30–33 Under the risk-based intervention scenario, we used individuals’ absolute CVD risks to determine whether they were affected by the interventions and assigned interventions (e.g. antihypertensive and statins) only to individuals who were not already receiving them. We applied smoking cessation to smokers irrespective of their absolute CVD risks. We note that the level of evidence supporting interventions varies: for example, the impact of population-wide interventions has only been estimated in observational studies,18, 21, 24 whereas the effect of statins on CVD has been consistently shown in many randomized trials.34 We also note that an individual may receive both population-wide and targeted interventions in practice, although these two types of interventions were analyzed separately here.
Statistical Analysis
We used risk prediction equations (or risk scores) for fatal CVD and for total CVD developed from 8 prospective cohorts in the US, as described elsewhere.29 Briefly, the models use four inputs to estimate individual-level 10-year risk: (1) the participants’ risk factor levels; (2) coefficients (i.e. log hazard ratios) for each risk factor estimated from the cohorts; (3) mean risk factor level for the same age-sex-race subgroup as the index participant; (4) average CVD event rate for the same age-sex-race subgroup as the index participant. The risk factors in the model were SBP, TC, diabetes and smoking. We used this new risk predication equation because it is based on data from multiple cohorts; it allows a straightforward recalibration by sex and race; it allows the age pattern of CVD risk to vary across race-sex subgroups; and it includes interactions between age and SBP, TC, diabetes and smoking and an interaction between sex and diabetes to account for the fact that the proportional effects of these risk factors on CVD vary by age and sex.35–39 For this application, we modified the risk scores to separate current from former smokers. The coefficients of the risk scores and the validation methods and results are presented in online-only Data Supplemental Table 1 and Supplemental Table 2. We assumed the same proportional associations between risk factors and fatal CVD risk for blacks and whites based on previous evidence.30–33 We relaxed this assumption by using race-specific coefficients in a sensitivity analysis (online-only Data Supplemental Table 3).
In the primary analysis, we first recalibrated the risk score by replacing the CVD event rate and mean risk factor levels with the observed age-sex-race-specific rates in the US population. We then used the recalibrated risk score and individual-level data from NHANES to estimate the 10-year risk of fatal CVD for each participant under the current risk factor levels. We report the mean predicted risk and number of events, as well as their relative or absolute differences between blacks and whites. We also present how the population and events were distributed by risk level in each sex-race subgroup. We further report the proportions of population and events at fatal CVD risk ≥ 2.5%, hereafter referred to as ‘moderate-risk’ and ≥ 6.67%, hereafter referred to as ‘high-risk’. As almost one-third of CVD events are fatal in the US,40 these risk thresholds approximately correspond to ≥ 7.5% (the AHA/ACC threshold15) and ≥ 20% (the ATP-III threshold16) for fatal and non-fatal CVD. In our secondary analysis, we used fatal-and-nonfatal CVD events as outcome. We calculated the age-sex-race-specific event rate of fatal-and-nonfatal CVD (CHD and stroke) using the corresponding death rate multiplying by the race-specific total-to-fatal event ratio. We used the total-to-fatal event ratios for CHD and stroke as reported in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) cohort40, 41 to account for the higher case fatality rates in blacks. We reported the proportions of population and events at total CVD risk ≥ 7.5% and ≥ 20%. In a sensitivity analysis, we calculated the 10-year CVD risk using the American College of Cardiology/American Heart Association (ACC/AHA) 2013 Pooled Cohort Equations.42
To estimate the effects of interventions on CVD risk and events, we first estimated their effects on risk factor(s), and then re-calculated the 10-year risk and events using the post-intervention risk factor levels. We chose this approach instead of directly applying the impact of interventions on CVD risk because for many of the interventions analyzed here, the outcome of epidemiological studies is risk factor level. For example, we estimated the impact of reducing incidence of diabetes from the Diabetes Prevention Program and combined that with evidence on the effect of diabetes on CVD from meta-analyses of observational studies.28, 35 As there are no studies that show a direct impact of diabetes prevention on CVD mortality, we conducted a separate analysis by removing diabetes prevention from the risk-based multiple risk factor intervention.
We quantified uncertainty by sampling repeated draws of different inputs to analysis, as described in the online-only Data Supplemental Text. All analyses were conducted using Stata 12.0 (StataCorp, College Station, Texas) and R 3.02. The study was approved by the institutional review board of the Harvard School of Public Health (Boston, MA, USA).
Results
We included 6,154 blacks and whites from 7 rounds of NHANES (online-only Data Supplemental Figure 1). About one-third of participants were black. TC levels were similar between blacks and whites, whereas other risk factor levels were higher in blacks (online-only Data Supplemental Table 4).
Mean 10-year risk of fatal CVD was 5.1% in black men versus 3.4% in white men (risk ratio (RR) of 1.49), and 3.0% in black women versus 1.7% in white women (RR of 1.79). This was equivalent to a 10-year CVD death rate of 5,052 per 100,000 in black men versus 3,393 in white men (rate difference (SD) of 1,659 per 100,000, and 2,989 in black women versus 1,669 in white women (RD of 1,320).
