Abstract
Hallucinogens fall into several different classes, as broadly defined by pharmacological mechanism of action, and chemical structure. These include psychedelics, entactogens, dissociatives, and other atypical hallucinogens. Although these classes do not share a common primary mechanism of action, they do exhibit important similarities in their ability to occasion temporary but profound alterations of consciousness, involving acute changes in somatic, perceptual, cognitive, and affective processes. Such effects likely contribute to their recreational use. However, a growing body of evidence indicates that these drugs may have therapeutic applications beyond their potential for abuse. This review will present data on several classes of hallucinogens with a particular focus on psychedelics, entactogens, and dissociatives, for which clinical utility has been most extensively documented. Information on each class is presented in turn, tracing relevant historical insights, highlighting similarities and differences between the classes from the molecular to the behavioral level, and presenting the most up-to-date information on clinically oriented research with these substances, with important ramifications for their potential therapeutic value.
Keywords: hallucinogen, psychedelic, dissociative, club drugs, drug policy
Introduction
Hallucinogens fall into several different classes, as broadly defined by pharmacological mechanism of action, and chemical structure (Nichols, 2004; Ray, 2010; Table 1). These include serotonin 2A receptor (5-HT2AR) agonists such as lysergic acid diethylamide (LSD), psilocybin, and N,N-dimethyltryptamine (DMT), often referred to as classic hallucinogens or psychedelics; mixed serotonin and dopamine reuptake inhibitors and releasers such as 3,4-methylenedioxy-methamphetamine (MDMA), referred to as empathogens or entactogens (Nichols, 1986); N-methyl-D-aspartate (NMDA) antagonists such as ketamine and dextromethorphan (DXM), also known as dissociative anesthetics (Morris & Wallach, 2014); as well as atypical hallucinogens such as the kappa opioid receptor (KOR) agonist salvinorin A, the indole alkaloid ibogaine, which affects multiple neurotransmitter systems, and the anticholinergics such as atropine and datura, also known as deliriants. Finally, cannabis is sometimes attributed hallucinogenic properties (Keeler et al., 1971), and will therefore be discussed briefly in this review.
Table 1.
Summary of hallucinogens and potential clinical applications.
| Subclass | Summary | |||
|---|---|---|---|---|
| Psychedelics | A group of serotonergic agonists that are currently classified as Schedule I substances. Some, including psilocybin, peyote (mescaline) and the DMT containing admixture ayahuasca, have been used for hundreds to thousands of years for religious purposes by indigenous cultures (Guerra-Doce, 2015). Preliminary evidence indicates one or more of these may be useful in treating cluster headache (Sewell et al., 2006), substance use disorders (Bogenschutz et al., 2015; Johnson et al., 2014; Krebs & Johanesen, 2012; Savage & McCabe, 1973), end-of-life anxiety and potentially depression (Carhart-Harris et al., 2016; Gasser et al., 2014; Grob et al., 2010), and obsessive compulsive disorder (Moreno et al., 2009). See below for examples. | |||
LSD a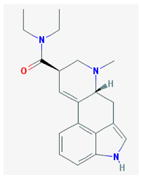
|
Psilocybin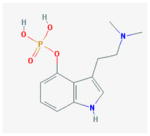
|
Mescaline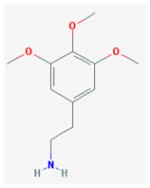
|
DMT b (ayahuasca)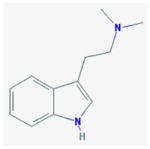
|
|
|
| ||||
| Entactogens | A group of Schedule I monoamine releasers and reuptake inhibitors known for their ability to evoke a sense of emotional openness and connection (Nichols, 1986). MDMA in particular has shown potential therapeutic benefits in post-traumatic stress disorder (PTSD; Mithoefer et al., 2011, 2012), and is being evaluated as a treatment for social anxiety in adults with autism spectrum disorders (Danforth et al., 2015), and end-of-life anxiety. See below for examples. | |||
MDMA c
|
MDA d
|
|||
|
| ||||
| Dissociatives | A group of glutamatergic NMDAe antagonists that are either unscheduled (DXM and N2O), or restricted but available for use as anesthetics (ketamine), and whose additional therapeutic uses are currently being explored in a variety of areas including depression (Abdallah et al., 2015a; Nagele et al., 2015; Nguyen et al., 2014), and substance use disorders (Krupitsky et al. 1992; 2007; Krupitsky & Grinenko, 1997; 1998). See below for examples. | |||
Ketamine
|
DXM f
|
Nitrous Oxide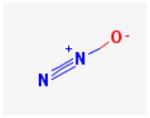
|
||
|
| ||||
| Atypical | A group of unrelated, pharmacologically distinct substances with some hallucinogenic properties, exhibiting diverse mechanisms of action, legal status, and therapeutic potentials. Ibogaine (Schedule I, US) acts as a serotonin 2A agonist, MOR g agonist, KOR h antagonist, and NMDA antagonist, displaying potentials as an anti-addiction agent, particularly for opioids (Alper et al., 1999; Schenberg et al., 2014). The unscheduled KOR agonist Salvinorin A exhibits preclinical evidence for potential in treating addiction (Butelman & Kreek, 2015; Freeman et al., 2014). The CB1 i receptor agonist THC is the main psychoactive chemical in cannabis, and has recognized therapeutic utility for nausea and vomiting due to chemotherapy, chronic neuropathic or cancer pain, and spasticity due to multiple sclerosis (Whiting et al., 2015). Many other cannabinoids and terpenes are present in whole plant cannabis and are being explored for their own therapeutic purposes (e.g., Devinsky et al., 2014). Cannabis and cannabinoids are currently undergoing a major shift in legal status, and range from Schedule I to unscheduled. See below for examples. | |||
Ibogaine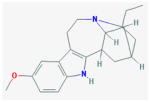
|
Salvinorin A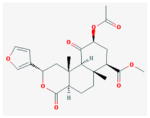
|
THC j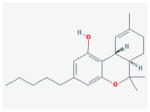
|
||
Note. Chemical structures retrieved from PubChem Compound Database: http://www.ncbi.nlm.nih.gov/pccompound, accessed Jan. 22, 2016. All legal status information refers to United Nations international drug control conventions unless otherwise noted.
LSD =lysergic acid diethylamide.
DMT = N,N-dimethyltryptamine.
MDMA = 3,4-methylenedioxy-methamphetamine.
MDA = 3,4-methylenedioxyamphetamine.
NMDA = N-methyl-D-aspartate.
DXM = dextromethorphan.
MOR= Mu opioid receptor.
KOR = Kappa opioid receptor.
CB1 = cannabinoid 1.
THC = Δ9-tetrahydrocannabinol.
Although these classes do not share a common primary mechanism of action, they do exhibit important similarities in their ability to occasion temporary but profound alterations of consciousness, including acute changes in somatic, perceptual, cognitive, and affective processes. Such effects likely contribute to their recreational use. However, a growing body of evidence indicates that these drugs may have other applications beyond their potential for abuse. A number of naturally occurring hallucinogens have a long history of use as religious sacraments dating back hundreds, and in some cases, thousands of years (El-Seedi et al., 2005; Guerra-Doce, 2015; Li, 1973). Furthermore, despite their current classification as controlled substances, recent analyses have found that psychedelics and cannabinoids in particular exhibit relatively lower risk of harm to the user and society than other currently available drugs such as alcohol and tobacco (Carhart-Harris & Nutt, 2013; Fantegrossi et al., 2004; Nutt et al., 2010; Van Amsterdam et al., 2010; 2011). In a burgeoning revival of clinical research, several hallucinogens have shown promise for a number of difficult to treat medical and psychological conditions, including chronic pain, cluster headache, post-traumatic stress disorder (PTSD), mood disorders, substance use disorders, and psychological distress associated with life-threatening illness, among others (Tupper et al., 2015).
Contemporary research has revisited the potential of hallucinogen-facilitated treatment paradigms, often involving use of these substances in conjunction with psychotherapy, to facilitate salient and cathartic emotional experiences, sometimes leading to lasting benefits. The present paper will offer an examination of several classes of hallucinogens with a particular focus on psychedelics, entactogens, and dissociatives, for which clinical utility has been most extensively documented. Information on each class is presented in turn, tracing relevant historical insights, highlighting similarities and differences between the classes from the molecular to the behavioral level, and presenting the most up-to-date information on clinically oriented research on these substances, with important ramifications for their potential utility to alleviate human suffering.
Psychedelics
From its inception, research with 5-HT2AR agonist hallucinogens was marked with considerable controversy surrounding the nature of these drugs and their effects. Early researchers struggled to create a context within which to understand these unusual substances (Osmond, 1957; Ruck et al., 1979). Many were intrigued by the psychosis mimicking or psychotomimetic properties of these compounds. Isbell et al. (1956) described LSD as, “the most effective and safest agent for inducing an experimental, but reversible, psychosis in nonpsychotic subjects” (p. 468). Others investigated these substances’ putative ability to generate insightful, therapeutic, and even spiritual1 experiences, leading to alternative characterizations such as phantastica (i.e., producing hallucinations and/or visionary states; Lewin, 1931; Stoll, 1947), psychedelic (i.e., mind-manifesting, or soul-revealing; Osmond, 1957, p. 429), and entheogenic (i.e., evoking the divine within; Ruck et al., 1979). For the purpose of the present article, the term psychedelic will be used to denote 5-HT2AR agonist hallucinogens (e.g., LSD, psilocybin, mescaline, DMT, and ayahuasca).
The first generation of clinical psychedelic research began in the mid-20th century and focused almost exclusively on LSD, mescaline, and psilocybin (e.g., Cohen, 1959; Evarts, 1957; Hollister & Hartman, 1962; Isbell et al., 1956; Isbell, 1959; Rinkel, 1957; Sandison et al., 1954; Stoll, 1947; Wolbach et al. 1962). At the time these compounds, LSD in particular, were seminal in the field of neurochemistry, where they helped advance our understanding of serotonin, and its role in the brain (Aghajanian & Marek, 1999; Brodie & Shore, 1957; Cozzi, 2013; Whitaker-Azmitia, 1999; Woolley & Shaw, 1954a, 1954b, 1957).
After extensive experimentation in humans, it became apparent that the pharmacology of psychedelics was not solely responsible for determining their subjective effects. The expectations and personal experiences of the individual taking them as well as the external environment were recognized as vitally important in influencing users’ experiences. These factors were respectively dubbed set and setting, and are now well-established elements of human hallucinogen research (Johnson et al., 2008; Fadiman, 2011; Leary et al., 1963). This insight also helps explain the widely varied reactions to these substances observed across different studies. For instance, one study of LSD for the treatment of alcoholism administered high doses (i.e., 800 micrograms) of the drug to patients strapped to a hospital bed, and without prior preparation (Smart et al., 1966). Such studies stand in stark contrast to research administering psychedelics in aesthetically pleasing, interpersonally supportive environs, which have generally been associated with more beneficial outcomes (Chwelos et al., 1959; Griffiths et al., 2006; Pahnke, 1969).
Lysergic Acid Diethylamide (LSD)
Background
LSD was first synthesized in 1938 by Albert Hofmann, a medicinal chemist employed at Sandoz Laboratory in Switzerland. Its psychoactive properties, however, were not discovered until five years later (Hofmann, 1979; 2013). One of the remarkable early findings concerning LSD was its incredible potency, the dose for minimal psychoactive effects being only 20 micrograms (μg; Greiner et al., 1958), and its therapeutic ‘optimal’ dose lying between 100 and 200 μg (Passie et al., 2008). For nearly 20 years LSD remained relatively obscure in the public sphere. While legitimate scientific research flourished during this period, LSD as a cultural phenomenon was not yet known.
