Abstract
Background
Two human herpesviruses, human herpesvirus 6 (HHV-6), and Epstein-Barr virus (EBV), have been repeatedly linked to multiple sclerosis (MS).
Objective
The aim of this study was to investigate HHV-6 and EBV reactive oligoclonal bands (OCBs), and viral DNA in the intrathecal compartment in MS.
Methods
The reactivity of OCBs in cerebrospinal fluid (CSF) for EBV and HHV-6 antigens and stability of virus reactive OCBs over time were studied in a well-characterized MS patient cohort. Associations between virus reactive OCBs and viral DNA in CSF (and any clinical and/or radiological findings) were investigated.
Results
Of patients with MS, 38% had OCBs reactive to either one of the viruses studied, compared to none in the patients with other inflammatory neurological diseases (p=0.005). The banding pattern of virus reactive OCBs remained the same over time. Furthermore, MS patients with viral DNA in CSF had more contrast enhancing lesions (CELs).
Conclusion
The stable presence of herpesvirus reactive OCBs in CSF further strengthens the association of MS with these viruses. The finding that herpesviruses might be linked to the appearance of active lesions warrants investigation of new therapeutic strategies to treat these viruses in MS.
Keywords: Multiple sclerosis, magnetic resonance imaging, immunology
Introduction
The etiology of multiple sclerosis (MS), the immune-mediated central nervous system (CNS) demyelinating disease, is unknown. Genetic involvement, associated with specific human leukocyte antigen (HLA) alleles, and environmental factors have been suggested to play important roles in disease development. Environmental factors include infectious agents, such as human herpesvirus 6 (HHV-6) and Epstein-Barr virus (EBV), geographical location, vitamin D levels and smoking.1 Disease course in MS is heterogeneous, making progression and treatment efficacy hard to predict. Therefore, there is a clear need for diagnostic, prognostic and treatment selection biomarkers in MS.
Although oligoclonal bands (OCBs) in MS were discovered decades ago, their specificity remains unknown. OCBs are useful for the diagnosis of MS,2 but they are not specific for this disease and have been demonstrated in infectious and autoimmune diseases of the CNS. It has been suggested that if MS has an infectious cause, the OCBs should include specific reactivity for the microbial agent. Furthermore, OCBs can have reactivity for Chlamydia pneumoniae,3,4 EBV5,6 and HHV-6.7
Here we studied the presence of EBV- and HHV-6-specific reactivity OCBs in the cerebrospinal fluid (CSF) of patients with MS and compared these findings to clinical and radiological findings. The specificity of the OCBs to viral antigens was confirmed by adsorbtion assay. In addition, we investigated the presence of herpesvirus reactive OCBs in longitudinal CSF samples. Finally, we studied the presence of viral DNA in cell-free CSF and determined if the herpesvirus reactive OCBs or viral DNA in CSF associate with clinical and/or radiological findings.
Methods
Patients
Paired CSF and serum samples were collected from 37 patients with MS (28 relapsing remitting MS (RRMS), 7 primary progressive MS (PPMS) and 2 secondary progressive MS (SPMS)) diagnosed according to 2010 revised McDonald’s criteria.2 MS patient demographics are pre sented in Table 1. All MS patients were off any immunomodulatory treatments at the time of study. CSF and sera from 15 patients with other inflammatory neurological disease (OIND) (seven patients with autoimmune encephalitis (courtesy of Josep Dalmau, University of Pennsylvania), six patients with HTLV-1 associated myelopathy (HAM), one patient with possible acute disseminated encephalomyelitis and one patient unknown) served as controls. Immunoglobulin G (IgG) was quantified by nephelometry (National Institutes of Health Clinical Laboratory). Informed consent was obtained from each subject in accordance with the Declaration of Helsinki. The study was reviewed and approved by the National Institute of Neurological Disorders and Stroke (NINDS) Institutional Review Board.
Table 1.
Multiple sclerosis (MS) patient demographics.