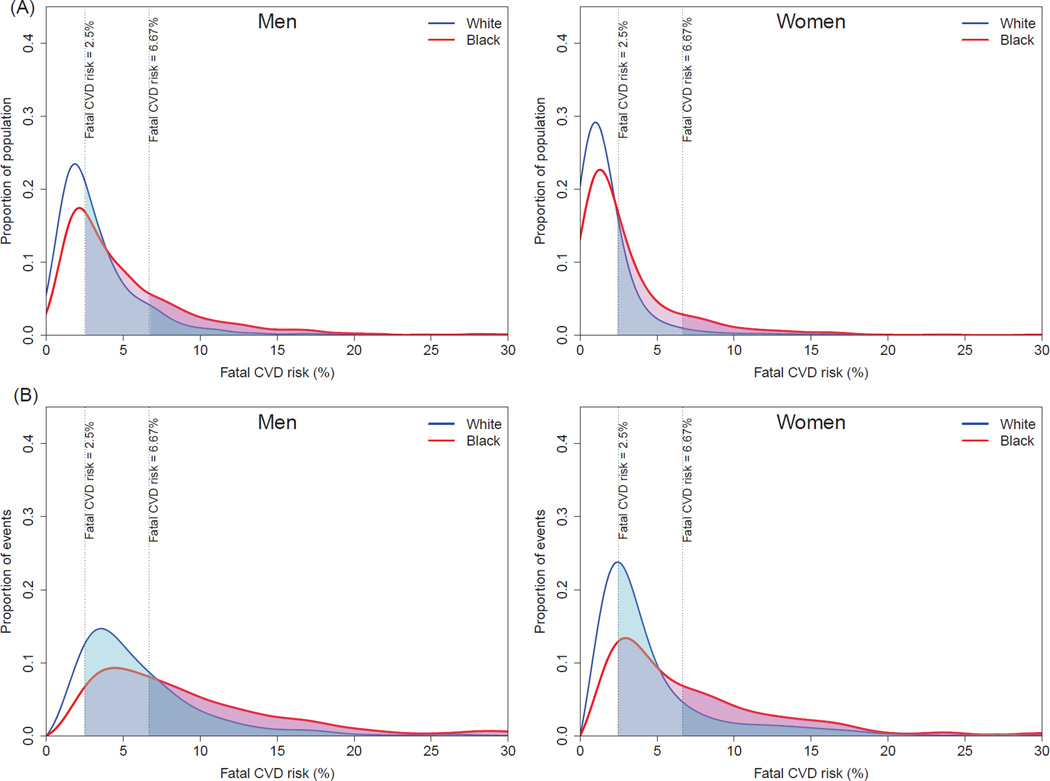
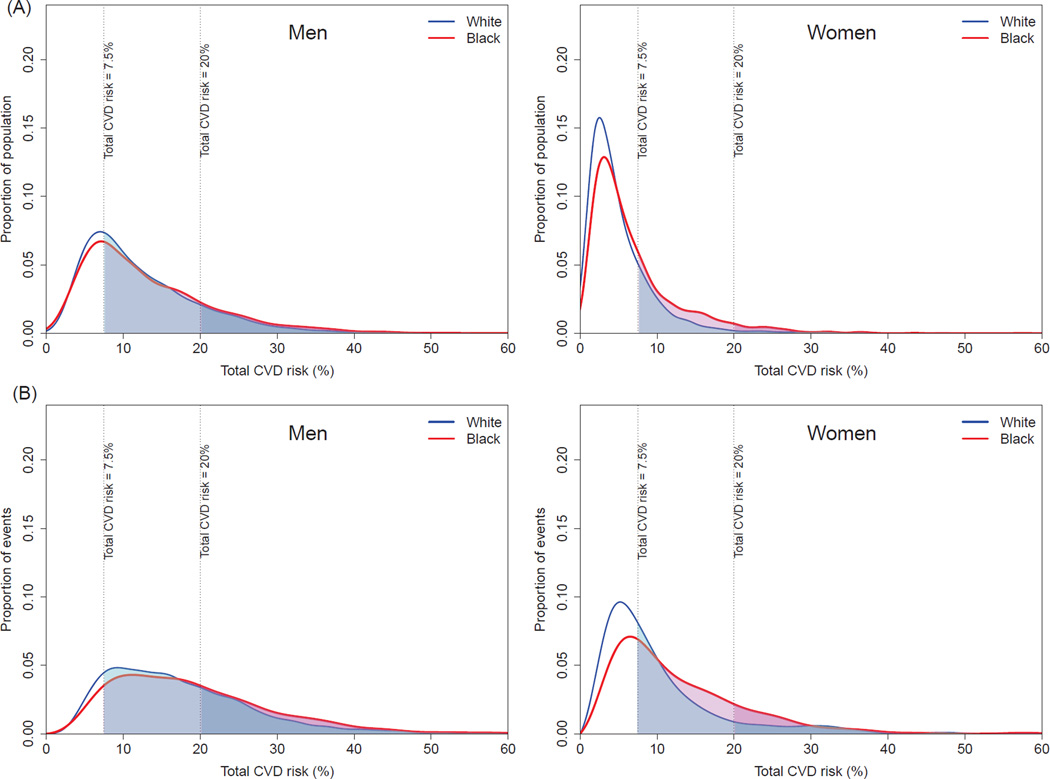
The distributions of both population and events by 10-year risk of fatal CVD were shifted to the right among blacks compared with whites; the distribution of events had a heavier tail than that of population as most of the events arise from the high-risk individuals (Figure 1). As a result, 25% (95% confidence interval 22–28) of black men were at high-risk (≥ 6.67% risk of fatal CVD in 10 year) compared with only 10% (8–12) of white men (Table 2 and online-only Data Supplemental Figure 2). This high-risk subgroup accounted for 55% (49–59) of CVD deaths in black men compared with 30% (24–35) in white men. For women, 12% (10–14) of blacks versus 3% (2–4) of whites at high-risk accounted for 42% (35–46) of CVD deaths in blacks versus 18% (13–22) in whites. Compared with the results of fatal CVD, black-white disparities in total CVD were substantially smaller for men (Figure 2, Table 3 and online-only Data Supplemental Figure 3). In women, disparities were only noticeable for those with ≥7.5% CVD risk where 30% (27–33) of blacks versus 19% (18–21) of white women accounted for 61% (57–63) of CVD events in blacks versus 46% (44–49) in whites.