In the 1950s, magazine articles chronicling the LSD experiences of highly visible journalists and movie stars such as Cary Grant began to be published (Bergquist, 1959; Katz, 1953). During this time, the Central Intelligence Agency (CIA) was allegedly sponsoring covert research with LSD as a potential tool for espionage and mind control (Lee & Shlain, 1992; Mashour, 2007), and in the early 1960s Harvard psychologists Richard Alpert and Timothy Leary began their now infamous experimentations with LSD and psilocybin, culminating in their departure from the university in 1963. In 1965, with the media craze reaching a fever pitch, Sandoz Laboratory immediately halted the production and distribution of LSD (Hofmann, 2013). Furthermore, the United Nations (UN) Single Convention on Narcotic Drugs in 1961, and UN Convention on Psychotropic Substances in 1971 placed tight restrictions on psychedelics and other drugs in 183 countries (Nutt, 2014); while in the US, the Controlled Substances Act was signed into law by President Richard Nixon in 1970. In this context, scientific research with LSD, psilocybin, DMT, and mescaline ground to a halt virtually overnight.
Prior to this, a large body of human subjects research with LSD was accumulated, including over 1,000 published papers by 1961 (Dyck, 2005), presenting data on an estimated 40,000 participants (Grinspoon & Bakalar, 1997; Masters & Houston, 2000; Nutt et al., 2013). Much of the earliest work with LSD centered on its psychotomimetic properties, see Osmond (1957) for a seminal review of this early period. However, some argued that to best understand these new compounds it would be necessary to transcend the pathological. In the words of early psychedelic researcher Humphry Osmond, “If mimicking mental illness were the main characteristic of these agents, “psychotomimetics” would indeed be a suitable generic term. It is true that they do so, but they do much more. Why are we always preoccupied with the pathological, the negative? Is health only the lack of sickness?” (1957, p. 429).
The work of Osmond (1957), Grof et al. (1972), and others significantly contributed to the development of a psychotherapeutic paradigm of psychedelic research (Dyck, 2005). Many studies in the late 1950s and 1960s examined LSD’s efficacy in the treatment of a broad variety of conditions including alcoholism (Smart et al., 1964; 1966), opioid dependence (Savage et al., 1973), pain (Kast & Collins 1964), neurosis (Cohen, 1959; Eisner, 1958), and cancer-related anxiety (Grof et al., 1972), among others. Researchers also examined LSD as an aid in facilitating creativity and problem solving in healthy volunteers (Harman et al., 1966; McGlothlin et al., 1967).
Use of LSD in the treatment of alcoholism was one of the most widely studied therapeutic applications of psychedelics. A recent meta-analysis of six double-blind, placebo controlled studies from this period (total N = 536) found that individuals receiving a single dose of LSD in the context of alcoholism treatment exhibited significantly reduced alcohol misuse at initial follow-up compared with patients receiving non-psychedelic control treatments (Krebs & Johansen, 2012). While much of the early research with LSD suffered from methodological shortcomings such as the absence of proper controls or blinding procedures, the sheer outpouring of research during this relatively short period serves as a useful metric for the considerable interest initially generated by LSD (Strassman, 1995a; Mangini, 1998; Dyck, 2005; Passie et al., 2008).
Pharmacology
Lysergic acid diethylamide (LSD) is a semi-synthetic tryptamine derived from the naturally occurring ergot alkaloid ergotamine (Nichols, 2004). LSD acts primarily as a serotonergic agonist, but also shows action at dopaminergic and adrenergic receptor sites (Halberstadt, 2014; Nichols, 2004). Substantial research in both animals and humans has implicated 5-HT2AR agonism as a primary mechanism for LSD and related psychedelics’ psychoactive effects (Glennon et al., 1983; Titeler et al., 1988; Vollenweider et al., 1998). In animal models this has been demonstrated with regard to particular behavioral effects such as the head-twitch response, as well as drug discrimination models (Fantegrossi et al., 2004, Carbonaro et al., 2014). However, differential actions at other serotonergic, dopaminergic, and downstream glutamatergic targets are known to modulate the effects of distinct psychedelics (Moreno et al., 2011; Nichols, 2016; Pieri et al., 1974; Ray, 2010; Vollenweider et al., 1999; Vollenweider & Kometer, 2010). Animal models have not found consistent self-administration of LSD in suggesting a low addictive potential for this drug class (Fantegrossi et al. 2008; Hoffmeister & Wuttke, 1975; Poling & Bryceland, 1979; Schuster & Thompson, 1969).
In humans, the subjective effects of LSD can last up to 12 hours, with rapid tolerance developed after repeated administration, and no evidence of withdrawal (Isbell et al., 1956; Schmid et al., 2015). Recent research has shown that LSD acutely increases plasma cortisol, prolactin, oxytocin, and epinephrine levels (Schmid et al., 2015). Subjective effects of LSD in humans last slightly longer than other psychedelics such as psilocybin and mescaline, though their effects are otherwise considered similar (Abramson et al. 1967; Wolbach et al., 1962). These effects can vary widely, but include altered mood, perception, cognition, the occurrence of elementary and complex hallucinations, as well as experiences described as insightful, transcendent, and/or mystical in nature (i.e., marked by a sense of all encompassing unity; Pahnke & Richards, 1966).
LSD has not been found to produce physiological toxicity, and there have been no documented human deaths from LSD overdose (Passie et al., 2008). However, drug effects can result in disorientation, anxiety, fear of insanity, and feelings that one is dying, which have been characterized colloquially as a “bad trip.” These effects typically resolve during the time course of acute drug action (i.e., within 12 hours; Cohen, 1960; McGlothlin & Arnold, 1971; Strassman, 1984). However, in some rare cases ongoing psychotic symptoms and other psychological sequelae have been reported (Glass & Bowers, 1970; Smart & Bateman, 1967). Although uncommon, it has been hypothesized that such persisting negative effects of hallucinogens may be related to personal or familial predisposition to psychotic disorders (Strassman, 1984), thus underscoring the importance of careful screening in clinical research.
Another potential persisting effect of LSD is hallucinogen persisting perceptual disorder (HPPD) or “flashback,” which is the intermittent reemergence of perceptual distortions weeks, months or longer after the drug’s effects have worn off. What little data are available on HPPD suggest that it occurs with very low prevalence (Baggott et al., 2011; Halpern & Pope, 2003). Data from a 10-year follow-up survey of 247 individuals who received LSD in experimental or psychotherapeutic settings in the 1960s found that 12 individuals (4.9%) reported instances of perceptual disturbances after the drug sessions had concluded, with individuals who revceived LSD on 10 or more occasions more likely to report such effects (McGlothlin & Arnold, 1971).
A more recent review of 20 studies confirmed that while HPPD is indeed reported as an adverse effect in some early studies of LSD, prevalence estimates vary widely (ranging from <5% to 77% of participants), in part because researchers had little or no prior knowledge of such a syndrome, and formal criteria were not devised until years later (Halpern & Pope, 2003). Nevertheless, the authors confirmed that prevalence appears higher among recreational psychedelic users than those administered the drugs in controlled settings, possibly as a result of careful screening procedures used in research settings (Halpern & Pope, 2003).
Early researchers found evidence suggesting that LSD may cause chromosomal damage, creating considerable concern about its use as a therapeutic agent (e.g., Auerbach & Rugowski, 1967; Cohen et al., 1967; Egozcue et al., 1968). In the early 1960s, the drug thalidomide was linked to thousands of birth defects and children’s deaths resulting in a massive public outcry (Lenz et al., 1962). Against this historical backdrop, preliminary reports of LSD’s effects on chromosomes generated a highly charged response from the media and public (Fort & Metzner, 1968). However, risks related to these early findings on LSD and chromosomal damage were later thought to be overstated, as in vitro chromosomal damage could not be consistently replicated in vivo in humans (Cohen & Shiloh, 1977; Dishotsky et al., 1971).
Contemporary Research
In 2014 a double-blind, randomized, active placebo-controlled study investigating LSD-assisted psychotherapy for anxiety associated with life-threatening illnesses was published, the first human trial of LSD in the 21st century (Gasser et al., 2014a). This study compared 20 μg LSD (i.e., active placebo) to 200 μg LSD using a crossover design in 12 patients with anxiety secondary to a life-threatening illness. Results showed that the 200 μg treatment condition significantly reduced state measures of anxiety up to 12 months post-treatment (Gasser et al., 2014a). Qualitative analysis of participant interviews at 12 months post-treatment found no evidence of lasting adverse effects, with participants largely reporting reduced anxiety (77.8%) and increased quality of life (66.7%) since their study participation (Gasser et al., 2014b).
Other recent pilot studies (N = 10) found that LSD may increase suggestibility and creative imagination in healthy volunteers (Carhart-Harris et al., 2014a), and can evoke heightened emotional responses to music (Kaelen et al., 2015). These results could have important implications for the therapeutic use of LSD, as suggestibility has been associated with enhanced treatment outcomes for a number of conditions including pain (Patterson & Jensen, 2003) and depression (Kirsch & Low, 2013). Furthermore, this work can contribute to guiding more refined application of musical stimuli in LSD-facilitated treatments (e.g., McKinney et al., 1997). Research on the neural mechanisms of LSD is currently underway at Imperial College in London and the University of Zurich, demonstrating a renewed interest in the basic science and potential therapeutic effects of this drug (Geyer, 2015).
Psilocybin
Background
Psilocybin is found in over 100 species of mushrooms (Stamets, 1996) and was first isolated in 1958 (Hofmann et al., 1958; Hofmann, 2013). The religious use of psilocybin containing mushrooms has been extensively studied with documented evidence of use throughout Mesoamerica as early as the arrival of Cortés in Mexico in 1519 (Metzner, 2004; Wasson, 1980; Schultes et al., 2001; Ott, 1993; McKenna & Riba 2015), and mushroom-shaped artifacts dating as far back as 500 BCE (Guerra-Doce, 2015). In Nahuatl, the language of the Aztecs, psilocybin-containing mushrooms are referred to as Teonanácatl or “divine flesh” (Schultes, 1940).
Pharmacology
Pharmacologically, psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) is most accurately characterized as a prodrug, and is dephosphorylated by hepatic first pass metabolism into the 5-HT2A, 1A and 2C receptor agonist psilocin (Presti & Nichols, 2004), with subjective effects lasting between 4 and 6 hours (Passie et al., 2002). Recent analysis has demonstrated a largely benign safety profile for psilocybin (van Amsterdam 2011), with independent analyses finding that psilocybin exhibits the least risk of harm to self or others when assessed relative to other commonly abused drugs (Carhart-Harris & Nutt, 2013; Nutt et al. 2010; van Amsterdam, 2010). Nevertheless, users may acutely experience moderate to severe disorientation, anxiety, or fear responses under the influence (Griffiths et al., 2006, 2011), a risk shared with LSD and the other psychedelics.
Early Research
The volume of clinical research with psilocybin during the 1950s and 1960s was nowhere near that of LSD. Many early researchers seemed more interested in studying the subjective effects of psilocybin simply as they compared to LSD (Malitz et al., 1960; Isbell, 1959; Hollister & Hartman, 1962). This period did however produce several landmark psilocybin studies including a 1963 study by Timothy Leary and colleagues remarking on the protective benefit of “set and setting” on the subjective effects of psilocybin (Leary et al., 1963), as well as Walter Pahnke’s (1963) oft-cited dissertation, commonly referred to as the Good Friday experiment.
The Good Friday experiment was an investigation of mysticism and psychedelics in which psychedelic-naive divinity students were administered either psilocybin or an active placebo in a religious chapel on Good Friday. Among a group of religiously inclined people in a setting designed to amplify religious sentiments, psilocybin (30 mg dose) was significantly more effective than an active placebo (i.e., nicotininc acid) at increasing measures of mystical experience and self-reported ratings of personal meaningfulness at 6-month follow-up (Pahnke, 1963).
Doblin (1991) provided a notable long-term follow up and methodological critique of the Good Friday experiment, which found that approximately 25 years later, among 16 of the original 20 participants who could be found, quantitative measures of mystical experience in the psilocybin group (n = 7) were still significantly greater than those of the control group (n = 9). Furthermore, interview data indicated that among those who had received psilocybin, the experiences retained a deep sense of meaningfulness, and were still considered “to have made a uniquely valuable contribution to their spiritual lives” (Doblin, 1991, p. 23) more than two decades later. However, Doblin (1991) also reported that in the original study two volunteers who received psilocybin had challenging experiences and attempted to leave the chapel where the experiment was being held, resulting in one of them being administered thorazine as a tranquilizer, details which were not fully acknowledged in earlier reports.