| Diagnosis | Sex | Age | Disease duration | EDSS | MRI
|
IgG Index | CSF IgG | WBC | Total OCB banding pattern | Viral CSF-bands | Viral DNA in CSF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CEL | Lesion load | |||||||||||
| PPMS | F | 59 | 6.9 | 5.5 | 0 | mild | 0.84 | 8.7 | 4 | II | – | – |
| PPMS | F | 57 | 0.3 | 5 | 0 | moderate | 1.9 | 4.6 | 6 | III | HHV-6 | – |
| PPMS | M | 51 | 15.2 | 7 | 0 | moderate | 0.62 | 5.5 | 3 | II | – | – |
| PPMS | F | 47 | 5.6 | 6 | 0 | mild | 0.88 | 2.2 | 3 | III | – | – |
| PPMS | F | 48 | 2.8 | 2 | 0 | mild | 0.97 | 5 | 8 | II | EBV | EBV |
| PPMS | M | 54 | 12 | 7 | 0 | moderate | 0.61 | 2.2 | 0 | II | – | – |
| PPMS | M | 51 | 5.7 | 6.5 | 0 | severe | 0.86 | 3.7 | 1 | II | EBV | – |
| RRMS | F | 28 | 1.1 | 0 | 0 | mild | 1.27 | 2.6 | 9 | II | HHV-6 | – |
| RRMS | M | 40 | 1.1 | 1 | 0 | moderate | 0.56 | 3 | 3 | I | HHV-6 | – |
| RRMS | M | 44 | 2.8 | 1 | 0 | mild | 1.72 | 2.1 | 0 | III | – | – |
| RRMS | F | 38 | 1.4 | 0 | 10 | moderate | 0.68 | 5.5 | 4 | II | – | – |
| RRMS | F | 29 | 0.5 | 0 | 0 | mild | 0.63 | 4.2 | 3 | II | – | – |
| RRMS | F | 25 | 5.5 | 2 | 0 | mild | 0.62 | 11.7 | 9 | I | – | – |
| RRMS | M | 24 | 0.2 | 1 | 0 | mild | 0.56 | 3.8 | 2 | II | – | HHV-6 |
| RRMS | F | 35 | 0.2 | 1.5 | 0 | mild | 1.6 | 3.3 | 9 | II | EBV | – |
| RRMS | M | 67 | 33 | 2 | 0 | moderate | 0.77 | 3.1 | 2 | II | EBV | – |
| RRMS | F | 25 | 0.3 | 0 | 1 | mild | 3.83 | 5.1 | 23 | III | HHV-6 | – |
| RRMS | F | 34 | 5 | 2 | 0 | moderate | 1.63 | 2.6 | 4 | II | – | – |
| RRMS | F | 49 | 14.8 | 2 | 0 | moderate | 0.51 | 4.1 | 0 | I | – | – |
| RRMS | F | 24 | 2.8 | 0 | 1 | moderate | 1.27 | 10.9 | 14 | II | – | – |
| RRMS | M | 37 | 8 | 5 | 0 | mild | 0.8 | 7.2 | 1 | III | HHV-6 | – |
| RRMS | F | 29 | 2 | 1 | 4 | moderate | 1.68 | 5.7 | 19 | III | – | EBV |
| RRMS | F | 41 | 0.3 | 1 | 0 | mild | 1.22 | 5.9 | 6 | III | HHV-6 | – |
| RRMS | M | 38 | 0.3 | 1.5 | 0 | mild | 0.68 | 3.3 | 2 | III | – | – |
| RRMS | M | 39 | 4.8 | 2.5 | 5 | moderate | 2.06 | 5.2 | 14 | II | – | HHV-6 |
| RRMS | M | 37 | 0.5 | 2 | 1 | mild | 0.84 | 15.2 | 9 | III | HHV-6 | – |
| RRMS | M | 40 | 7.7 | 2.5 | 3 | moderate | 0.75 | 4.3 | 4 | II | – | – |
| RRMS | M | 52 | 5 | 6 | 2 | severe | 0.64 | 2.6 | 4 | II | – | – |
| RRMS | M | 39 | 10 | 0 | 0 | mild | 0.96 | 6 | 1 | II | – | – |
| RRMS | F | 42 | 1.4 | 1 | 1 | mild | 0.91 | 15.3 | 5 | II | EBV | – |
| RRMS | M | 51 | 0.3 | 2 | 3 | severe | 0.62 | 2 | 1 | II | – | HHV-6 |
| RRMS | F | 50 | 1.2 | 0 | 0 | mild | 0.52 | 4.3 | 1 | II | – | – |
| RRMS | M | 59 | 8 | 1 | 0 | mild | 0.82 | 2.6 | 1 | III | – | – |
| RRMS | M | 28 | 8 | 1 | 4 | mild | 0.53 | 2.1 | 1 | IV | – | HHV-6, EBV |
| RRMS | M | 29 | 2 | 6 | 4 | moderate | 0.75 | 3.5 | 4 | II | – | – |
| SPMS | F | 56 | 15 | 6.5 | 0 | moderate | 0.68 | 3.6 | 0 | III | HHV-6 | – |
| SPMS | M | 49 | 13 | 6 | 2 | moderate | 0.5 | 2.5 | 0 | II | HHV-6 | HHV-6 |
PPMS: primary progressive MS; RRMS: relapsing remitting MS; SPMS: secondary progressive MS CEL: contrast enhancing lesion; CSF: cerebrospinal fluid; EDSS: Expanded Disability Status Scale; IgG: immunoglobulin G; MRI: magnetic resonance imaging; OCB: oligoclonal band.
Viral antigens
EBV producing cells (B95-8) and SupT1 cells were cultured in RPMI-1640. SupT1 cells were infected with HHV-6A (strain U1101) or HHV-6B (strain Z-29). B95-8 or HHV-6 infected SupT1 cells were collected and 2×107 cells (containing 10–1000 viral copies per cell) were resuspended in 1 ml of cold lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100 and complete protease inhibitors (Roche)) and incubated 20 min on ice. Cell debris was removed and 20 μg of viral or control cell lysate per cm2 of membrane was used for coating.