Figure 1.
Distributions of predicted 10-year risk of fatal CVD in the population (A) and among cases (B).
Table 2.
Proportion of population and proportion of fatal CVD events occurring among high-risk individuals by sex and race.
| Fatal CVD risk ≥ 2.5%* | Fatal CVD risk ≥ 6.67%* | |||
|---|---|---|---|---|
| Proportion of population (%) |
Proportion of event (%) |
Proportion of population (%) |
Proportion of event (%) |
|
| Men | ||||
| White | 50 (47–52) | 77 (75–78) | 10 (8–12) | 30 (24–35) |
| Black | 66 (62–69) | 88 (87–90) | 25 (22–28) | 55 (49–59) |
| Women | ||||
| White | 17 (15–18) | 49 (46–52) | 3 (2–4) | 18 (13–22) |
| Black | 36 (33–39) | 74 (72–76) | 12 (10–14) | 42 (35–46) |
Figure 2.
Distributions of predicted 10-year risk of fatal-and-nonfatal CVD in the population (A) and among cases (B).
Table 3.
Proportion of population and proportion of fatal-and-nonfatal CVD events occurring among high-risk individuals by sex and race.
| Fatal-and-nonfatal CVD risk ≥ 7.5% |
Fatal-and-nonfatal CVD risk ≥ 20% |
|||
|---|---|---|---|---|
| Proportion of population (%) |
Proportion of event (%) |
Proportion of population (%) |
Proportion of event (%) |
|
| Men | ||||
| White | 68 (66–71) | 86 (85–87) | 15 (13–16) | 32 (29–34) |
| Black | 70 (66–73) | 87 (86–89) | 18 (15–20) | 37 (32–40) |
| Women | ||||
| White | 19 (18–21) | 46 (44–49) | 2 (1–3) | 10 (6–13) |
| Black | 30 (27–33) | 61 (57–63) | 4 (3–5) | 16 (12–19) |
Population-wide interventions (i.e. salt reduction, improving diet, WHO EMPOWER tobacco control policies, increasing price of sugar-sweetened beverages) and targeted interventions on single risk factors (i.e. antihypertensive and statins treatment, referral for quitting smoking, diabetes prevention program) were estimated to reduce the 10-year CVD death rate by at most 440 per 100,000 in men and 290 in women. The risk-based multiple risk factor intervention was estimated to reduce the average CVD death rate by 1,086 per 100,000 in men and 669 in women for blacks, and 671 per 100,000 in men and 246 in women for whites (Table 4).
Table 4.
Impact of interventions on 10-year rate of fatal CVD (per 100,000) by sex and race
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10-year CVD death rate |
Rate ratio (black vs. white) |
Rate difference |
Absolute change in rate difference |
Relative change in rate difference |
10-year CVD death rate |
Rate ratio (black vs. white) |
Rate difference |
Absolute change in rate difference |
Relative change in rate difference |
|||
| Black | White | - | - | Black | White | |||||||
| Current | 5,052 | 3,393 | 1.49 | 1,659 | NA | NA | 2,989 | 1,669 | 1.79 | 1,320 | NA | NA |
| Population-wide interventions | ||||||||||||
| 1) Salt reduction | 4,939 | 3,329 | 1.48 | 1,610 | –49 | –3% | 2,945 | 1,644 | 1.79 | 1,301 | −19 | −1% |
| 2) Community-based dietary improvement to reduce serum cholesterol |
4,906 | 3,290 | 1.49 | 1,616 | −44 | −3% | 2,893 | 1,618 | 1.79 | 1,276 | −45 | −3% |
| 3) MPOWER package for smoking | 4,939 | 3,356 | 1.47 | 1,583 | −77 | −5% | 2,934 | 1,647 | 1.78 | 1,287 | −34 | −3% |
| 4) Increasing price of sugar-sweetened beverages for diabetes prevention |
4,972 | 3,362 | 1.48 | 1,609 | −50 | −3% | 2,931 | 1,651 | 1.77 | 1,280 | −41 | −3% |
| 5) Multiple interventions (1–4) | 4,612 | 3,151 | 1.46 | 1,461 | −198 | −12% | 2,729 | 1,549 | 1.76 | 1,179 | −141 | −11% |
|
Single raised risk factor interventions |
||||||||||||
| 6) Treatment for hypertension | 4,885 | 3,290 | 1.48 | 1,595 | −64 | −4% | 2,906 | 1,629 | 1.78 | 1,277 | −43 | −3% |
| 7) Treatment for dyslipidaemia | 4,687 | 3,143 | 1.49 | 1,544 | −116 | −7% | 2,731 | 1,554 | 1.76 | 1,177 | −144 | −11% |
| 8) Referral for quitting smoking | 4,875 | 3,342 | 1.46 | 1,533 | −126 | −8% | 2,926 | 1,643 | 1.78 | 1,283 | −38 | −3% |
| 9) Diabetes prevention program | 4,704 | 3,187 | 1.48 | 1,517 | −142 | −9% | 2,699 | 1,551 | 1.74 | 1,148 | −173 | −13% |
| Risk–based interventions | ||||||||||||
| 10) Multiple risk factors (6–9 if risk of CVD death ≥ 2.5%) * |
3,966 | 2,722 | 1.46 | 1,244 | −415 | −25% | 2,320 | 1,423 | 1.63 | 897 | −423 | −32% |
| 11) Multiple risk factors (10 without diabetes prevention) |
4,296 | 2,882 | 1.49 | 1,414 | −246 | −15% | 2,601 | 1,495 | 1.74 | 1,106 | −214 | −16% |
The eligibility for each intervention was defined based on risk of CVD death ≥ 2.5% except for smoking cessation. Smoking cessation was provided to all current smokers irrespective of his/her predictive risk.