Another noteworthy early study examined psilocybin-facilitated treatment for rehabilitation of incarcerated men (Leary et al., 1965; Leary, 1969). In this study, 32 male inmates at the Massachusetts Correctional Institute at Concord underwent a psilocybin-facilitated group therapy program focused on reducing subsequent recidivism. Volunteers received two to four administrations of psilocybin with doses ranging from 20–70 mg during a six-week group therapy intervention, and were monitored after parole to determine effects on recidivism. Leary (1969) reported that 73% of the study sample managed to avoid parole violation and new crimes leading to arrest and re-incarceration during the follow-up period. However, in a subsequent long-term follow-up to this study conducted by Doblin (1998) some 34 years after the original study, recidivism rates of 59–71% were reported among experimental participants approximately 2.5 years post-release, roughly equivalent to that of the larger prison population, thus indicating no significant effects of the intervention. Doblin’s (1998) results should be interpreted with caution, as data were limited to only 21 of the original 32 participants whose records could be located.
Leary maintained that these results were not due to treatment failure per se, but could be attributed to lack of structured support after parole, which make it very difficult for such programs to have lasting effects (Doblin, 1998; Leary et al., 1965; Leary, 1969). Nevertheless, as Doblin remarked, “Whether a new program of psilocybin-assisted group psychotherapy and post-release programs would significantly reduce recidivism rates is an empirical question that deserves to be addressed within the context of a new experiment” (1998, p. 425).
A recent longitudinal study of over 25,000 individuals in the criminal justice system in the Southeastern US reported some pertinent findings in this area (Hendricks et al., 2014). All participants had a history of drug use, and were under community corrections supervision. Results found that among the sample, hallucinogen use was significantly associated with reduced rates of supervision failure (i.e., parole or probation violations), whereas use of other drugs such as cocaine predicted increased supervision failure. These findings suggest additional potential for psychedelics in rehabilitation programs among correctional populations (Hendricks et al., 2014).
Contemporary Research
Psilocybin has received renewed attention in the 21st century with multiple studies in various populations. Results from some of the first human laboratory research with psilocybin in decades found that in healthy normal volunteers, 30 mg/ 70 kg psilocybin facilitated mystical-type experiences with sustained meaning and persisting beneficial effects (Griffiths et al., 2006; 2008), consistent with earlier findings from Pahnke (1963) and Doblin (1991). Furthermore, pooled analyses revealed that psilocybin-occasioned mystical experiences were significantly correlated with persisting increases in the personality domain of openness (MacLean et al., 2011), representing the first discrete laboratory manipulation shown to elicit significant, lasting changes in personality, a construct which is considered generally stable throughout adulthood (Terracciano et al., 2005).
Participants also reported experiences of a challenging and sometimes frightening nature under the influence. However, these acute dysphoric responses were well managed with interpersonal support, and resolved by the end of the day-long session. Importantly, even in cases where individuals reported strong ratings of fear or anxiety, the majority of these sessions were still judged as personally meaningful, and no volunteer rated the experience as having decreased their sense of well being or life satisfaction (Griffiths et al., 2006; 2011).
A recent meta-analysis of eight double blind, placebo-controlled experiments conducted in a single laboratory over the course of 10 years analyzed the acute and persisting effects of 227 psilocybin sessions (dose range from 0.115 – 0.315 mg / kg) across 110 healthy volunteers (Studerus et al., 2011). In line with recent work on long lasting personal meaningfulness, 60% of the volunteers in that analysis rated their psilocybin experience “very enriching” and 90% rated it enriching to at least a medium degree between 8 and 16 months after administration (Studerus et al., 2011). The most common adverse side effect of psilocybin was found to be mild and well-tolerated headache (e.g., Johnson et al., 2012), and lethargy immediately after psilocybin administration with normal function largely restored after 24 hours. Interestingly, psilocybin and LSD have been implicated as potential treatments for cluster headache, a highly debilitating pain syndrome; however, most data in this area remain anecdotal in nature, and controlled studies are necessary to assess efficacy (Schindler et al., 2015; Sewell et al., 2006).
Regarding Hallucinogen-Persisting Perception Disorder (HPPD) or related symptoms, Studerus et al. (2011) reported that at baseline nine of the 110 volunteers (10%) reported lifetime prevalence of non-distressing, spontaneously occuring altered states of consciousness (e.g., perceptual alterations), and after study participation, eight (9%) reported recurrences of such instances. That sample was comprised of 60% hallucinogen naieve individuals, and 40% volunteers with prior hallucinogen use, thus data indicate no significant increase in spontaneous perceptual disturbances after experimental drug sessions (Studerus et al., 2011). Additional studies administering from 5 to 30 mg / 70kg of psilocybin to healthy volunteers reported no bothersome or clinically significant perceptual phenomena at 14-months post-session (Griffiths et al., 2008; 2011).
A related meta-analysis examined psilocybin effects across 23 trials (dose range from 0.115 – 0.315 mg / kg) in a sample of 261 healthy volunteers, finding that drug dose, the personality trait absorption, a positron emission tomography (PET) scanning environment, and age were signifncant factors predicting response to psilocybin (Studerus et al., 2012). Specifically, higher dose predicted greater overall drug effects, greater personality absorption predicted more mystical-type effects, and lower age and PET scanner environment predicted greater anxiety (Studerus et al., 2012). Participant gender was not found to have any significant effects on psilocybin response (Studerus et al., 2012), consistent with the limited human data examining sex differences in classic psychedelics’ effects (e.g., Leary et al., 1963). Studerus and colleagues noted that similarity of psychedelic effects across males and females may be attributable to lack of sex differences in 5-HT2AR binding in the cortex (Adams et al., 2004).
Clinical Research
The 21st century has also seen a new wave of clinical research with psilocybin (Table 2) including pilot studies of psilocybin for the treatment of anxiety secondary to a cancer diagnosis (Grob et al., 2011), for obsessive-compulsive disorder (Moreno et al., 2006), for treatment-resistant depression (Carhart-Harris et al., 2016), for smoking cessation (Johnson et al., 2014; Garcia-Romeu et al., 2014), and for alcoholism (Bogenschutz et al., 2015). Grob and colleagues administered 0.2 mg / kg psilocybin and a placebo in counter-balanced order to 12 individuals with cancer, and found significant reductions in anxiety at one month and 3 months post-psilocybin administration, and reductions in depression 6 months post-psilocybin administration (Grob et al., 2011). In keeping with these results Carhart-Harris et al. (2012) conducted functional magnetic resonance imaging (MRI) in 15 healthy volunteers during intravenous (i.v.) psilocybin administration2 (2 mg in 10 mL saline infusion), finding decreased activity in the medial prefrontal cortex (mPFC), an area known to exhibit increased activation in individuals suffering from depression (Drevets et al., 2008). Additionally, Kraehenmann et al., (2014) found reduced amygdala activity in response to negative stimuli and increased positive mood during 0.16 mg / kg psilocybin effects in healthy volunteers, indicating possible neural mechanisms for psilocybin in treatment of mood disorders.
Table 2.
Summary of clinical research with psychedelics.
| Study | Drug (dose) / Design | Total N (no. females) / Diagnosis | Key Findings |
|---|---|---|---|
| Bogenschutz et al., 2015 | Psilocybin (0.3 – 0.4 mg/kg) with MET a / open label | 10 (4) / Alcohol dependence | Significant reduction in self-reported drinking days and heavy drinking days for 32 weeks after psilocybin administration compared to baseline (p < 0.05). |
| Carhart-Harris et al., 2016 | Psilocybin (10 mg and 25 mg) in a supportive setting, open-label | 12 (6) / Treatment-resistant unipolar major depression | Significant reductions in baseline Quick Inventory of Depressive Symptoms (QIDS) scores from one week (p = 0.002; Hedges’ g = 3.1) to 3 months (p = 0.003; Hedges’ g = 2) after 25 mg psilocybin. Beck Depression Inventory (BDI) showed complete remission in 8 (67%) participants at one week, and 5 (42%) participants at 3 months after 25 mg psilocybin, |
| Gasser et al., 2014a | LSD (200 μg) with psychotherapy / randomized double-blind active placebo (20 μg LSD), cross-over | 11 (4) / Anxiety associated with life-threatening illness | Significant reductions in State-Trait Anxiety Inventory (STAI) scores at 2 months post-drug administration, with sustained decrease in STAI scores to 12-month follow-up (p < 0.05). |
| Grob et al., 2011 | Psilocybin (0.2 mg/kg) / randomized double-blind active placebo (niacin) | 12 (11) / Anxiety associated with advanced cancer | Significant reductions in STAI trait anxiety at 1 and 3 months post-treatment (p < 0.05). Significant reductions in Beck Depression Inventory scores at 6-months post-treatment (p < 0.05). |
| Johnson et al., 2014 | Psilocybin (20 and 30 mg / 70 kg) with CBT b / open label | 15 (5) / Tobacco dependence | Biologically verified smoking abstinence in 80% (n = 12) of volunteers at 6-month follow-up, as assessed by exhaled breath carbon monoxide and urine cotinine levels. |
| Krebs & Johansen, 2012 | LSD (200 – 800 μg) with counseling / meta-analysis of controlled trials | 536 (2) / Alcohol dependence | Individuals receiving a single dose of LSD in the context of alcoholism treatment exhibited significantly reduced alcohol misuse at initial follow-up compared with patients receiving non-psychedelic control treatments (OR, 1.96; 95\36–2.84; p = 0.0003) |
| Moreno et al., 2006 | Psilocybin (0.025 – 0.3 mg/kg) / double-blind dose escalation | 9 (2) / Obsessive compulsive disorder | Marked reductions on Yale-Brown Obsessive Compulsive Scale (YBOCS) scores for all participants during one or more psilocybin sessions, ranging from 23 – 100% decrease in YBOCS score, with effects generally lasting more than 24 hours post-drug administration. |
| Osório et al., 2015 | Ayahuasca (2.2 mL/kg)1 / open label | 6 (4) / Recurrent major depressive disorder | Significant reductions in Hamilton Rating Scale for Depression (HAM-D) and Montgomery-Åsberg Depression Rating Scale (MADRS) scores between baseline and 1, 7, and 21 days after ayahuasca administration. |
Note.
MET = Motivational Enhancement Therapy.
CBT = Cognitive behavioral therapy.
Ayahuasca contained: 0.8 mg/mL dimethyltryptamine, 0.21 and mg/mL harmine, and no harmaline.
Another pilot study examining psilocybin in nine individuals with treatment-resistant obsessive-compulsive disorder (OCD) found significant improvement on measures of OCD symptoms at four, eight and twenty-four hours post-psilocybin administration across a range of doses from 0.025 mg / kg (very low dose) to 0.3 mg / kg (Moreno et al., 2006). Furthermore, Carhart-Harris and colleagues (2016) recently published results from an open-label pilot study in which 12 participants with unipolar treatment-resistant major depression received a low (10 mg) and high (25 mg) dose of psilocybin in a supportive setting a week apart. On average, scores on the Quick Inventory of Depressive Symptoms (QIDS) showed significant reductions relative to baseline QIDS scores, from one week (p = 0.002; Hedges’ g = 3.1) to 3 months (p = 0.003; Hedges’ g = 2) after high-dose psilocybin administration (Carhart-Harris et al., 2016). Scores on the Beck Depression Inventory showed complete remission in 8 (67%) participants at one week, and 5 (42%) participants at 3 months after high-dose psilocybin administration, suggesting psilocybin may function as a rapid acting anti-depressant with sustained therapeutic benefits (Carhart-Harris et al., 2016).
Pilot studies examining psilocybin as an aid in treating substance use disorders have also shown promise. In one open-label pilot study, Johnson and colleagues found that two to three doses of psilocybin (20 mg /70 kg and 30 mg /70 kg) in combination with cognitive-behavioral therapy for smoking cessation resulted in an 80% success rate at 6 months, with 12 of 15 participants demonstrating biologically verified smoking abstinence (Johnson et al., 2014). Secondary analyses found a significant correlation between acute mystical-type effects of psilocybin and treatment outcomes at 6-month follow-up (Garcia-Romeu et al., 2014). Similarly, in another open-label pilot study (N = 10), Bogenschutz and colleagues reported that one to two administrations of psilocybin (0.3 mg / kg and 0.4 mg / kg) in the context of motivational enhancement therapy for alcoholism significantly increased abstinence up to 36 weeks later (Bogenschutz et al., 2015). Results from these studies are limited, as both were conducted open-label with small samples, and did not employ a control condition. However, further studies of psilocybin-facilitated treatment of substance use disorders are currently in progress, including randomized controlled trials investigating psilocybin as an aid in smoking cessation and alcoholism treatment, as well as a pilot study of psilocybin for cocaine dependence.