Isoelectric focusing (IEF) and immunoblot
Serum and CSF samples were diluted to 5 mg/dl, or if the IgG concentration of CSF was less than 5 mg/dl, serum was diluted to the same concentration as CSF. Five μl of each sample was applied on the Isogel® Agarose IEF Plates pH 3–10 (Lonza, Basel, Switzerland) using sample applicator foil (GE Healthcare, Waukesha, Wisconsin, USA). Samples were prefocused with 1 W for 10 min and focused 45 min at 25 W (limits 1000 V and 10 mA) using Multiphor™ II system (GE Healthcare) with cooling. After focusing, gel was overlaid with nitrocellulose membrane and covered with blotting papers and a 1 kg weight for 30 min. After transfer, the membrane was blocked with 5% milk. IgG was detected using alkaline phosphatase conjugated anti-human IgG at a 1:25000 dilution (Sigma-Aldrich, St. Louis, Missouri) and visualized using NBT/BCIP substrate solution (Roche).
Detection of virus reactive oligoclonal bands
After isoelectric focusing the gel was overlaid with a membrane coated with viral antigens as described previously.6,7 Monoclonal antibody to HHV-6 92/98 kDa glycoproteins (HHV-6 Foundation, Santa Barbara, California, USA) and monoclonal antibody to EBNA-1 (courtesy of Jeffrey Cohen, National Insitute of Allergy and Infectious Diseases) were used as positive controls for HHV-6 and EBV antigens, respectively. Antibodies were isoelectrically focused and transferred to either uncoated or virus-coated membrane. HRP-conjugated anti-mouse IgG (1:2000, Biorad, Hercules, California, USA) and ECL detection kit (SuperSignal West Pico, Pierce, Rockford, Illinois, USA) were used for the detection. The monoclonal antibody to HHV-6 reacted only with HHV-6 antigen-coated membrane, not with EBV or SupT1 coated membrane (Figure 1(d)). Although not all herpesviruses were included in the study, for convenience the term ‘herpesviruses’ is used to describe these two viruses throughout the text.
Figure 1.
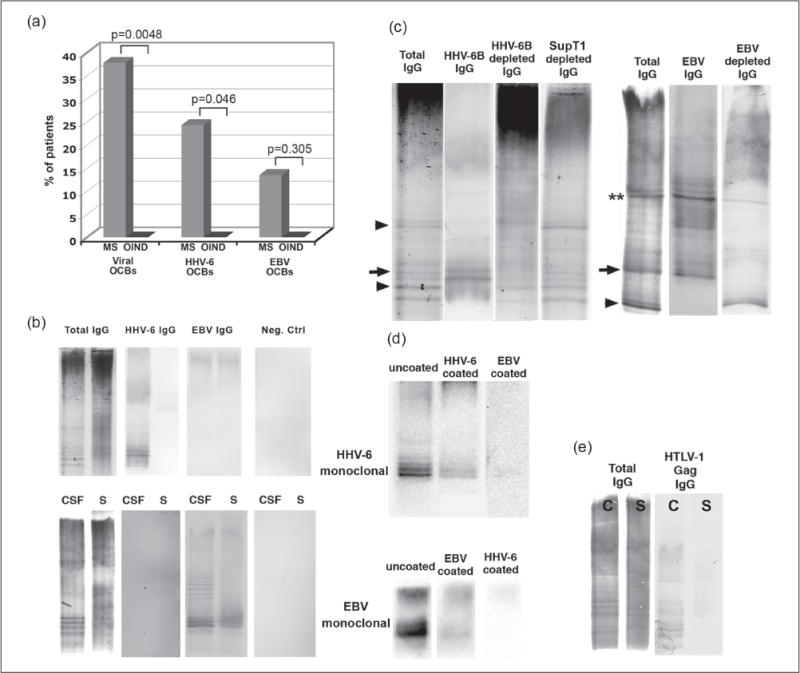
Herpesvirus reactive oligoclonal bands (oligoclonal bands) in multiple sclerosis (MS). (a) Herpesvirus reactive OCBs were significantly more common in cerebrospinal fluid (CSF) of MS cases than cases with other inflammatory neurological disease (OIND), a difference that was also noted for human herpesvirus 6 (HHV-6) reactive but not for Epstein-Barr virus (EBV) reactive OCBs. Fisher’s exact test was used. (b) Identical amounts of serum and CSF immunoglobulin G (IgG) were isoelectrically focused and transferred to uncoated membrane or membrane coated with viral antigens or mock-infected control antigens. A representative image from a patient with HHV-6 reactive (upper panel) and EBV reactive (lower panel) OCBs is shown. (c) To demonstrate the reactivity of the CSF OCBs to herpesviruses, HHV-6 or EBV antigen was coupled to sepharose beads and CSF IgG was incubated with beads to adsorb virus-specific OCBs. Arrows indicate bands that disappeared totally and two stars bands that disappeared partially when CSF was pre-incubated with viral antigen. Some of the bands, however, remained in the CSF even after pre-incubation with HHV-6B viral antigen (arrowhead). (d) Anti-HHV-6 and anti-EBV monoclonal antibodies were isoelectrically focused and transferred to uncoated, HHV-6 or EBV coated membranes. (e) Serum and CSF from a patient with HTLV-1 associated myelopathy was used as a positive control. Several of the OCBs in CSF were reactive to HTLV-1 gag protein.