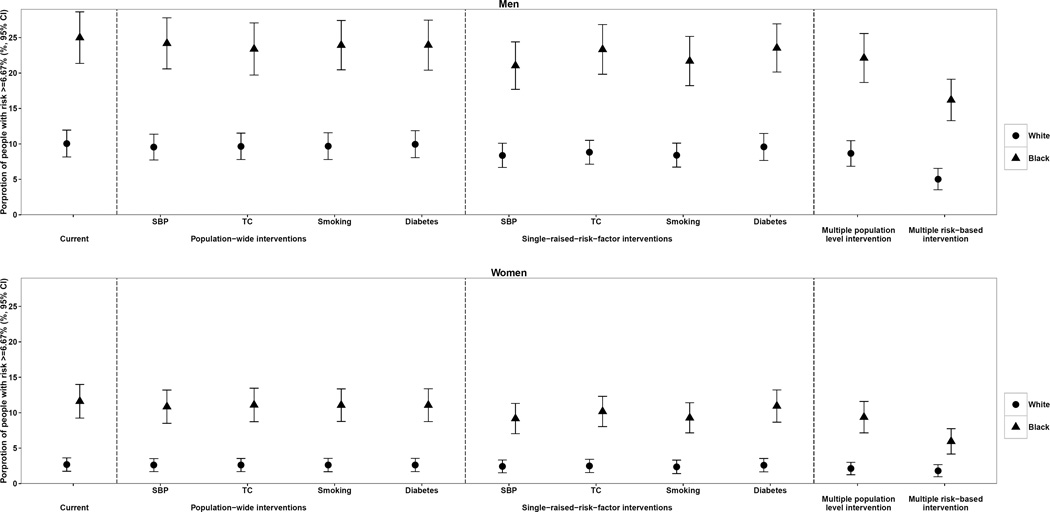
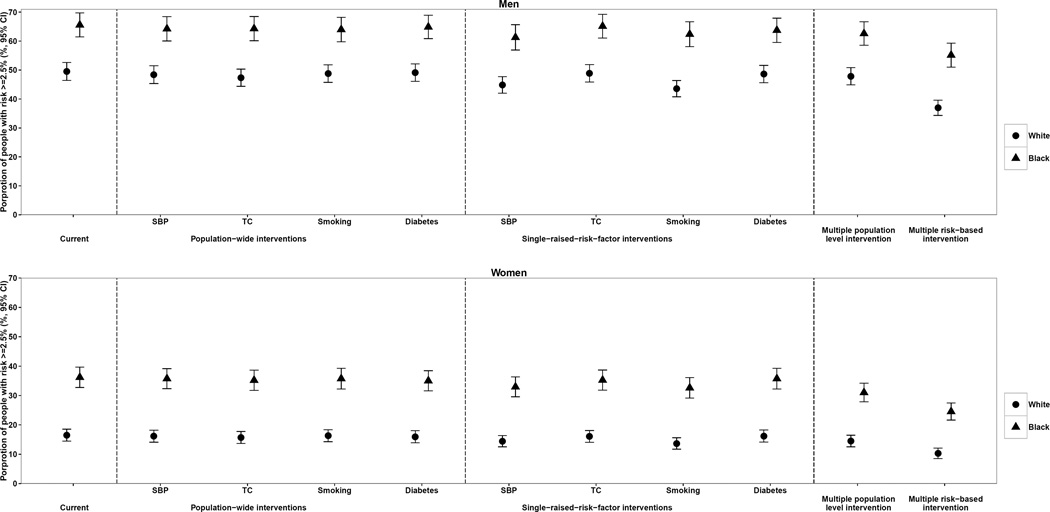
Population-wide interventions and targeted interventions on single risk factors did not substantially reduce the proportion of population at high risk of fatal CVD (≥ 6.67% risk of fatal CVD in 10 years) in either whites or blacks (Figure 3). In contrast, the risk-based multiple risk factor intervention was estimated to reduce the proportion of high-risk population by at most 12 percentage points for men and 6 percentage points for women. Results were similar for moderate-risk group (≥ 2.5% risk of fatal CVD in 10 years), where the risk-based multiple risk factor intervention was estimated to reduce the moderate-or-high-risk proportion by at most 13 percentage points for men and 9 percentage points for women compared with at most 6 and 4 percentage points in population-wide or targeted interventions (Figure 4). Our sensitivity analysis using separate fatal CVD risk scores for blacks and whites showed similar results (online-only Data Supplemental Figure 4 and Supplemental Figure 5).