Mescaline
Background
Mescaline was isolated from Lophophora williamsii, a small cactus native to northern Mexico and the southwestern United States, in 1896 by German chemist Arthur Heffter. It was the first naturally occurring psychedelic alkaloid to be isolated in the laboratory (Heffter, 1896). The Lophophora williamsii cactus has a long history of religious use among the indigenous peoples of North and South America, and is often referred to using the Nahuatl term péyotl (aka peyote; Prue, 2013). Religious use of peyote has been estimated to extend back more than 5,700 years (Bruhn et al., 2002; El-Seedi et al., 2005). Despite its Schedule I classification, peyote use is constitutionally protected in the US on the basis of religious freedom when used by the Native American Church (NAC; de Verges, 1974).
Why mescaline never attracted significant cultural attention while LSD would go on to galvanize an entire nation remains an interesting historical question. Despite its comparable obscurity, Aldous Huxley stimulated scientific and artistic curiosity about mescaline when he wrote of his experiences with the drug in The Doors of Perception (Huxley, 1954). Nevertheless, contemporary research with mescaline has remained limited relative to the other psychedelics, possibly due to its tendency to induce nausea (Deniker, 1957), or its longer duration of action and lesser potency compared to psilocybin and LSD (Wolbach et al., 1962).
Later in the 20th century, chemist and pharmacologist Alexander Shulgin’s pioneering work included modification of the mescaline molecule to create many highly potent phenethylamine hallucinogens such as 4-Bromo-2,5-dimethoxyphenethylamine (2C–B) and 2,5-Dimethoxy-4-Methylamphetamine (DOM) among many others, some of which are growing increasingly popular as recreational drugs in recent years (Shulgin, 1973; Shulgin & Shulgin, 1995; Shulgin & Nichols, 1978; Faillace et al., 1970; Caudevilla-Gálligo et al., 2012).
Pharmacology
Mescaline (3,4,5-trimethoxy-β-phenethylamine) is a naturally occurring psychedelic found in a number of cacti including peyote (Lophophora williamsii), and San Pedro cactus (Echinopsis pachanoi), and derived from the amino acid phenylalanine. The first generation of scientists to conduct human subjects mescaline research seemed most interested in mescaline simply as it compared to LSD, similar to early research with psilocybin, and these findings confirmed that the effects and risks of mescaline are largely comparable to those of LSD and psilocybin (Rinkel, 1957; Hollister & Hartman, 1962). Although some research has been forthcoming examining the effects of mescaline in humans as a model psychosis (Hermle et al., 1992; 1998), clinical research investigating mescaline as a potential therapeutic aid has been lacking. However, research examining the indigenous use of peyote holds some clinical relevance.
Early Research
Early observational studies of peyote use by the Native American Church (NAC) concluded that religious use of peyote seemed safe and may prove effective in the treatment of alcoholism (Albaugh & Anderson, 1974; Bergman, 1971; Garrity, 2000; Prue, 2013). These findings are limited due to their reliance on naturalistic observation rather than human laboratory administration. However, more recent data seem to corroborate these results. One study compared the mental health of long-term peyote using NAC members who reported minimal use of any other drugs (n = 61) with a median of 300 lifetime episodes of peyote use, to Native Americans with a history of alcohol abuse (n = 36), and non-drug using Native American controls (n = 79). Results found that long-term peyote users demonstrated no cognitive deficits compared to non-drug controls. Additionally peyote users showed significantly greater psychological well-being and general positive affect than non-drug using controls, whereas individuals in the chronic alcohol use group exhibited significant cognitive and neuropsychological deficits compared to both control and peyote using participants (Halpern et al., 2005). These data, though limited in scope, suggest a potential role for mescaline, and possibly other phenethylamines, as therapeutic agents warranting further investigation.
DMT
Background
The subjective effects of N, N-Dimethyltryptamine (DMT) were discovered in 1956 (Szara, 1956). Early research on DMT focused on the basic physiological effects and psychotomimesis of DMT as well as the synthesis of several new DMT analogs such as N, N-Diethyltryptamine (DET) and N, N-dipropyltryptamine (DPT; Böszörményiet al., 1959). DMT has become increasingly visible in recent years due to its coverage in several popular online media outlets and in film (e.g., Barclay, 2012). Recent survey data from the Global Drug Survey suggests DMT may be growing in popularity as a recreational drug and is almost always vaporized and inhaled when not prepared as ayahuasca, a South American DMT containing brew which will be covered in the following subsection (Winstock et al., 2013).
Pharmacology
While similar to LSD and psilocybin in many regards including molecular composition and affinity for the 5-HT2AR, DMT also possesses many unique characteristics. This first became evident in 1965 when DMT was detected in the urine and blood of healthy adults (Franzen & Gross, 1965). Since then, many studies have identified DMT in the body fluids both of schizophrenics and healthy, non-drug using individuals (Barker et al., 2012). DMT has also been identified in whole rat brain homogenate and more recently in rat pineal gland (Barker et al., 1980; 2013; Christian et al., 1977). Besides the serotonin 2A, 2C, and 1A receptors, DMT also displays affinity and agonist activity at the sigma-1 and trace amine associated receptors, among others (Bunzow et al., 2001; Fontanilla et al., 2009).
Indolethylamine-N-methyyltransferase (INMT), the enzyme responsible for synthesizing DMT from tryptamine is widely expressed in the body including in the lungs, thyroid, adrenal glands, placenta, skeletal muscle, heart, small intestine, stomach, pancreas and lymph nodes (Thompson et al., 1999). Many suggestions have been put forth regarding DMTs physiological role in the body, though there is no widely accepted consensus (Burchett & Hicks, 2006; Callaway, 1988; Frecska et al., 2013; Jacob & Presti, 2005; Su et al., 2009). For instance, DMT has been implicated as a mediator of consciousness and perception, particularly visual perception, via interactions with trace amine associated recptors (Wallach, 2009).
Other researchers have proposed an immunomodulatory role of DMT, suggesting that “while DMT is a substance which produces powerful psychedelic experiences, it is better understood not as a hallucinogenic drug of abuse, but rather an agent of significant adaptive mechanisms that can also serve as a promising tool in the development of future medical therapies” (Frecska et al., 2013, p. 1295). Along these lines, Szabo (2015) suggested a possible therapeutic role for psychedelics in modulating immune function and reducing inflammation, potentially through sigma-1 receptor mediated pathways. These claims are consistent with preclinical data showing administration of the 5-HT2AR agonist (R)-2,5-dimethoxy-4-iodoamphetamine (R-DOI) greatly suppresses tumor necrosis factor alpha (TNF-α) induced inflammation in vitro (Yu et al., 2008), and in vivo (Nau et al., 2013). Furthermore, these results have been found to generalize to a rodent model of allergic asthma, indicating considerable clinical potential (Nau et al., 2015).
Early Research
Early human subjects research with DMT focused largely on basic psychopharmacology, and psychotomimetic effects of this substance (e.g., Gillin, et al., 1976). Like other classic hallucinogens, research with DMT ceased with the passage of the Controlled Substances Act, and was never investigated as an aid in clinical treatment to the extent LSD was. However, research with dipropyltryptamine (DPT), a closely related synthetic analog of DMT, revealed some promise as an adjunct to psychotherapy both with alcoholics (Grof et al., 1973; Rhead et al., 1977; Soskin et al., 1973), and those with anxiety associated with a terminal cancer diagnosis (Richards et al., 1977; 1980; Richards, 1978). Contemporary survey data from a sample of 121 Australian recreational DMT users found that 31.1% of lifetime DMT users claimed psychotherapeutic benefits as a reason for DMT use, and 75.5% reported psychospiritual insight as their primary motivation for DMT use (Cakic et al 2010), consistent with earlier research on DPT as an adjunct to psychotherapy.
Contemporary Research
Human subjects research with DMT resumed in 1990 after a long hiatus, with several experiments performed to assess the basic pharmacological and subjective effects of DMT in experienced hallucinogen users (Strassman, 1995b; Strassman & Qualls, 1994; Strassman et al., 1994a; Strassman et al., 1996). Major contributions of this work included the finding that i.v. administration of DMT to carefully screened volunteers was physiologically and psychologically well tolerated. Unlike other 5-HT2AR agonists, closely spaced administrations of DMT did not result in psychological tolerance.
More recently, researchers have administered DMT and ketamine to experienced drug users in order to better characterize serotonergic and glutamatergic models of psychosis (Daumann et al., 2008; 2010; Gouzoulis-Mayfrank et al., 2005; Heekeren et al., 2007). Results showed that in healthy subjects, DMT was associated with incidence of positive symptoms of schizophrenia (e.g., thought disorder, inappropriate affect), while ketamine was associated with more negative symptoms (e.g., catatonia, body perception disturbances), suggesting differentially mediated effects of serotonin and glutamate in the manifestation of psychotic disorders (Gouzoulis-Mayfrank et al., 2005). Data additionally showed that DMT had no significant effect on pre-pulse inhibition (PPI) of the acoustic startle reflex in healthy subjects, and ketamine increased PPI, responses which are inconsistent with the diminished PPI startle reflex observed in schizophrenia (Heekeren et al., 2007).
Another study found differential alterations in neural correlates related to visual and auditory processing tasks during acute DMT and ketamine administration (Daumann et al, 2008; 2010). These results highlight some noteworthy differences between hallucinogen-induced model psychoses, and naturally occurring schizophrenia, and furthermore make the case for continued human subjects hallucinogen research as a means of enhancing our understanding of organically occurring psychotic disorders and symptoms. Nevertheless, while data on pure DMT as a clinical aid are still lacking, ayahuasca, an indigenous DMT-containing formulation is receiving increasing attention as a potential tool in therapy.
Ayahuasca
Background
Western scientific knowledge of ayahuasca, a Quechua term for “vine of the soul” (McKenna, 2004) dates to the 19th century when it was first described by pioneering botanist Richard Spruce on a botanical expedition to the Amazon (Spruce, 1873). We now know that what we call ayahuasca is a loosely defined admixture that has been documented to contain “more than 90 different plant species from 38 plant families” (Ott, 1993, pg. 221). Luna (1986a; 1986b) identified 72 indigenous groups reported to use ayahuasca and 42 different indigenous names for the beverage. While the use of ayahuasca by indigenous populations dates back thousands of years (Naranjo, 1979), its broader use in a syncretic religious context is a product of the 20th century. The oldest syncretic ayahuasca church, Santo Daime, was founded in the 1930s. By 2005 Santo Daime had at least one church in 23 different countries (Labate et al., 2008; MacRae, 1992). Religious ayahuasca use was legalized in Brazil in 1987 (Grob et al., 1996). In 2006, O Centro Espirita Beneficente Uniao do Vegetal (UDV) another prominent ayahuasca church, was granted the legal right to conduct ayahuasca ceremonies in the United States as an expression of religious freedom, despite the Schedule I status of DMT, which is contained in ayahuasca (Bullis, 2008). In 2008, ayahuasca was formally declared a part of the national cultural heritage of Peru by the Peruvian government (Insituto Nacional de la Cultura, 2008).
Pharmacology
Despite their chemical and botanical diversity, the majority of ayahuasca brews are characterized by the combination of DMT, the only major alkaloid present in the leaves of Psychotria viridis, and the β-carboline alkaloids harmine, tetrahydroharmine, and harmaline present alongside additional trace alkaloids in the bark and stems of the vine Banisteriopis caapi (McKenna et al., 1984a; 1984b). DMT is rapidly metabolized by monoamine oxidase (MAO) in the gut following oral administration, however, the β-carboline alkaloids harmine, tetrahydroharmine and harmaline are potent MAO inhibitors (MAOIs), preventing the first-pass oxidative deamination of DMT (Callaway et al., 1999; McKenna & Towers, 1984).