Herpesviral IgG depletion from CSF
Either B95-8 EBV producing, HHV-6A or HHV-6B infected, or uninfected SupT1 cells were washed twice with phosphate buffered saline (PBS), suspended to a concentration of 2×107 cells/ml and homogenized using a dounce homogenizer. Large cell particles were removed by low-speed centrifugation (400 g, 2 min) and protein concentration was determined. Virus antigen was coupled to Cyanogen bromide-activated Sepharose 4b beads (Sigma) overnight at +4°C. CSF samples were incubated overnight at +4°C with beads, centrifuged and supernatant was concentrated using Amicon ultra 100 kDa concentrators. Concentrate was used for IEF and immunoblotting analysis.
Electrochemiluminescence-based antibody assay for HHV-6 and EBV
Antibody assays for detection of anti-HHV-6 and EBV antibodies were done as previously described.8 Briefly, a 96-well plate was coated with HHV-6B or EBV lysate (0.2 mg/ml), blocked with Blocker A solution and washed with PBS. Duplicate CSF samples diluted in MSD Antibody Diluent (1:20) were applied to the wells and incubated on a shaker at room temperature for 1 h. After washing, Sulfo-Tag-conjugated goat anti-human IgG was added and incubated on the shaker for 1 h. After washes, MSD Read Buffer T was added and the plate was read on an MSD PR400 plate reader. All reagents were from Meso Scale Discovery, Gaithersburg, Maryland, USA.
Nested PCR detection of HHV-6 or EBV DNA
Total nucleic acids were extracted from 200 μl of CSF or serum using QIAmp UltraSens Virus kit (Qiagen) and eluted to 60 μl of water. Ten μl of elution was used for nested PCR detection using HHV-6 major capsid protein (MCP)8 or EBV p239 specific primers as described previously. DNA extractions and PCR reactions were prepared in separate rooms and negative controls were added to each run to control contaminations. All samples were run at least two times.
Brain magnetic resonance imaging (MRI)
Spin-echo and gradient-echo T1-weighted images following intravenous administration of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist; Berlex) were collected on both 1.5T and 3T scanners (GE Medical Systems) using 8-channel phased-array head coils (Invivo). Three scans were performed over a two-month period and the one closest to lumbar puncture was chosen for the study. An experienced neuroradiologist (DSR.) counted the number of CELs with reference to pre-contrast T1-weighted as well as T2-weighted (both fast-spin-echo and fluid-attenuated inversion recovery) and proton-density-weighted images blindly from virological or antibody findings. The number of CELs in each scan as well as the overall white matter lesion burden characterized by number and confluency of lesions were recorded as published.10
Statistical analysis
Fisher’s exact test was used to determine whether the proportion of cases with virus reactive OCBs differed between MS and OIND. Linear regression model with unequal variance was applied to examine if herpesvirus reactive OCBs or viral DNA in CFS (with four categories) associated with clinical or radiological variables, such as EDSS, IgG index and white blood cell count (WBC), where age, gender and disease duration were considered as covariates (if significant at p<0.1). Inverse and logarithm transformation were applied to IgG index and WBC respectively. For the number of CELs, since none of covariates was shown significant at p<0.1, the Kruskal-Wallis test was used to evaluate the association with herpesvirus reactive OCBs (with two categories) or viral DNA in CFS (with two categories), and the combined variable (with four categories) followed by pair-wise comparisons with Bonferroni correction. Spearman’s rank correlation coefficient was used in the correlation analyses. A p-value of 0.05 or below was regarded as statistically significant. SAS version 9.2 was used for statistical analyses.
Results
Total IgG oligoclonal bands
As described previously,11 CSF and serum bands can be divided into four different OCB patterns: no bands in CSF (pattern 1), bands only in CSF (pattern 2), bands in CSF and serum with unique bands in CSF (pattern 3), and similar bands in CSF and serum (pattern 4). In this study 33 of 37 (89%) patients with MS had CSF-restricted OCBs (i.e. pattern 2 or 3) (Table 1.). Three patients with RRMS did not have bands at all, and one patient had banding pattern 4. In 11 cases (8 RRMS, 2 PPMS and 1 SPMS), there were bands in both CSF and serum with additional bands in CSF (pattern 3). In the remaining 22 cases (16 RRMS, 5 PPMS and 1 SPMS), only CSF bands were observed (pattern 2). The total number of OCBs in CSF in patients with positive OCB finding varied from 6 to 22 (median 12). WBC count in CSF correlated with IgG index, but neither WBC counts in CSF nor IgG index correlated with the total number of IgG OCBs in CSF (Supplementary Material, Figure 1). Fourteen of 15 (93%) OIND patients had CSF-specific OCBs (Supplementary Material, Table 1).
Higher prevalence of herpesvirus reactive OCBs in MS than in OIND
Of the 37 MS CSF samples, 14 (38%) had intrathecal OCBs reactive for HHV-6 or EBV. By contrast, none of OIND samples reacted with EBV or HHV-6 (p=0.0048) (Figure 1(a)). Nine MS cases had OCBs reactive for HHV-6 (p=0.046) and five had OCBs reactive for EBV in the CSF (p=0.305) (Figure 1(a)). All the MS cases with CSF reactivity for herpesvirus were specific for either HHV-6 or EBV but never both (Figure 1(b)). No reactivity to SupT1 was seen.