Figure 3.
Impact of population-wide, single raised risk factor, and risk-based interventions on proportion of population with ≥ 6.67%* 10-year risk of fatal CVD. * This threshold approximately equals to ≥ 20% for risk of fatal-and-nonfatal CVD given one third of CVD events are fatal in the US.40
Figure 4.
Impact of population-wide, single raised risk factor, and risk-based interventions on proportion of population with ≥ 2.5%* 10-year risk of fatal CVD. * This threshold approximately equals to ≥ 7.5% for fatal-and-nonfatal CVD given one third of CVD events are fatal in the US.40
None of the interventions analyzed here had a potential to reduce black-vs-white fatal CVD rate ratios (Table 4). When we considered disparities in absolute CVD rates, combining the four selected population-wide interventions was estimated to reduce black-white disparities by 198 per 100,000 (12% of total absolute disparity) in men and 141 (11%) in women.
Among targeted single-risk interventions, the diabetes prevention program had the largest potential, with an estimated reduction in absolute disparity by 142 (9%) in men and 173 (13%) in women. The risk-based multiple risk factor intervention had much larger potential and could reduce absolute disparities by 415 per 100,000 (25%) in men and 423 (32%) in women. Removing diabetes prevention from the risk-based multiple risk factor intervention reduced the estimated impact of risk-based intervention by 41% to 50% but this intervention still had the largest potential for reducing absolute black-white disparities.
For fatal-and-nonfatal CVD rates, there were no significant disparities between blacks and whites in men. The estimated black-white disparities in women could be reduced by 217 per 100,000 (13% of total disparity in absolute risk) through a combination of four population-wide interventions. Implementing a diabetes prevention program alone was estimated to reduce disparities by 412 (24%) and the risk-based multiple risk factor intervention by 491 (29%) (Table 5). The sensitivity analysis using ACC/AHA 2013 Pooled Cohort Equations also showed consistent with the main analysis (online-only Data Supplemental Table 5 and Supplemental Table 6).
Table 5.
Impact of interventions on 10-year rate of fatal-and-nonfatal CVD (per 100,000) by sex and race
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10-year CVD event rate |
Rate ratio (black vs. white) |
Rate difference |
Absolute change in rate difference |
Relative change in rate difference |
10-year CVD event rate |
Rate ratio (black vs. white) |
Rate difference |
Absolute change in rate difference |
Relative change in rate difference |
|||
| Black | White | - | - | Black | White | |||||||
| Current | 13,082 | 12,343 | 1.06 | 739 | NA | NA | 6,868 | 5,179 | 1.33 | 1,688 | NA | NA |
| Population-wide interventions | ||||||||||||
| 1) Salt reduction | 12,881 | 12,189 | 1.06 | 691 | −48 | −6% | 6,791 | 5,123 | 1.33 | 1,668 | −20 | −1% |
| 2) Community-based dietary improvement to reduce serum cholesterol |
12,703 | 11,965 | 1.06 | 739 | −1 | 0% | 6,645 | 5,011 | 1.33 | 1,634 | −55 | −3% |
| 3) MPOWER package for smoking | 12,852 | 12,224 | 1.05 | 629 | −110 | −15% | 6,748 | 5,144 | 1.31 | 1,604 | −84 | −5% |
| 4) Increasing price of sugar- sweetened beverages for diabetes prevention |
12,962 | 12,264 | 1.06 | 697 | −42 | −6% | 6,736 | 5,151 | 1.31 | 1,585 | −104 | −6% |
| 5) Multiple interventions (1–4) | 12,204 | 11,613 | 1.05 | 590 | −149 | −20% | 6,358 | 4,886 | 1.30 | 1,471 | −217 | −13% |
|
Single raised risk factor interventions |
||||||||||||
| 6) Treatment for hypertension | 12,829 | 12,144 | 1.06 | 686 | −54 | −7% | 6,739 | 5,106 | 1.32 | 1,633 | −56 | −3% |
| 7) Treatment for dyslipidaemia | 12,147 | 11,347 | 1.07 | 800 | 61 | 8% | 6,329 | 4,819 | 1.31 | 1,510 | −179 | −11% |
| 8) Referral for quitting smoking | 12,816 | 12,190 | 1.05 | 626 | −113 | −15% | 6,719 | 5,130 | 1.31 | 1,589 | −99 | −6% |
| 9) Diabetes prevention program | 12,449 | 11,868 | 1.05 | 581 | −158 | −21% | 6,290 | 5,013 | 1.25 | 1,277 | −412 | −24% |
| Risk–based interventions | ||||||||||||
| 10) Multiple risk factors (6–9 if risk of CVD death ≥ 2.5%) * |
10,791 | 10,246 | 1.05 | 546 | −193 | −26% | 5,740 | 4,542 | 1.26 | 1,197 | −491 | −29% |
| 11) Multiple risk factors (10 without diabetes prevention) |
11,430 | 10,654 | 1.07 | 776 | 37 | 5% | 6,135 | 4,683 | 1.31 | 1,452 | −236 | −14% |
The eligibility for each intervention was defined based on risk of total CVD ≥ 7.5% except for smoking cessation. Smoking cessation was provided to all current smokers irrespective of his/her predictive risk
Discussion
We found that a substantially larger proportion of blacks (25% of men and 12% of women) in the US had a high risk of fatal CVD than their white counterparts (10% of men and 3% of women). These high-risk individuals bore about half of the burden of fatal CVD events in the population. An intervention that could identify high-risk individuals and treat multiple risk factors could both deliver large total benefits and substantially reduce the absolute black-white disparities. Population-wide and targeted interventions on single risk factors had smaller potential on reducing racial disparities in CVD compared with a risk-based intervention on multiple risk factors. Total CVD risks were similar in black versus white men and the disparity between black and white women could be substantially reduced by a risk-based multiple risk intervention.