Concentrations of DMT and β-carboline alkaloids vary widely by batch, however, laboratory analysis of one batch of Santo Daime Brazilian ayahuasca contained 0.53 mg / mL DMT, 0.9 mg / mL harmine, 0.06 mg / mL harmaline and 0.72 mg / mL tetrahydroharmine. The above study considered a high dose of ayahuasca to be 0.85 mg / kg bodyweight of DMT, 1.4 mg / kg of harmine, 0.09 mg / kg of harmaline, and 1.16 mg / kg of tetrahydroharmine (Riba et al., 2003). The same study identified peak plasma concentrations of DMT at 1.5 hours, peak plasma levels of harmaline at 2 hours and peak plasma levels of tetrahydroharmine at 3 hours and undetectable plasma levels of harmine in blood plasma after a high dose. Peak subjective effects coincided with peak plasma DMT levels at both dose ranges. Cardiovascular effects were modest with a statistically significant increase in diastolic blood pressure (9 mm / Hg at 75 minutes) in the high dose condition only, and no significant effect on systolic blood pressure or heart rate at any dose (Riba et al., 2003).
Contemporary Research
Although research with pure DMT has remained largely focused on pharmacology and psychotomimetic effects, the rapid growth of ayahuasca as a cultural phenomenon and religious sacrament has put increased pressure on researchers around the world to study its effects scientifically. A seminal study of ayahuasca use among 15 adult male members of the UDV was initiated in 1993, and subsequently known as the ‘Hoasca Project’ (McKenna, 2004; McKenna et al., 1998; Grob et al., 1996). Results indicated that structured ayahuasca consumption was medically safe (Callaway et al. 1996; 1999), and exhibited a potential protective psychological effect. Semi-structured clinical interviews found higher rates of adverse psychiatric diagnoses and symptoms including violent behavior, substance abuse, depression, and anxiety disorders in UDV members as compared to non-ayahuasca using controls prior to their initiation into the UDV, and near complete remission of all pathological behaviors after their long-term involvement with the UDV (Grob et al., 1996). Later research has confirmed and elaborated on the medical safety and pharmacology of ayahuasca use (Barbosa et al., 2012; Bouso et al., 2012; Dos Santos et al., 2007, 2011, 2012; Gable, 2007; Riba et al., 2001; Riba & Barbanoj, 2005).
Initial research on the long-term neuropsychological effects of ayahuasca (e.g., Grob et al., 1996) has continued in the 21st century with several teams conducting similar lines of research on a variety of ayahuasca using populations. As a whole, this literature has shown decreases in measures of psychopathology and increases in performance on cognitive tasks in a Brazilian cohort of adult ayahuasca users as compared to matched non-ayahuasca using controls at baseline and 1 year follow up (Bouso et al., 2012); decreases in alcohol consumption and measures of addiction severity in both urban and rural ayahuasca users as compared to matched controls (Fábregas et al., 2010); decreased rates of psychopathology as well as alcohol and amphetamine use among Brazilian adolescent religious ayahuasca users compared to matched controls (Da Silveira et al., 2005; Doering-Silveira et al., 2005a, 2005b); and decreased substance use and psychopathology among a group of American religious ayahuasca users (Halpern et al., 2008).
One recent study of note compared 22 regular ayahuasca with 22 matched controls, finding that ayahuasca users exhibited significantly greater self-transcendence (ST), a personality trait related to spirituality, and significantly less cortical thickness in the posterior cingulate than non-users (Bouso et al., 2015). Furthermore, an inverse correlation between ST and cortical thickness in the posterior cingulate cortex was found, suggesting a compelling link between psychedelic use, brain structure, and spiritual attitudes. Additionally, Alonso and colleagues reported that ayahuasca’s acute effects may in part be associated with alterations of functional coupling and information flow between brain regions particularly, potentially through enhancement of bottom-up information transfer (Alonso et al., 2015). This work represents some of the most clinically relevant findings among a rapidly growing literature on 5-HT2AR agonists and other hallucinogens’ effects on the brain and neurological function (e.g., Carhart-Harris et al., 2012; 2014b; Palhano-Fontes et al., 2015; Petri et al., 2014; Roseman et al., 2014; Tagliazucchi et al., 2014), a full review of which lies outside the scope of the current paper.
Addiction
Interest in ayahuasca as a potential aid in the treatment of substance abuse has been encouraged by observational findings including decreased rates of alcohol use among current users of ayahuasca, as well as reduction of substance abuse upon initiation of religious ayahuasca use have been reported in multiple studies (Bouso & Riba, 2014; Grob et al., 1996; Halpern et al., 2008; Da Silveira et al., 2005; Doering-Silveira et al., 2005a, 2005b; Fábregas et al., 2010; Labate et al., 2010). Multiple rehabilitation centers structured around religious ayahuasca use for the treatment of substance abuse have opened in Brazil, Peru, Argentina, Uruguay and Chile (Mabit, 2002; 2007; Prickett & Liester, 2014). While intriguing, none of these centers have been examined by any independent researchers. An internal report from one such center in Peru documents their activities from the year 1992 to 1998, during which time 380 patients were admitted, stating that 62% claimed to have benefitted in some capacity from their treatment model (Mabit, 2002). Data from two recent pilot studies of ayahuasca-assisted treatment in drug dependent individuals further suggest ayahuasca’s potential for enhancing psychological well-being and decreasing problematic substance use among these populations (Fernández et al., 2014; Thomas et al., 2013). However, larger, more carefully controlled studies are necessary before results can be deemed conclusive.
Depression
Interest in ayahuasca for the treatment of major depressive disorder (MDD) has also been forthcoming, and stems largely from its influence on serotonergic neurotransmission, where both DMT and β-carboline alkaloids have demonstrated activity (de Lima et al., 2011; Palhano-Fontes et al., 2014). One of the seminal findings in this area was the discovery that long-term ayahuasca use was correlated with an increased density of serotonin transporters in platelets (Callaway et al., 1994; McKenna 2004), deficits of which have been implicated in aggression, substance abuse, and MDD (Gorwood et al., 2000; Hallikainen et al., 1999; Tiihonen et al., 1997).
Additionally, ayahuasca has demonstrated effects in other biological systems implicated in depression. These include effects on hypothalamic-pituitary-adrenal (HPA) axis function (Dos Santos et al. 2011; 2012), which regulates the production and transmission of hormones throughout the brain and body, mediating the body’s stress response through hormones such as cortisol and adrenaline, which also affect the latency and duration of rapid eye movement (REM) sleep (Buckley & Schatzberg, 2005). Ayahuasca has been found to inhibit REM sleep and increase slow-wave activity without reducing subjective sleep quality (Barbanoj et al., 2008). These effects are largely consistent with those exhibited by approved serotonergic anti-depressants such as mirtazapine, which down-regulate HPA axis hyperactivity in depressed patients and inhibit REM sleep (Mayers & Baldwin, 2005; Schüle, 2007; Tsuno et al., 2005). Furthermore, β-carbolines found in ayahuasca (e.g., harmine) have also demonstrated anti-depressant properties in rodent models of depression (Aricioglu & Altunbas 2003; Farzin & Mansouri 2005; Fortunato et al. 2009; 2010).
Pilot findings have demonstrated acute reductions in hopelessness and panic-like symptoms among a small sample of religious ayahuasca users (N = 9) during active ayahuasca administration compared with placebo control conditions (Dos Santos et al., 2007), as well as statistically significant reductions in depression scores for up to 21 days after administration of a single dose of ayahuasca in an open-label pilot of six patients with a current depressive episode (Osório et al., 2015). Furthermore, ayahuasca has recently been reported to significantly increase mindfulness related capacities, reducing participants’ judgment and reactivity towards inner experiences, 24 hours after ayahuasca use in a sample of 25 healthy volunteers (Soler et al., 2015). Such effects may indicate a potential psychological mechanism mediating therapeutic effects of ayahuasca, as successful mindfulness-based treatments for depression have been shown to decrease brooding, in part by enhancing nonjudgmental awareness (Shahar et al., 2010).
While the majority of this research suggests ayahuasca use is safe, has a low abuse potential, does not exhibit many of the problems typically associated with drug abuse, and may even be useful in the treatment of psychiatric conditions (Barbosa et al., 2012; Osório et al., 2015), methodological shortcomings, and the possibility of self-selection bias should also be noted. Future research should make an effort to study individuals who used ayahuasca in religious contexts for a period of time before abandoning the practice to compare to active users. Thus far, only a small number of studies have been published studying individuals before and after participating in their first ayahuasca ceremony, much of which has focused on qualitative data and motivation for using ayahuasca for the first time (Barbosa et al., 2005, 2009; Harris & Gurel, 2012; Trichter et al., 2007).
Entactogens
The term entactogen, from the Greek meaning, “to touch within” was coined by Nichols (1986) to describe the psychoactive effects of the synthetic drugs 3,4-methylenedioxy-methamphetamine (MDMA), 3,4-methylenedioxy-amphetamine (MDA) and 3,4-methylenedioxy-N-ethyl-amphetamine (MDEA; Nichols, 1986). Entactogens combine the catecholaminergic effects of methamphetamine, from which they are derived, with the serotonergic effects of psychedelics, exhibiting a unique profile of prosocial and interpersonal effects. Evidence for the distinction of entactogens from both methamphetamine and psychedelics comes from studies of molecular structure-activity relationships and animal models of self-administration (Nichols, 1994; Nichols & Oberlender, 1989) indicating the robustness of the drug family. The main focus of the following section is MDMA, by far the most widely studied and recreationally used entactogen (Freudenmann et al. 2006; McDowell & Kleber, 1994).
3, 4-methylenedioxymethamphetamine (MDMA)
Background
3,4-methylenedioxymethamphetamine (MDMA) is a methamphetamine derivative first synthesized in 1912 for use as an intermediary in the production of other chemicals (Karch, 2011). While the first half of the 20th century produced some preliminary human and animal data on MDMA, it did not rise from scientific and cultural obscurity until the second half of the 20th century. In 1976, Dr. Leo Zeff became the first psychotherapist in the United States to incorporate MDMA into his private practice psychotherapy, conducting hundreds of sessions and proselytizing for its therapeutic use among fellow therapists. On the East coast of the U.S. especially, MDMA became an increasingly popular tool for therapists who believed it encouraged the psychotherapeutic alliance, increased empathy and openness, and allowed for the expression of highly charged and traumatic emotional material (Greer & Tolbert, 1986; Pentney, 2001; Stolaroff, 1997). One MDMA psychotherapist estimated that as many as 4,000 therapists were introduced to MDMA during this period (Holland, 2001).
During the late 1970s and early 1980s MDMA began to gain popularity for recreational use. By 1981 MDMA acquired the street name ‘Ecstasy,’ and in 1985 was classified as a Schedule I controlled substance during an emergency session of the DEA (Riedlinger, 1985). The first peer-reviewed papers on MDMA psychotherapy were not published until after its emergency scheduling (Greer & Tolbert, 1986; 1990; 1998). It is estimated that during the period from 1990 to 1995 global MDMA use increased by 4,000 percent (Holland, 2001). In the first five months of the year 2000 over four million doses of the drug were confiscated by United States authorities alone (Holland, 2001). This explosion of recreational use was accompanied by an entirely new youth culture, the rave and electronic dance music culture (Arria et al., 2002).
Pharmacology
Pharmacologically, MDMA possesses properties of both methamphetamine and mescaline. Like methamphetamine, MDMA is a potent releaser of catecholamine neurotransmitters (i.e., epinephrine, norepinephrine, and dopamine) via action at presynaptic reuptake sites. MDMA is also a potent releaser of pre-synaptic serotonin (De la Torre et al., 2004; Nichols & Oberlender 1989; 1990; Nichols, 1994). Like mescaline and other classic hallucinogens, the subjective effects of MDMA are also attenuated by the 5-HT2AR antagonist ketanserin (Liechti et al., 2000).
MDMA is usually taken orally, however, limited i.v. use has been observed among some experienced users with an eventual return to oral use in most cases (Topp et al., 1999). Peak plasma levels occur within 1 to 2 hours, with subjective effects lasting between 2 and 4 hours (De la Torre et al., 2004). The full range of subjective and cardiovascular effects are evident at doses at or above 1.0 mg / kg (Dumont & Verkes, 2006). Of potential relevance to its therapeutic utility, MDMA has been shown to have dramatic effects on plasma levels of oxytocin, cortisol, and prolactin (Dumont & Verkes 2006; Dumont et al., 2009; Harris et al 2002; Parrott et al., 2013; Wolff et al., 2006, 2012).