Depletion of herpesviral IgG from CSF confirms the specificity of OCBs to herpesviruses
To demonstrate that a fraction of total IgG OCBs are virus reactive, we used EBV- or HHV-6-coupled Sepharose beads to adsorb virus-specific antibodies from the CSF. Two untreated CSF samples and one CSF sample treated with virus-coupled beads from the same patient were isoelectrically focused. Untreated CSF was transferred to uncoated membrane to detect the total IgG (Figure 1(c), lane 1) and the other CSF to viral antigen-coated membrane to detect virus reactive OCBs (Figure 1(c), lane 2). CSF treated with virus-coupled beads was transferred to uncoated membrane to detect total IgG lacking virus reactive IgG (Figure 1(c), lane 3). As shown in Figure 1(c), some of the bands seen in total IgG blots aligned with the bands seen in virus IgG blots and disappeared when treated with virus coupled beads (arrows). Other bands seen in total IgG blots that were not seen in virus IgG blots were, as expected, present after the treatment with virus-coupled beads (arrowheads).
Longitudinal analysis of herpesvirus reactive OCBs in MS
Longitudinal samples were available for 11 MS cases and 2 OIND cases. Although small changes were observed in intensity of some of the bands, the banding pattern remained constant in each longitudinal CSF sample pair, consistently with earlier reports12 (Supplementary Material, Figure 2(a)). Of the 11 MS cases, four were positive for HHV-6 and none were positive for EBV. The banding pattern remained the same in all HHV-6 positive samples (Supplementary Material, Figure 2(b)). In addition, the levels of CSF viral antibodies measured by electrochemiluminescence-based antibody assay did not significantly change over time (p=0.53 and p=0.44 for HHV-6 and EBV antibodies, respectively) (Supplementary Material, Figure 2(c) and 2(d)).
Figure 2.
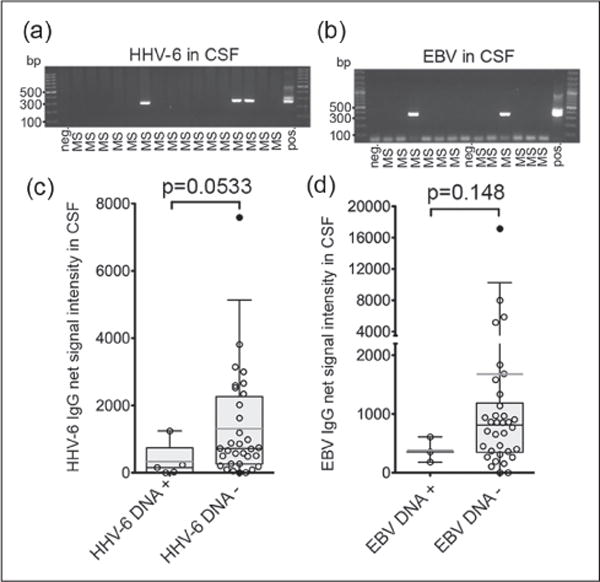
Low levels of herpesviral antibodies might be associated with detection of herpesvirus DNA in cerebrospinal fluid (CSF).
The presence of (a) human herpesvirus 6 (HHV-6) and (b) Epstein-Barr virus (EBV) DNA in cell-free CSF from patients with multiple sclerosis (MS) was studied by nested polymerase chain reaction (PCR).
(c) Patients with HHV-6 DNA in CSF trended to have less HHV-6 antibodies in CSF and (d) patients with EBV DNA in CSF trended to have less EBV antibodies in CSF. Box plot with 5% and 95% percentiles. Light line: average, dark line: median. Kruskall-Wallis test was used.
Presence of herpesviral DNA in CSF might be associated with lower levels of herpesvirus antibodies in CSF
Patients without herpesviral bands had lower levels of herpesvirus antibodies in CSF than those that had herpesvirus reactive bands (Supplementary Material, Figure 3). Since herpesviral DNA has been detected in MS CSF,13 it was of interest to determine if the presence of herpesvirus reactive OCBs associate with herpesvirus DNA in CSF. From 37 MS CSF samples, five (13.5%) were found to have HHV-6 DNA, and three (8.1%) were found to have EBV DNA (Figure 2(a) and (b)). Patients with HHV-6 DNA in CSF had lower levels of HHV-6 antibodies (Figure 2(c)) and patients with EBV DNA in CSF had lower levels of EBV antibodies (Figure 2(d)), although the findings were not statistically significant.
Figure 3.
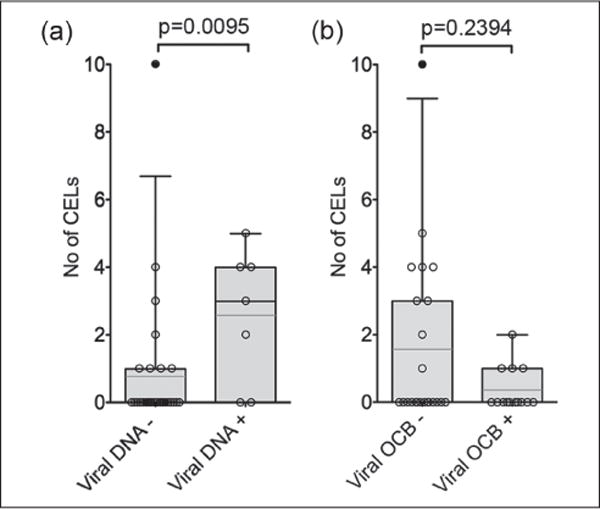
Herpesviral DNA in cerebrospinal fluid (CSF) associates with magnetic resonance imaging (MRI) findings. Number of contrast enhancing lesions (CELs) was significantly elevated in MRIs from patients with viral DNA in CSF as compared to those who did not have viral DNA. (A) No significant difference was found in CELs between patients with or without virus reactive oligoclonal bands (OCBs). (B) Box plots with 5% and 95% percentiles. Light line: average, dark line: median. Kruskall Wallis test was used.