Our results on the disparities in risk factor exposure, and in their role as a cause of racial disparities in CVD, are consistent with those of previous analyses.1, 3, 4, 9, 11, 13 A previous study proposed that population-wide interventions have a larger effect on health disparities than interventions that target high-risk individuals, but the two scenarios were only qualitatively compared.10 Other studies quantified the effects of hypothetical risk factor reductions on disparity in mortality without considering specific interventions.3, 4, 9, 11, 13 In addition, previous studies often used a single risk factor to identify high-risk individuals and considered interventions on one risk factor at a time.11, 43
A key strength of our analysis is that we have assessed not only the aggregate risk and events within each group but also how risk and events were distributed, providing important information on who needs intervention and what the expected impact of intervention is. In addition, we compared the total and disparity impacts of a wide range of population-wide and targeted interventions using consistent methods and data. Risk factor distributions were from a nationally representative survey, mortality data were from vital registration system, and effect sizes for interventions were obtained from large meta-analyses of randomized trials or observational studies that had adjusted for important confounders. We also systematically quantified the uncertainty as a result of the sampling variability in the national surveys and the uncertainty of coefficients from the risk prediction equations. Finally, our primary model included age interaction between risk factors and CVD incorporating evidence from many prospective studies.35
Our study has some limitations. First, although we estimated the risk distributions for both fatal CVD and total CVD, reliable national data on total CVD incidence is not available for model recalibration, especially by race. Recent data from a large prospective cohort (REGARDS) shows that black men have higher incidence of fatal CHD and lower incidence of non-fatal CHD than white men, resulting in similar incidence of total CHD for black and white men.40 Using estimates of case fatality rates from REGARDS to recalibrate model for total CVD risk eliminated much of the racial disparity in total CVD risk and thus it is expected that the interventions evaluated here would have minimal impact on racial disparities in total CVD risk. Second, our analysis focused on primary prevention of CVD. However, patients with history of CVD have a high risk of subsequent cardiovascular events and should receive treatments for risk factors. In the US, 9% of blacks and 6% of whites aged 50–69 in the 2011–2012 NHANES survey had a history of CVD. Were these proportions to be added to our estimates of prevalence of high-risk status, disparities would be slightly larger than our estimates. Our analysis did not include patients with CVD because existing risk scores for these patients require data on predictors such as electrocardiography (ECG) results, coronary imaging and biomarkers that are not measured in NHANES.44, 45 Third, we assumed that compliance with interventions would be similar to those observed in the randomized trials and observational studies used to generate the intervention effects, which may lead to overestimating the impact of interventions on black-white disparities. Although compliance may vary by race, prior work suggested that non-compliance is likely due to barriers of access to and poor quality of healthcare.46, 47 If insurance coverage and healthcare quality were similar across races, it is unlikely that compliance would differ substantially, as has been observed for antiretroviral therapy.48 Fourth, smoking cessation interventions have been shown to affect disadvantaged populations more strongly. However, detailed data on the differential impacts of smoking cessation by race is not available. Therefore, our estimates for the impact of smoking cessation on black-white disparities in CVD risk should be considered conservative. Fifth, there is limited evidence on direct impact of diabetes prevention on CVD and it remains unclear whether the Diabetes Prevention Program prevents or delays the onset of diabetes. Our sensitivity analyses of removing diabetes prevention from the risk-based multiple risk factor intervention confirmed the largest impact on reducing the absolute back-white disparities still came from the risk-based intervention. Finally, the effects of some interventions (e.g. reducing salt in package food, WHO’s MPOWER tobacco control policies) may be cumulative over decades. Our analyses did not incorporate the cumulative effects and hence may underestimate the long-term effect of these interventions.
In conclusion, although prevention and treatment have helped reduce CVD rates over the past few decades in the US, mortality rates remain higher in blacks than whites.1, 2, 40 Eliminating racial disparities in health is one of the overarching goals of the Healthy People 2020 agenda.49 As disparities in CVD are caused by disparities in broader social, economic and environmental determinants, policies and strategies are needed to address these factors and to facilitate healthy life-style and environment. Meanwhile, our findings suggest a much larger proportion of blacks are at high risk of fatal CVD than whites, and this high-risk subpopulation is bearing almost half of the deaths in the population. Therefore, by targeting this sick subpopulation with combination risk-based therapy, we can reduce a large share of events. While such approach has been advocated for the US population as a whole,15 achieving its potential as a means to reduce racial disparities will require increasing health insurance coverage and a strong primary care system that is equipped with well-trained health workers and appropriate infrastructure to provide low-cost essential drugs. Previous research has shown that universal health insurance over age 65 in the US is associated with lower racial differences in cardiovascular risk factors.50 An accessible and high-quality primary care program has also successfully reduced cardiovascular health inequality in other countries.51 The window of opportunity for addressing cardiovascular health disparity lies in the Affordable Care Act of 201052 that has already shown promise in improving access to primary care services53 and commits to eliminate barriers to health for disadvantaged communities, along with the new guideline for risk-based multidrug treatment for CVD.15 Their intersection could help identify important opportunities to improve the access and affordability of risk-based treatment for CVD in underserved population, and finally improve cardiovascular health for all.