Safety
The safety of MDMA continues to be an area of intense controversy, the breadth of which is outside the scope of this review. However, for a thorough review of this topic see Parrott (2013; 2014); Doblin et al. (2014); Nutt (2009); and Henry and Rella (2001). It is important to make a distinction between the medical risks of controlled MDMA use and recreational use which is often associated with prolonged dancing and physical exertion, lack of proper hydration, and additional confounds such as poly-drug use and the fact that black market ‘Ecstasy’ is sometimes adulterated with other potentially dangerous drugs such as amphetamine, ephedrine, ketamine, methamphetamine, MDA, MDEA, and caffeine, and may not contain any MDMA at all (Sherlock et al., 1999; Cole et al., 2002).
In laboratory studies of human volunteers, MDMA exposure is associated with increased heart rate, increased systolic and diastolic blood pressure, accelerated breathing, jaw clenching, thirst and increases in core body temperature (Dumont & Verkes, 2006; Freedman et al., 2005; Kirkpatrick et al., 2014a; Liechti et al., 2001). In combination with strenuous activity and warm, poorly ventilated indoor areas which are often encountered in recreational settings, MDMA use can result in hyperthermia which in extreme cases can lead to hospitalization, organ failure and even death (Chadwick et al., 1991). Recent research using animal models has demonstrated a pronounced effect of ambient temperature on MDMA induced hyperthermia. For a review on the thermal effects of MDMA see Parrott (2012).
Recreational ‘Ecstasy’ use has also been associated with cognitive deficits, including memory deficits (Laws & Kokkalis 2007; Nulsen et al., 2010; Rogers et al., 2009). Interpretation of this literature is sometimes complicated by poly-drug use and the unknown content and dosage of black market ‘Ecstasy’. In an attempt to address this issue, one study compared 12 moderate ‘Ecstasy’ users (22–50 lifetime uses), and 11 heavy users (60–450 uses) who self-reported minimum exposure to any other drugs including alcohol, and 16 individuals reporting no lifetime ‘Ecstasy’ use. Findings showed no significant differences between moderate users and non-drug controls on a battery of cognitive tasks. Significant differences were found between heavy users and non-drug controls, with heavy users exhibiting decreased mental processing speed and greater impulsivity (Halpern et al., 2004). However, as Lyvers and Hasking (2004) noted, Halpern et al.’s (2004) results may have been overstated, as the study used a large number of measures (39 total) in a relatively small sample (N = 39), performing 117 between-group comparisons without adjusting for Type I error, and furthermore cannot demonstrate causation as such between group differences may have been pre-existing, potentially contributing to heavy users’ more extensive recreational drug use.
Two similar studies in larger samples examined cognitive function in current and ex-‘Ecstasy’ users, poly-drug users, and drug-naive controls (Hoshi et al., 2007; Roiser et al., 2007). The first (N = 109) found no significant differences in cognitive processing between current ‘Ecstasy’ users and drug-naive controls; however among both current- and ex-‘Ecstasy’ users greater impulsivity was correlated with greater cognitive impairment, a relationship which was not apparent among controls, suggesting impulsivity as a mediator of vulnerability to cognitive deficits in chronic ‘Ecstasy’ users (Roiser et al., 2007). Another comparable study (N = 109) reported that while current- ‘Ecstasy’ users and poly-drug users showed subtle impairment in verbal learning and memory, as well as response inhibition in a Go/No-go task, the majority of assessments did not show significant differences from drug-naive controls, indicating that recreational drug use, and particularly recent drug use were more likely associated with the observed cognitive deficits rather than ‘Ecstasy’ use per se (Hoshi et al., 2007).
The debate on MDMA neurotoxicity goes back over 20 years (Baggott & Mendelson, 2001; Baumann et al., 2007; McKenna & Peroutka, 1990; Parrot, 2002; 2013; 2014). Extensive animal data has shown reduced levels of serotonin (5-HT), the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) and the 5-HT transporter (SERT) after exposure to MDMA, as well as damage and or loss of serotonergic axon fibers after exposure to MDMA (O’hearn et al., 1988; Scallet et al. 1987; Ricaurte et al., 1999). While compelling, many unresolved questions cloud interpretation of this literature, including determination of comparable doses of MDMA in non-human animals, the validity of using extremely high doses and exposing the test animal to multiple doses in a short amount of time, and questions such as the lack of a clear and unambiguous definition of neurotoxicity (Baumann et al., 2007; Easton & Marsden 2006). In a review of the rodent literature Baumann and colleagues point out that 1–2 mg / kg doses of MDMA elicit comparable neurochemical effects in rats and humans, and thus doses do not necessarily need to be adjusted between these species (Baumann et al., 2007). Furthermore, while relatively high doses of MDMA (e.g., 10–20 mg / kg) in rodent models show evidence of serotonin depletion, other markers of neurotixicity such as cell death are not reliably evoked (Baumann et al., 2007). Nevertheless, the presence of persisting anxiety-like behavior in rats adminstered moderate doses (5–7.5 mg / kg) of MDMA do pose a potential risk in addition to concerns about neurotoxicity (Baumann et al., 2007).
Recently, some researchers have proposed that rather than MDMA neurotoxicity, the observed effects represent a neuroadaptive response to a foreign stimulus (e.g., Kindlundh-Högberg et al., 2008). Taken together, evidence indicates that chronic recreational ‘Ecstasy’ users may experience some lasting functional impairment (Parrott, 2013), though such effects are not known to generalize to individuals undergoing limited exposure to MDMA of established dosage and purity in controlled settings for MDMA-assisted treatment paradigms (Doblin et al., 2014).
Contemporary Research
In recent years laboratory data on subjective effects of MDMA, and in particular social cognitive effects of potential clinical relevance, have begun to accumulate (Bedi et al., 2009; Danforth et al., 2015; Kamilar-Britt & Bedi, 2015; Kirkpatrick et al., 2014a; 2014b; 2015; Wardle & de Wit, 2014; Wardle et al., 2014). Such findings include acute reduction in the recognition of fearful faces and identification of negative emotions in an ‘eyes only’ emotion recognition task (Bedi et al., 2010; Hysek et al., 2012), and slowed perception of angry expressions and an increased psychophysiological response to happy expressions after MDMA administration (Wardle & de Wit, 2014). Utilizing measures of facial emotion recognition in a functional MRI paradigm, researchers observed a decreased amygdala response to angry faces and increased ventral striatum activity in response to happy faces suggesting an enhanced response to rewarding social cues (Bedi et al., 2009). MDMA has also been demonstrated to acutely increase social cognitive factors such as generosity, communicativeness, and self-compassion (Baggott et al., 2015; Kamboj et al., 2015; Kirkpatrick & de Wit, 2015).
Unlike the classic psychedelics, MDMA has shown significant sex differences in human subjects (Liechti et al., 2001). In a pooled analysis of three double-blind placebo controlled trials Liechti et al (2001) compared the effects of MDMA (dose range 75–150 mg) in 54 male and 20 female volunteers, finding that subjective effects of MDMA were more intense in women, with female volunteers exhibiting greater perceptual changes, thought disturbances, and fear of loss of body control, as well as more adverse effects and sequelae post-drug administration. MDMA has also been found to increase measures of emotional empathy and prosocial behavior in men, while impairing identification of negative emotions in women (Hysek et al., 2013). Such differential effects may have significant ramifications for use of MDMA and other entactogens as therapeutic agents, and warrant further investigation.
Multiple studies of social behavior in rodents after MDMA exposure have reported significant prosocial effects (Kamilar-Britt & Bedi, 2015), including decreased aggressive behavior and increased social interaction (Morley & McGregor, 2000). Some of these effects have been shown to be attenuated by pre-treatment with an oxytocin receptor antagonist (Thompson et al., 2007). Recent neurobiological models for MDMA in the treatment of anxiety disorders have drawn on this body of work proposing three mechanisms by which MDMA may be an effective pharmacotherapy in the treatment of anxiety disorders. The authors suggest that increased oxytocin levels may account for the strengthening of the psychotherapeutic alliance first described by the earliest MDMA psychotherapists. Furthermore, increased ventromedial prefrontal activation and decreased amygdala activation were hypothesized to improve emotional response to emotionally difficult material. Finally, acute increases in cortisol levels under the influence of MDMA may facilitate the extinction of learned fear associations within a therapeutic context (Johansen & Krebs 2009).
Clinical Research
The clinical research available on MDMA-assisted psychotherapy consists of data collected by MDMA therapists in the U.S. before the scheduling of MDMA in 1985 (Greer & Tolbert, 1986), data from a Swiss team collected between 1988 and 1993 (Gasser, 1994), and a more recent wave of preliminary clinical trials in the 21st century. The early data consists largely of retrospective, qualitative analyses of MDMA-assisted psychotherapy sessions, which were proposed to “reduce or somehow eliminate the neurophysiological fear response to a perceived threat to one’s emotional integrity” (Greer & Tolbert, 1998, p. 377), thereby facilitating therapeutic outcomes. Follow-up data collected from 29 subjects who underwent MDMA-assisted psychotherapy found the most commonly reported benefits to be positive changes in attitudes or feelings, expanded mental perspective, increased insight into personal problems, and positive changes in their relationships. Common negative effects of MDMA-assisted psychotherapy included undesirable emotional symptoms such as anxiety during and following the session and undesirable physical symptoms such as jaw clenching. Consistent with psychedelic research from two decades prior, the importance of set, setting, and careful preparation were also cited as crucial factors in effective MDMA-assisted psychotherapy (Greer & Tolbert, 1986).
Additionally, a team of Swiss researchers collected quantitative long-term follow up data on 121 patients who had undergone at least one session of MDMA-assisted psychotherapy, with 84.3% of completers reporting increased quality of life and 90.9% reporting good or slight improvement in their condition after MDMA-assisted psychotherapy (Gasser, 1994). The mean number of MDMA-assisted sessions was 6.8. It should be noted that 71% of participants who were mailed the questionnaire completed and returned it, making positive selection bias a possibility. Additionally, both the American and the Swiss teams reported decreased drug use after MDMA-assisted psychotherapy, particularly with regards to alcohol and cannabis (Gasser, 1994; Greer & Tolbert 1986).
More recent clinical MDMA research has largely focused on treatment-resistant posttraumatic stress disorder (PTSD) with three double-blind, placebo-controlled pilot trials of MDMA-assisted psychotherapy for PTSD and one long-term follow up study published since 2008, and several more underway. Of these, one study conducted by Bouso et al. (2008) was cut short before its completion. Of the two remaining studies, each used the Clinician Administered PTSD Scale (CAPS) as the primary outcome measure (Table 3).
Table 3.
Summary of clinical research with entactogens.
| Study | Drug (dose) / Design | Total N (no. females) / Diagnosis | Key Findings |
|---|---|---|---|
| Bouso et al., 2008 | MDMA a (50 – 75mg) with psychotherapy / not completed | 6 (6) / chronic PTSD b after sexual assault | Low doses (50 – 75mg) of MDMA were physiologically and psychologically well-tolerated in the study sample. However the study was not completed and therefore statistical analyses could not be conducted. |
| Mithoefer et al., 2011, 2012 | MDMA (125 – 187.5mg) with psychotherapy / randomized double-blind placebo-controlled cross-over | 20 (17) / chronic treatment-resistant PTSD | Significant decreases in Clinician-Administered PTSD Scale (CAPS) scores from baseline to 2 months post-treatment, with sustained decreases in CAPS scores in 16 volunteers who completed a long-term follow-up 17 – 74 months post-treatment. |
| Oehen et al., 2013 | MDMA (125 – 187.5 mg) with psychotherapy / randomized double-blind active placebo (25 – 37.5 mg MDMA) | 12 (10) / chronic treatment-resistant PTSD | Decreases in CAPS scores that did not reach statistical significance (p = 0.066) at 2 months post-treatment. Clinically and statistically significant self-reported improvement on Posttraumatic Diagnostic Scale (p = 0.014) at 2 months post-treatment. CAPS scores improved further at 12-month follow-up. Three MDMA sessions were more effective than two (p = 0.016). |
Note.