The association of virus antibodies or DNA in CSF with clinical characteristics and MRI in MS
There was no difference in WBC count in CSF, IgG index, or EDSS between patients with herpesvirus reactive OCBs and herpesviral DNA, patients without herpesvirus reactive OCBs but with herpesvirus DNA, patients with herpesvirus reactive OCBs but without herpesviral DNA or patients without herpesvirus reactive OCBs and herpesviral DNA (Table 2), but there was a statistically significant difference in CELs between these patients (p=0.0409). Thirteen of 37 (35%) MS patients had CELs, representing blood-brain barrier permeability at the time of lumbar puncture.
Table 2.
Association of clinical or radiological findings with virus reactive oligoclonal bands (OCBs) and virus DNA in cerebrospinal fluid (CSF).
| Viral-OCB+/Viral-DNA+ (n=2) | Viral-OCB–/Viral-DNA+ (n=5) | Viral OCB+/Viral-DNA–(n=12) | Viral-OCB–/Viral-DNA– (n=18) | p-value | |
|---|---|---|---|---|---|
| Mean EDSS (SEM)a | 3.75 (2.25) | 1.50 (0.32) | 2.63 (0.70) | 2.60 (0.55) | 0.7335 |
| Mean no of CELs (SEM)b | 1.00 (1.00) | 3.20 (0.86) | 0.25 (0.13) | 1.33 (0.55) | 0.0409 |
| Mean IgG Index (SEM)c | 0.74 (0.24) | 1.09 (0.32) | 1.27 (0.26) | 0.87 (0.09) | 0.5156 |
| Mean WBC (SEM)d | 4.00 (4.00) | 7.40 (3.80) | 6.17 (1.80) | 3.71 (0.87) | 0.6234 |
CEL: contrast enhancing lesion; EDSS: Expanded Disability Status Scale; IgG: immunoglobulin G; SEM: standard error of the mean; WBC: white blood cell count.
age was used as covariate;
Kruskal-Wallis test: pair-wise comparison found significant difference between ‘−/+’ and +/−’ with p=0.013 (with Bonferroni correction);
sex was used as covariate;
sex and age were used as covariates.
MS cases with EBV/HHV-6 DNA in CSF had significantly more CELs than patients with EBV/HHV-6 OCBs or those without EBV/HHV-6 DNA (Figure 3(a)). There was no significant difference in the number of CELs between patients with or without virus reactive OCBs (Figure 3(b)).
Discussion
From a large number of infectious candidates linked to MS, HHV-6 and EBV have garnered the most attention. However, the evidence supporting these linkages differs for the two viruses. The most compelling evidence associating HHV-6 to MS is the presence of the virus at the site of demyelination. Detection of HHV-6 DNA14,15 or protein14,16,17 in MS brain plaques has been reported. HHV-6 is ubiquitous and has been found in normal-appearing white matter of MS brains and in normal control brains,18 although at significantly lower frequencies than in MS plaques.15 HHV-6 transcripts indicating active viral replication have been detected in the sera of MS cases during clinical relapses.19 In addition, intrathecal antibody responses to HHV-6 have been reported in approximately 20% of MS cases.20,21
By contrast, the most compelling evidence linking EBV to MS is epidemiological. EBV, like HHV-6, is ubiquitous with a reported seroprevalence in MS cases of 99.5% (compared to 94.2% in age-matched controls).22 Also, symptomatic EBV infection (infectious mononucleosis), has been suggested as a risk factor for MS (relative risk 2.17).23 Although EBV is not commonly detected in MS or control brains,24 EBV infected B-cell follicles in MS brains, particularly in progressive forms of MS, have been reported.25 While some studies have found intrathecal antibodies to EBV predominantly in pediatric MS26 or early in the disease course,27 others have not.28 Furthermore, EBV-specific OCBs have also been reported5,25,29, although most were not CSF-specific.6
In support of earlier studies,5–7 we detected both EBV and HHV-6 reactive OCBs in MS CSF. In an earlier study,6 EBV reactive OCBs were found mainly as a mirror pattern in CSF and serum and rarely only in CSF. In our study, most of the patients with EBV reactive OCBs had bands both in CSF and serum, but in five MS samples there was at least two bands in CSF not present in serum (i.e. intrathecal synthesis). It has been reported that MS OCBs can have reactivity to several viruses, such as measles, HSV-1, mumps and rubella.30 However, in our previous study we did not find any reactivity to HSV-1 antigen7 and in this study we have shown OCB reactivity to either HHV-6 or EBV but never reactivity to both. In addition, we did not find any reactivity to HTLV-1 antigens (data not shown). These observations suggest that the reactivity of OCBs might be more restricted than previous studies have suggested. This difference could be due to the methods used and the different viruses studied. While we have shown OCB selectivity for the two herpesviruses analyzed in this study, it remains to be determined if there are reactivities to other non-herpesviruses.30 We also demonstrated that HHV-6 reactive OCBs, like total IgG OCBs, are constant in CSF over time. Interestingly, MS cases with herpesvirus reactive OCBs did not have more WBCs in CSF or a higher IgG index suggesting that herpesvirus reactive OCBs do not relate to the high polyclonal IgG response. We were also able to deplete a fraction of the total IgG OCBs by incubating CSF samples with herpesviral antigens.