Supplementary Material
Clinical Perspective.
1) What is new?
We investigated how risk of fatal and fatal-plus-nonfatal cardiovascular disease (CVD), estimated using a risk prediction model, is distributed among whites and blacks in the US and how population-wide or targeted interventions on CVD risk factors would reduce these racial disparities.
We used a nationally representative sample of adults aged 50 to 69 years in the US and a CVD risk prediction model that was recalibrated separately for blacks and whites.
2) What are the clinical implications?
Our results indicated that there are substantial disparities in risk of fatal CVD.
A large proportion of fatal CVD events among blacks were concentrated among a small proportion of the population; in contrast, racial disparities in risk of fatal-and-nonfatal CVD were only noticeable among women.
Population-wide and targeted interventions on single risk factors did not reduce black-white disparities in fatal CVD risk substantially.
An intervention that focused on high-risk individuals and reduced multiple risk factors simultaneously could reduce black-white disparities in fatal CVD risk by a quarter in men and a third in women.
Focusing preventive interventions on the high-risk individuals has a large potential to improve overall CVD health and reduce racial disparities.
Acknowledgments
Data for prospective cohorts were obtained from the National Heart Lung and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center. This study does not necessarily reflect the opinions or views of the cohorts used in the analysis, or the NHLBI.
Funding Sources: ME is supported by UK Medical Research Council, National Institute for Health Research Comprehensive Biomedical Research Centre at Imperial College Healthcare National Health Service Trust. GD is supported by US NIH (NIDDK: 1R01-DK090435). YL is supported by a Harvard/ Robert Wood Johnson Foundation Health & Society seed grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, Hogelin G, Marler J, McGovern P, Morosco G, Mosca L, Pearson T, Stamler J, Stryer D, Thom T. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 2.Harper S, Rushani D, Kaufman JS. Trends in the black-white life expectancy gap, 2003–2008. JAMA. 2012;307:2257–2259. doi: 10.1001/jama.2012.5059. [DOI] [PubMed] [Google Scholar]
- 3.Danaei G, Rimm EB, Oza S, Kulkarni SC, Murray CJ, Ezzati M. The promise of prevention: the effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States. PLoS Med. 2010;7:e1000248. doi: 10.1371/journal.pmed.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects--Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 5.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 6.Di Cesare M, Khang YH, Asaria P, Blakely T, Cowan MJ, Farzadfar F, Guerrero R, Ikeda N, Kyobutungi C, Msyamboza KP, Oum S, Lynch JW, Marmot MG, Ezzati M Lancet NCD Action Group. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381:585–597. doi: 10.1016/S0140-6736(12)61851-0. [DOI] [PubMed] [Google Scholar]
- 7.Lorenc T, Petticrew M, Welch V, Tugwell P. What types of interventions generate inequalities? Evidence from systematic reviews. J Epidemiol Community Health. 2013;67:190–193. doi: 10.1136/jech-2012-201257. [DOI] [PubMed] [Google Scholar]
- 8.Durkin S, Brennan E, Wakefield M. Mass media campaigns to promote smoking cessation among adults: an integrative review. Tob Control. 2012;21:127–138. doi: 10.1136/tobaccocontrol-2011-050345. [DOI] [PubMed] [Google Scholar]
- 9.Bajekal M, Scholes S, Love H, Hawkins N, O'Flaherty M, Raine R, Capewell S. Analysing recent socioeconomic trends in coronary heart disease mortality in England, 2000–2007: a population modelling study. PLoS Med. 2012;9:e1001237. doi: 10.1371/journal.pmed.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capewell S, Graham H. Will cardiovascular disease prevention widen health inequalities? PLoS Med. 2010;7:e1000320. doi: 10.1371/journal.pmed.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kivimaki M, Shipley MJ, Ferrie JE, Singh-Manoux A, Batty GD, Chandola T, Marmot MG, Smith GD. Best-practice interventions to reduce socioeconomic inequalities of coronary heart disease mortality in UK: a prospective occupational cohort study. Lancet. 2008;372:1648–1654. doi: 10.1016/S0140-6736(08)61688-8. [DOI] [PubMed] [Google Scholar]
- 12.McLaren L, McIntyre L, Kirkpatrick S. Rose's population strategy of prevention need not increase social inequalities in health. Int J Epidemiol. 2010;39:372–377. doi: 10.1093/ije/dyp315. [DOI] [PubMed] [Google Scholar]
- 13.Khang YH, Lynch JW, Yun S, Lee SI. Trends in socioeconomic health inequalities in Korea: use of mortality and morbidity measures. J Epidemiol Community Health. 2004;58:308–314. doi: 10.1136/jech.2003.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, Lopez AD. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet. 2006;368:367–370. doi: 10.1016/S0140-6736(06)68975-7. [DOI] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 17.He FJ, Pombo-Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ open. 2014;4:e004549. doi: 10.1136/bmjopen-2013-004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J Global Burden of Diseases Nutrition Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 19.Puska P, Stahl T. Health in all policies-the Finnish initiative: background, principles, and current issues. Annual review of public health. 2010;31:315–328. doi: 10.1146/annurev.publhealth.012809.103658. 3 p following 328. [DOI] [PubMed] [Google Scholar]