MDMA = .3,4-Methylenedioxymethamphetamine.
PTSD = Post-traumatic stress disorder.
The first study administered placebo or MDMA (125 mg with optional supplemental dose of 62.5 mg approximately 2 hours later) during two 8–10 hour sessions approximately four weeks apart, under double-blind conditions in conjunction with psychotherapy (11 sessions) to individuals with chronic PTSD (N = 20). Results found significant reductions in CAPS scores in the MDMA group as compared to placebo 4 days after each session and 2 months following the second session. Four days after the second session 10 of 12 (83.3%) participants in the MDMA condition showed clinically significant reductions in CAPS score, as opposed to 2 of 8 (25%) participants in the placebo condition (Mithoefer et al., 2011). Furthermore, 7 of the 8 participants who received placebo opted to participate in an open-label crossover, in which they received MDMA-assisted psychotherapy for PTSD after completing their initial 2-month follow-up. All of these individuals showed a clinically significant reduction in CAPS score after their MDMA-assisted treatment.
The long-term follow up to this study invited all original participants who received MDMA-assisted psychotherapy (N = 19) to complete a long-term follow-up questionnaire, and a reassessment of current CAPS score, taking place between 17 and 74 months after the final MDMA session (Mithoefer et al., 2012). For the 16 individuals who agreed to repeat the follow-up CAPS, results showed that long-term post-treatment CAPS scores showed no significant differences from CAPS score administered 4 days after the second MDMA session of the original study. Furthermore, a persisting effects questionnaire inquiring into long-term benefits of their participation found that 89% of subjects endorsed both a general well-being and increased self-awareness and understanding as a benefit of their participation while 79% endorsed less excessive vigilance and less avoidance of people or places as a persisting benefit.
Additionally, a more recent study published in this area (N = 12) found less dramatic results, with reductions in CAPS scores that failed to reach statistical significance (p = 0.066; Oehen et al., 2013), though a secondary outcome measure, the Posttraumatic Diagnostic Scale did show significant post-treatment reductions (p = 0.014; Oehen et al., 2013). Among these three clinical trials of MDMA for PTSD, no serious drug-related adverse events were reported. Further trials in this area are ongoing, as well as novel research investigating the use of MDMA-assisted therapy for autistic adults with social anxiety (Danforth et al., 2015), and individuals suffering from anxiety at the end-of life, whose results are forthcoming. Despite the risks associated with chronic recreational and black-market use, clinical research with MDMA in controlled settings represents an important direction in treating a number of clinical syndromes such as PTSD, which currently lack highly efficacious treatment models.
Dissociative Anesthetics
Dissociative anesthetics comprise a large drug family including phencyclidine (PCP), ketamine, dextromethorphan (DXM) and nitrous oxide, among others (Morris & Wallach, 2014). While not true of all dissociative anesthetics, each compound listed above acts as an antagonist at the N-methyl-D-aspartate (NMDA) glutamate receptor as a key mechanism of action, which may contribute to the well-documented rapid antidepressant effects of ketamine (Abdallah et al., 2015b), as well as the potential antidepressant action of both nitrous oxide (Nagele et al., 2015), and DXM (Lauterbach, 2011; Nguyen et al., 2014; Nguyen & Matsumoto, 2015). The anesthetic properties of this drug class have earned several of these drugs important roles in medicine, most notably ketamine and nitrous oxide. However, their marked perception altering and dissociative effects have also made them popular with recreational users (Addy, 2007; Corazza et al., 2013). Recent research with ketamine and DXM have set them apart as drugs with novel clinical potential that will be discussed in greater depth in the following sections (Table 4).
Table 4.
Summary of selected clinical research with dissociatives.
| Study | Drug (dose) / Design | Total N (no. females) / Diagnosis | Key Findings |
|---|---|---|---|
| Berman et al., 2000 | Ketamine (0.5 mg/kg, i.v.) / randomized double-blind placebo-controlled | 9 (5) / MDD a (n=8); BPD b (n=1) | Significant decrease in depression from 4 to 72 hours post-ketamine infusion, with mean Hamilton Depression Rating Scale (HDRS) scores decreased by 14 ± SD 10 points vs. 0 ± 12 points, respectively during active and sham treatment (p = 0.02). |
| Cummings et al., 2015 | DXM (20–60 mg daily) + quinidine (10–20 mg daily) / randomized double-blind placebo-controlled | 220 (126) / AD d with agitation | Significantly reduced Neuropsychiatric Inventory Agitation / Aggression scores for dextromethorphan-quinidine vs. placebo (p < 0.001). |
| Diazgranados et al., 2010b | Ketamine (0.5 mg/kg, i.v.) / randomized double-blind placebo-controlled | 18 (12) / treatment-resistant BPD, current depressive episode | Significant decrease in Montgomery-Åsberg Depression Rating Scale (MADRS) scores from 40 minutes to 3 days post-ketamine infusion (p < 0.001); 71% of volunteers responded to ketamine vs. 6% responded to placebo. |
| Feder et al., 2014 | Ketamine (0.5 mg/kg, i.v.) / randomized double-blind active placebo (midazolam) | 41 (19) / chronic PTSDd | Significant and rapid reduction in PTSD symptom severity on the Impact of Event Scale in ketamine vs. midazolam at 24 hours post-treatment (p = 0.02). |
| Krupitsky & Grinenko, 1997 | Ketamine (2.5 mg/kg, i.m) with psychotherapy / open label vs. control (TAU) e | 211 (0) / chronic alcohol dependence | Significantly greater abstinence in ketamine group (65.8%) vs. treatment as usual control group (24%) at 12-month follow-up (p < 0.01). |
| Krupitsky et al., 2002 | Ketamine (2.0 mg/kg, i.m) with psychotherapy / randomized double-blind active placebo (0.2 mg/kg ketamine, i.m.) | 70 (15) / opioid dependence | Significantly greater biochemically verified opioid abstinence in ketamine group vs. active-placebo control group at 12-month and 24-month follow-up (p < 0.05). |
| Murrough et al., 2014 | Ketamine (0.5 mg/kg, i.v.) / randomized double-blind active placebo (midazolam) | 73 (37) / treatment-resistant MDD | Significantly greater decrease in MADRS scores in the ketamine group than control group 24 hours post-treatment (p ≤ 0.002); 64% of volunteers responded to ketamine vs. 28% to placebo at 24 hours post-treatment. |
| Nagele et al., 2015 | N2O f (50% + 50% oxygen inhalation for 60min) / randomized double-blind placebo-controlled cross-over | 20 (12) / treatment-resistant MDD | Significantly greater decrease in HDRS scores at 2 hours and 24 hours after N2O vs. placebo (p < 0.001). 20% of volunteers responded to N2O vs. 5% responded to placebo at 24 hours post-treatment. |
| Rodriguez et al., 2013 | Ketamine (0.5 mg/kg, i.v.) / randomized double-blind placebo-controlled cross-over | 15 (7) / OCD g | Significantly greater decrease in Yale-Brown Obsessive Compulsive Scale scores in ketamine than placebo group (p < 0.01); 50% of volunteers responded to ketamine vs. 0% responded to placebo at 7 days post-treatment. |
| Zarate et al., 2006 | Ketamine (0.5 mg/ kg, i.v.) / randomized double-blind placebo-controlled | 18 (12) / treatment-resistant MDD | Significant decrease in HDRS scores from 110 minutes to 7 days post-ketamine infusion vs. placebo (p < 0.05); 71% of volunteers responded to ketamine vs. 0% placebo at 24 hours post-treatment. |
Note.
MDD= Major depressive disorder.
BPD = Bipolar disorder.
AD = Alzheimer’s disease.
PTSD = Post-traumatic stress disorder.
TAU = Treatment as usual.
N2O = Nitrous oxide.
OCD = Obsessive-compulsive disorder.
Ketamine
Background
Ketamine is an N-methyl-D-aspartate receptor (NMDAR) antagonist with dissociative, anesthetic, analgesic, and hallucinogenic properties first synthesized in 1962 from phencyclidine (PCP; Wolff & Winstock, 2006). It first saw medical use in the mid 1960s when it was used extensively as a battlefield anesthetic in the Vietnam War, and remains the most widely used veterinary anesthetic today (Morgan & Curran, 2012). The first recorded evidence of recreational ketamine abuse dates to 1971, with anecdotal evidence reaching back into the late 1960s. Ketamine abuse, however, did not reach appreciable levels until its adoption as a ‘club drug’ in the 1990s (Morgan & Curran, 2012). As a result, ketamine was classified as a schedule III drug in the US in 1999, and is still used in medicine as an anesthetic in humans.
Pharmacology
Ketamine can be administered via a variety of routes including oral, intravenous (i.v.), and intramuscular (i.m.), though the majority of recreational users report intranasal use (Morgan & Curran, 2012). Depending on the route administered, the subjective effects of ketamine last between ten minutes and four hours. As a general rule, recreational doses fall between 10% and 25% of an anesthetic dose in non-chronic, tolerant users, or roughly 350–500 mg taken orally, 50–150 mg i.m., or 200 mg intranasally (Jansen, 2000). Anesthetic doses of ketamine typically fall in the 1–4.5 mg / kg, i.v. dose range (Miller et al., 2011), while subanesthetic doses used for antidepressant purposes are generally around 0.5 mg / kg, i.v. (Abdallah et al, 2015a).
Chronic ketamine abuse is known to cause serious toxicity to both the gastrointestinal and urinary track with ulcerative cystitis, hepatic dysfunction and abdominal cramps among the most common medical problems associated with chronic use (Bokor & Anderson 2014). Withdrawal symptoms among chronic users are less extensively studied, however, craving, sweating, shaking and palpitations have been documented among a small sample of chronic users (Morgan & Curran, 2012). Recreational ketamine use has also been linked to persisting cognitive deficits (Morgan et al., 2004; Morgan et al., 2010), however, to date controlled administration of ketamine in medical and research settings has not been linked to lasting complications such as ketamine abuse or cognitive impairment (e.g., Krupitsky et al., 2002).
At low doses (~150 mg intranasal), ketamine induces visual distortions along with mild dissociative and stimulant effects, while at higher doses the dissociative effects intensify and predominate with many users claiming complete mental dissociation from the physical body (Muetzelfeldt et al., 2008; Wolff & Winstock 2006). A qualitative interview study of 90 infrequent, frequent, and ex-ketamine users revealed the most appealing aspects of ketamine use to be melting into the surroundings, visual hallucinations, out-of-body experiences, and “giggliness” (Muetzelfeldt et al., 2008). The profound dissociative effects of ketamine have led to comparisons both to lucid dreaming and near death experiences (Jansen, 2000; Krupitsky & Grinenko 1997). The dissociative and perceptual effects, importance of set and setting, and possibility of personal meaningfulness of ketamine have led some to classify it as a psychedelic drug (Bowdle et al., 1998; Jansen, 2000; Krupitsky & Grinenko 1997). Ketamine’s psychedelic-like effects were systematically characterized by Bowdle and colleagues (1998), who found a strong correlation between ketamine plasma concentration and subjective drug effects, and a psychometric profile on the Hallucinogen Rating Scale comparable to that of DMT. A within-subjects comparison of DMT and ketamine administration examined the glutamate and serotonin models of schizophrenia, finding administration of ketamine to be associated with negative symptoms of schizophrenia including catatonic behavior and psychomotor retardation, while the 5-HT2AR agonist DMT was associated with more positive symptoms of schizophrenia such as thought disorder and inappropriate affect, suggesting distinct roles for the glutamate and serotonin systems in the manifestation of psychotic symptoms and psychedelic effects (Gouzoulis-Mayfrank et al., 2005).