The data in this report showing a higher presence of herpesviral DNA in patients with CELs are consistent with earlier publications15,17 showing higher prevalence of HHV-6 in active MS plaques, and suggests an activation of herpesviruses that may be associated with MS lesion formation. By contrast, EBV is usually not detected in MS plaques.24,31 Although increased intrathecal antibodies to many neurotropic viruses is commonly interpreted as part of polyspecific response in MS,24,32,33 herpesvirus reactive OCBs may also be a reflection of a robust humoral immune response that serves to control the virus, particularly within the CNS. The failure to control the virus in the CNS, reflective of low intrathecal herpesvirus antibodies and increased herpesvirus DNA in CSF, might lead to localized tissue damage and/or activation of local immune cells that further attracts (auto) reactive lymphocytes to the brain parenchyma. This failure could be, in part, genetically determined and associated with genes related to immunological control of common viral infections. It is known that T-cells are important in clearing viral infections and maintaining latency in the host.34 However, anti-viral antibodies also play a role in maintaining latency and limiting the dissemination of viral reactivation of betaherpesvirus35 and gammaherpesvirus.36 Viral reactivation in MS CNS in some patients might lead to, or be a consequence of, inflammation which is a hallmark of active plaques in MS brain tissue.
Collectively, our data together with earlier reports strengthens the hypothesis that these viruses may be associated with MS disease pathogenesis. We acknowledge that the sample size in this study is relatively small and findings should be validated in independent larger patient populations. The finding of herpesvirus reactive OCBs together with viral DNA in CSF, and their relation to the MRI activity could be used to stratify patients to an antiviral intervention. To test this hypothesis further, we will need well-designed antiviral clinical trials with safe, efficient and CNS-penetrable antiviral drugs.
Supplementary Material
Acknowledgments
Bibiana Bielekova, Irene Cortese, Joan Ohayon and Neuroimmunology Branch clinical group at NINDS evaluated the study participants and facilitated collection of biological samples. Anna Abrams language edited the manuscript. Josep Dalmau (University of Pennsylvania) provided samples from encephalitis patients. Tianxia Wu helped with the statistical analysis.
Funding
This study was funded by NINDS Intramural Program. JO Virtanen has been supported by the Academy of Finland, Orion-Farmos Foundation and Instrumentarium Science Foundation.
Footnotes
Conflicts of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Handel AE, Giovannoni G, Ebers GC, et al. Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol. 2010;6:156–166. doi: 10.1038/nrneurol.2010.1. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derfuss T, Gurkov R, Then Bergh F, et al. Intrathecal antibody production against Chlamydia pneumoniae in multiple sclerosis is part of a polyspecific immune response. Brain. 2001;124:1325–1335. doi: 10.1093/brain/124.7.1325. [DOI] [PubMed] [Google Scholar]
- 4.Fainardi E, Castellazzi M, Tamborino C, et al. Chlamydia pneumoniae-specific intrathecal oligoclonal antibody response is predominantly detected in a subset of multiple sclerosis patients with progressive forms. J Neurovirol. 2009;15:425–433. doi: 10.3109/13550280903475580. [DOI] [PubMed] [Google Scholar]
- 5.Cepok S, Zhou D, Srivastava R, et al. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest. 2005;115:1352–1360. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franciotta D, Di Stefano AL, Jarius S, et al. Cerebrospinal BAFF and Epstein-Barr virus-specific oligoclonal bands in multiple sclerosis and other inflammatory demyelinating neurological diseases. J Neuroimmunol. 2011;230:160–163. doi: 10.1016/j.jneuroim.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Virtanen JO, Pietilainen-Nicklen J, Uotila L, et al. Intrathecal human herpesvirus 6 antibodies in multiple sclerosis and other demyelinating diseases presenting as oligoclonal bands in cerebrospinal fluid. J Neuroimmunol. 2011;237:93–97. doi: 10.1016/j.jneuroim.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Yao K, Honarmand S, Espinosa A, et al. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65:257–267. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villegas E, Santiago O, Carrillo JA, et al. Low intrathecal immune response of anti-EBNA-1 antibodies and EBV DNA from multiple sclerosis patients. Diagn Microbiol Infect Dis. 2011;70:85–90. doi: 10.1016/j.diagmicrobio.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27:455–461. [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch Neurol. 2005;62:865–870. doi: 10.1001/archneur.62.6.865. [DOI] [PubMed] [Google Scholar]