- 20.Jackson R, Beaglehole R. Trends in dietary fat and cigarette smoking and the decline in coronary heart disease in New Zealand. Int J Epidemiol. 1987;16:377–382. doi: 10.1093/ije/16.3.377. [DOI] [PubMed] [Google Scholar]
- 21.Levy DT, Ellis JA, Mays D, Huang AT. Smoking-related deaths averted due to three years of policy progress. Bull World Health Organ. 2013;91:509–518. doi: 10.2471/BLT.12.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. doi: 10.1136/bmj.h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh GM, Micha R, Khatibzadeh S, Shi P, Lim S, Andrews KG, Engell RE, Ezzati M, Mozaffarian D Global Burden of Diseases N and Chronic Diseases Expert G. Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PLoS One. 2015;10:e0124845. doi: 10.1371/journal.pone.0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 26.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. The Cochrane database of systematic reviews. 2005;2:CD001007. doi: 10.1002/14651858.CD001007.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajifathalian K, Ueda P, Lu Y, Woodward M, Ahmadvand A, Aguilar-Salinas C, Cifkova R, Cesare M, Eriksen L, Farzadfar F, Ikeda N, Khang Y, Lanska V, León-Muñoz L, Magliano D, Msyamboza K, Oh K, Rodríguez-Artalejo F, Shaw J, Stevens G, Tolstrup J, Zhou B, Salomon J, Ezzati M, Danaei G. A novel risk score for predicting coronary heart disease risk in national populations: a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes & Endocrinology. 2015;3:339–355. doi: 10.1016/S2213-8587(15)00081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Y, McGee DL, Cooper RS. Prediction of coronary heart disease mortality in blacks and whites: pooled data from two national cohorts. Am J Cardiol. 1999;84:31–36. doi: 10.1016/s0002-9149(99)00187-3. [DOI] [PubMed] [Google Scholar]
- 31.Keil JE, Sutherland SE, Knapp RG, Lackland DT, Gazes PC, Tyroler HA. Mortality rates and risk factors for coronary disease in black as compared with white men and women. N Engl J Med. 1993;329:73–78. doi: 10.1056/NEJM199307083290201. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell BD, Haffner SM, Hazuda HP, Patterson JK, Stern MP. Diabetes and coronary heart disease risk in Mexican Americans. Annals of epidemiology. 1992;2:101–106. doi: 10.1016/1047-2797(92)90043-p. [DOI] [PubMed] [Google Scholar]
- 33.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 34.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn S, Lawes CM, Ali MK, Mozaffarian D, Ezzati M. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 37.Lawes CM, Bennett DA, Parag V, Woodward M, Whitlock G, Lam TH, Suh I, Rodgers A. Blood pressure indices and cardiovascular disease in the Asia Pacific region: a pooled analysis. Hypertension. 2003;42:69–75. doi: 10.1161/01.HYP.0000075083.04415.4B. [DOI] [PubMed] [Google Scholar]
- 38.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 39.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–1980. doi: 10.1016/S0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 40.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G, Investigators R. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 43.Manuel DG, Lim J, Tanuseputro P, Anderson GM, Alter DA, Laupacis A, Mustard CA. Revisiting Rose: strategies for reducing coronary heart disease. BMJ. 2006;332:659–662. doi: 10.1136/bmj.332.7542.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrow DA. Cardiovascular risk prediction in patients with stable and unstable coronary heart disease. Circulation. 2010;121:2681–2691. doi: 10.1161/CIRCULATIONAHA.109.852749. [DOI] [PubMed] [Google Scholar]
- 45.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 46.Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44:1410–1421. doi: 10.1345/aph.1P150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker EA, Molitch M, Kramer MK, Kahn S, Ma Y, Edelstein S, Smith K, Johnson MK, Kitabchi A, Crandall J. Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care. 2006;29:1997–2002. doi: 10.2337/dc06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, Wilson K, Buchan I, Gill CJ, Cooper C. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.US Department of Health Human Services. Healthy People 2020. [Accessed on December 30, 2015];2011 https://wwwhealthypeoplegov/2020/about/foundation-health-measures/Disparities.
- 50.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of medicare coverage. Ann Intern Med. 2009;150:505–515. doi: 10.7326/0003-4819-150-8-200904210-00005. [DOI] [PubMed] [Google Scholar]
- 51.Farzadfar F, Murray CJ, Gakidou E, Bossert T, Namdaritabar H, Alikhani S, Moradi G, Delavari A, Jamshidi H, Ezzati M. Effectiveness of diabetes and hypertension management by rural primary health-care workers (Behvarz workers) in Iran: a nationally representative observational study. Lancet. 2012;379:47–54. doi: 10.1016/S0140-6736(11)61349-4. [DOI] [PubMed] [Google Scholar]
- 52.Department of Health and Human Services. The Affordable Care Act. [Accessed on December 30, 2015];2010 http://www.healthcare.gov/law/introduction/index.html.
- 53.Sommers BD, Gunja MZ, Finegold K, Musco T. Changes in Self-reported Insurance Coverage, Access to Care, and Health Under the Affordable Care Act. JAMA. 2015;314:366–374. doi: 10.1001/jama.2015.8421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.