- 12.Olsson JE, Link H. Immunoglobulin abnormalities in multiple sclerosis. Relation to clinical parameters: exacerbations and remissions. Arch Neurol. 1973;28:392–399. doi: 10.1001/archneur.1973.00490240052009. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Lafuente R, Garcia-Montojo M, De Las Heras V, et al. Herpesviruses and human endogenous retroviral sequences in the cerebrospinal fluid of multiple sclerosis patients. Mult Scler. 2008;14:595–601. doi: 10.1177/1352458507086425. [DOI] [PubMed] [Google Scholar]
- 14.Challoner PB, Smith KT, Parker JD, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cermelli C, Berti R, Soldan SS, et al. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis. 2003;187:1377–1387. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- 16.Virtanen JO, Zabriskie JB, Siren V, et al. Co-localization of human herpes virus 6 and tissue plasminogen activator in multiple sclerosis brain tissue. Med Sci Monit. 2005;11:BR84–BR87. [PubMed] [Google Scholar]
- 17.Goodman AD, Mock DJ, Powers JM, et al. Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis. 2003;187:1365–1376. doi: 10.1086/368172. [DOI] [PubMed] [Google Scholar]
- 18.Tuke PW, Hawke S, Griffiths PD, et al. Distribution and quantification of human herpesvirus 6 in multiple sclerosis and control brains. Mult Scler. 2004;10:355–359. doi: 10.1191/1352458504ms1057oa. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Lafuente R, De Las Heras V, Bartolome M, et al. Human herpesvirus 6 and multiple sclerosis: A one-year follow-up study. Brain Pathol. 2006;16:20–27. doi: 10.1111/j.1750-3639.2006.tb00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derfuss T, Hohlfeld R, Meinl E. Intrathecal antibody (IgG) production against human herpesvirus type 6 occurs in about 20% of multiple sclerosis patients and might be linked to a polyspecific B-cell response. J Neurol. 2005;252:968–971. doi: 10.1007/s00415-005-0794-z. [DOI] [PubMed] [Google Scholar]
- 21.Virtanen JO, Farkkila M, Multanen J, et al. Evidence for human herpesvirus 6 variant A antibodies in multiple sclerosis: Diagnostic and therapeutic implications. J Neurovirol. 2007;13:347–352. doi: 10.1080/13550280701381332. [DOI] [PubMed] [Google Scholar]
- 22.Ascherio A, Munger KL, Lennette ET, et al. Epstein-Barr virus antibodies and risk of multiple sclerosis: A prospective study. JAMA. 2001;286:3083–3088. doi: 10.1001/jama.286.24.3083. [DOI] [PubMed] [Google Scholar]
- 23.Handel AE, Williamson AJ, Disanto G, et al. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One. 2010;5:e12496. doi: 10.1371/journal.pone.0012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis SN, Stadelmann C, Rodig SJ, et al. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain. 2009;132:3318–3328. doi: 10.1093/brain/awp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serafini B, Rosicarelli B, Franciotta D, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohl D, Rostasy K, Jacobi C, et al. Intrathecal antibody production against Epstein-Barr and other neurotropic viruses in pediatric and adult onset multiple sclerosis. J Neurol. 2010;257:212–216. doi: 10.1007/s00415-009-5296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaquiery E, Jilek S, Schluep M, et al. Intrathecal immune responses to EBV in early MS. Eur J Immunol. 2010;40:878–887. doi: 10.1002/eji.200939761. [DOI] [PubMed] [Google Scholar]
- 28.Jafari N, van Nierop GP, Verjans GM, et al. No evidence for intrathecal IgG synthesis to Epstein Barr virus nuclear antigen-1 in multiple sclerosis. J Clin Virol. 2010;49:26–31. doi: 10.1016/j.jcv.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Sargsyan SA, Shearer AJ, Ritchie AM, et al. Absence of Epstein-Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology. 2010;74:1127–1135. doi: 10.1212/WNL.0b013e3181d865a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rostrom B, Link H, Laurenzi MA, et al. Viral antibody activity of oligoclonal and polyclonal immunoglobulins synthesized within the central nervous system in multiple sclerosis. Ann Neurol. 1981;9:569–574. doi: 10.1002/ana.410090610. [DOI] [PubMed] [Google Scholar]
- 31.Peferoen LA, Lamers F, Lodder LN, et al. Epstein Barr virus is not a characteristic feature in the central nervous system in established multiple sclerosis. Brain. 2010;133:e137. doi: 10.1093/brain/awp296. [DOI] [PubMed] [Google Scholar]
- 32.Owens GP, Bennett JL. Trigger, pathogen, or bystander: The complex nexus linking Epstein- Barr virus and multiple sclerosis. Mult Scler. 2012;18:1204–1208. doi: 10.1177/1352458512448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petereit HF, Reske D. Expansion of antibody reactivity in the cerebrospinal fluid of multiple sclerosis patients - follow-up and clinical implications. Cerebrospinal Fluid Res. 2005;2:3. doi: 10.1186/1743-8454-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddehase MJ, Mutter W, Munch K, et al. CD8-positive T lymphocytes specific for murine cytomegalovirus immediateearly antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonjic S, Pavic I, Polic B, et al. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim IJ, Flano E, Woodland DL, et al. Antibody-mediated control of persistent gamma-herpesvirus infection. J Immunol. 2002;168:3958–3964. doi: 10.4049/jimmunol.168.8.3958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