Ketamine for Addiction
The marked subjective effects of ketamine first began to be noted within the scientific literature at approximately the same time as the scheduling of the serotonergic psychedelics, around 1970 (Krupitsky & Grinenko 1997). Reports of ketamine’s psychedelic-like effects, together with the increased restrictions on serotonergic psychedelics, led to ketamine’s incorporation into psychotherapy in 1973 (Khorramzadeh & Lofty, 1973). The most relevant of this initial work explored ketamine psychotherapy for the treatment of addiction. In the 1980s and 90s, over 1,000 alcoholic patients were treated with ketamine psychedelic therapy (KPT) without any complications such as psychosis or ketamine abuse (Krupitsky et al 1992; Krupitsky & Grinenko, 1997; 1998). The KPT method developed during this period employed intramuscular injections of ketamine in a supervised environment, and anticipated many contemporary recommendations for human hallucinogen research (e.g., Johnson et al., 2008), including extensive preparation for the psychedelic sessions as well as post-session integration. Follow-up data on a subset of participants showed increased rates of alcohol abstinence in a KPT group (n = 111) compared to a control group (n = 100) at one year post-treatment (65.8% vs. 24%), as well as continued abstinence in 40.7% of the KPT group at two years post-treatment (n = 81) and 33.3% at three years post-treatment (n = 42). Two and three year follow-up data were not collected from the control group due to funding limitations. Secondary findings included internalization of the locus of control as well as positive changes in life values associated with KPT (Krupitsky & Grinenko, 1997; 1998).
This approach was later applied in the treatment of heroin dependence. The first study utilized a single session of KPT (2 mg / kg im; n = 35) compared with a very low (non-hallucinogenic) dose of ketamine (0.2 mg / kg im; n = 35). High dose ketamine was found to produce greater levels of biologically verified heroin abstinence in all but two of sixteen follow-up visits including the final follow-up at 24 months after treatment. Both doses of ketamine also reduced craving for heroin from baseline measures as evaluated by a visual analog scale, however, the high dose was significantly more effective in reducing cravings than the low dose (Krupitsky et al., 2002). A second study (N = 59) examined the possible benefits of multiple sessions of KPT as opposed to a single session (Krupitsky et al., 2007). Participants randomized to the multiple session treatment had three sessions of drug-assisted KPT in total, and showed significantly greater abstinence at one year follow-up (50%) than those randomized to the single-session group (22.2%; p < 0.05).
More recent work probing the psychological dimensions of ketamine’s anti-addictive properties in eight non-treatment seeking cocaine-dependent volunteers have demonstrated that a single i.v. ketamine infusion (0.41 mg / kg) was effective in increasing motivation to quit cocaine 24 hours post-infusion as well as reducing cue-induced cravings for cocaine as compared to an active placebo (lorazepam 2 mg), with a second ketamine infusion (0.71 mg / kg) resulting in further decreases in cue-induced craving. Additionally, four out of the eight (50%) individually achieved biologically verified abstinence from cocaine two weeks after their ketamine infusions even though no outpatient treatment was provided (Dakwar et al., 2013).
A secondary analysis found that ketamine produced dose-related increases in a measure of mystical experience which were significantly correlated with changes in motivation to quit (Dakwar et al., 2014), similar to findings from psilocybin-facilitated addiction treatment pilot studies (Bogenschutz et al., 2015; Garcia-Romeu et al., 2014). Furthermore, mystical experience predicted motivation to quit with greater accuracy than a clinician administered scale of dissociative symptoms, suggesting a mediating role of mystical-type effects in ketamine’s addiction treatment outcomes (Dakwar et al., 2014).
Current methods of evaluating the subjective and mystical-type effects of hallucinogens, as well as their relative contribution to clinical treatment outcomes are still in need of further refinement. Quantitative assessments such as the Hallucinogen Rating Scale (Strassman et al., 1994b), APZ (altered states of consciousness) Questionnaire (Dittrich, 1998), Hood Mysticism Scale (Hood, 1975), and more recently the Mystical Experience Questionnaire (Barrett et al., 2015; MacLean et al., 2012) have been used to characterize halluicnogen effects. However, these scales often focus on particular qualities or features of the drug experience such as intensity, affective response, feelings of unity, or a sense of oceanic boundlessness, to the detriment of other effects that are not specifically queried. Thus, while such scales have been helpful in quantifying some aspects of hallucinogens’ subjective effects, the variability of responses to hallucinogens make this an ideal area for qualitative approaches, which allow participants to describe experiences in their own words (Addy et al., 2015; Gasser et al., 2014b). By expanding on current methodologies of studying subjective drug effects, we may gain a more nuanced and well-formulated understanding of the role of mystical and other types of drug experiences in hallucinogen-facilitated treatments.
Ketamine as Antidepressant
Ketamine has additionally shown promise as a rapid-acting antidepressant, with multiple studies showing robust effects in treatment-resistant populations (Abdallah et al., 2015b; Berman et al., 2000; Diazgranados et al., 2010b; Murrough et al., 2014; Valentine et al., 2011; Zarate et al., 2006; 2012). Research has found that i.v. administration of a single subanesthetic infusion of ketamine (0.5 mg / kg over 40 minutes) can attenuate depressive symptoms within 4 hours of administration (Berman et al., 2000).
Ketamine’s antidepressant effects generally last from 3 to 7 days, and have been observed to persist as long as two weeks in some cases (e.g., Diazgranados et al., 2010b). However, ketamine is largely eliminated from the body within 3 hours of administration (Mion & Villevieille, 2013), suggesting a significant role for downstream mechanisms triggered by ketamine in mediating its antidepressant effects. Ketamine has shown a clinical response rate of 40% to 60% at 24 hours post-infusion in treatment-resistant populations suffering from both unipolar (MDD) and bipolar depression (Abdallah et al., 2015b), and has additionally exhibited notable antisuicidal properties (Price et al., 2009; 2014; Larkin et al. 2011; Diazgranados et al., 2010a).
Converging evidence from animal models and human research suggest the observed rapid antidepressant effects of ketamine are due in part to a cascade of activity beginning with a surge of glutamate, leading to activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and downstream activity resulting in neuroplastic changes including synaptogenesis, or the formation of new neural pathways (Abdallah et al., 2015a; Li et al., 2010; Zhou et al., 2014). Ketamine’s antidepressant effects have additionally been linked to increases in brain-derived neurotrophic factor (BDNF; Haile et al., 2014), and enhanced cortical excitability (Cornwell et al., 2012), suggesting further potential mechanisms of action. Preclinical models have demonstrated sex differences in ketamine effects, with female rats showing greater sensitivity to ketamine’s antidepressant properties than male rats, and the hormones estrogen and progesterone mediating ketamine’s antidepressant effects in female rats (Carrier & Kabbaj, 2013). While these sex differences in the effects of ketamine and other dissociatives have yet to be thoroughly examined in human subjects, they represent an important area requiring additional attention in future research.
Ketamine’s hallucinogenic properties have been implicated as a mediator of antidepressant effects in some, but not all research. For instance, in a double-blind, crossover, placebo-controlled clinical trial (N=27), Sos and colleagues (2013) found a significant correlation between acute psychotomimetic effects (e.g., perceptual disturbances, euphoria) during ketamine infusion, and decreased depression scores at 7 days post-infusion (r= −0.40, p=0.04). Similarly, Luckenbaugh et al. (2014) found that acute dissociative effects of ketamine were significantly associated with decreased depression scores at 230 min (r= −0.35, p=0.007) and 7 days post-infusion (r= −0.41, p=0.01) in a sample of treatment-resistant inpatients with MDD or bipolar depression (N=108). These results suggest that the acute subjective effects of ketamine may be linked to later efficacious treatment outcomes, consistent with preliminary results from related hallucinogen-facilitated addiction treatment paradigms (Bogenschutz et al., 2015; Dakwar et al., 2014; Garcia-Romeu et al., 2014). Despite a possible association with favorable outcomes, these subjective effects are generally referred to as psychotomimetic in the literature, and otherwise considered as undesirable, adverse side effects.
In addition to ketamine’s potential as an aid in addiction treatment and antidepressant, recent studies have shown some promise for ketamine as a treatment for obsessive-compulsive disorder (Bloch et al., 2012; Rodriguez et al., 2013), and post-traumatic stress disorder (Feder et al. 2014; Zeng et al., 2013), representing novel avenues for research with NMDAR antagonist pharmacotherapies. Evidence regarding ketamine’s efficacy for treating obsessive-compulsive disorder is limited, however Rodriguez and colleagues (2013) found significant anti-obsessive effects of a single subanesthetic ketamine infusion in a small sample of individuals (N=15) suffering from near constant, intrusive obsessions, with effects lasting as long as 7 days in some participants.
Furthermore, Feder et al. (2014) studied the effects of 0.5 mg / kg infusion of ketamine over 40 minutes vs. the active comparator midazolam (a benzodiazepine), in a randomized double-blind crossover clinical trial (N=41). Ketamine showed a significant and rapid decrease in PTSD symptoms compared to midazolam at 24 hours post-infusion, with some patients showing clinically significant decreases in PTSD symptoms for as long as 2 weeks. Taken together, these findings suggest that ketamine, and potentially other NMDAR antagonists, deserve consideration for further clinical development and proliferation for a number of conditions. Some issues that will need to be addressed include the relatively short duration of ketamine’s efficacy for treatment of mood disorders, the role of so called psychotomimetic subjective effects in observed therapeutic effects, as well as its potential for toxicity in repeated administration models seeking to extend beneficial effects, and its abuse liability. Nevertheless, this remains a very promising area for future research.
Dextromethorphan (DXM)
Background
Dextromethorphan (DXM) is an analogue of the opioid analgesic levorphanol (Bem & Peck, 1992). DXM is a non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor (Zhou & Musacchio, 1991), which also acts upon the serotonin transporter (SERT), sigma-1 receptors (Werling et al., 2007), and α3β4 nicotinic acetylcholine receptors (Hernandez et al. 2000). At low doses in the range of 10 to 30 mg (usually administered orally), DXM is an effective cough suppressant that has been available as an FDA approved over-the-counter medication since 1958 (Carter et al., 2013; Morris & Wallach, 2014). DXM is widely used, and has a high margin of safety, and low abuse liability at recommended antitussive doses (Bem and Peck, 1992; Gutstein and Akil, 2001). Supratherapeutic doses of DXM have been abused recreationally since the 1970s (Addy, 2007).
Contemporary Research
Human laboratory research has shown that at supratherapeutic doses (e.g., 200–800 mg / 70kg), DXM exhibited psychedelic-like effects in a sample of 12 healthy volunteers with history of recreational hallucinogen use (Reissig et al., 2012). Participants self-reported beneficial persisting effects at 1 month after multiple high-dose administrations of DXM, including increased spirituality, and positive changes in attitudes, mood, and behavior (Reissig et al., 2012). High doses of DXM in the range of 400–800 mg / 70kg (i.e., ≥10–30 times the therapeutic dose of DXM) were also shown to elicit acute cognitive impairment in attention, memory, and metacognition comparable to a large dose (0.5 mg / 70kg) of the benzodiazepine triazolam under double-blind conditions, with doses in the 100–300 mg / 70kg range (i.e., 3–10 times the therapeutic dose of DXM) showing relatively milder impartment, indicating a broad therapeutic window for DXM (Carter et al., 2013).
Though clinical research with high-dose DXM has been limited, using modified dosing regimens, DXM has shown safety and preliminary feasibility as an aid in pain management (Siu & Drachtman, 2007; Weinbroum et al., 2000), and in attenuating opioid withdrawal (Koyuncuoğlu & Saydam, 1990; Koyuncuoğlu, 1991). Additionally, because DXM is rapidly metabolized by cytochrome P450 2D6 (CYP2D6), low-doses (~10 mg) of the CYP2D6 inhibitor quinidine have been added to novel drug formulations with DXM in order to alter the drug’s pharmacokinetic and pharmacodynamic profile, allowing for more prolonged exposure to DXM in the central nervous system (Doody et al., 2015). This DXM / quinidine combination has shown promise as a treatment for agitation in Alzheimer’s patients (Cummings et al., 2015), and pseudobulbar affect in dementia patients (Doody et al., 2015).
It has been hypothesized that DXM may possess rapid-acting antidepressant properties (Lauterbach, 2011, 2012), based on its pharmacological similarities to the NMDA antagonist ketamine, which has demonstrated robust rapid-acting antidepressant properties. Furthermore, recent preclinical data have demonstrated that DXM exhibits antidepressant effects in animal models mediated by its activity at Sigma-1 and glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Nguyen et al., 2014; Nguyen & Matsumoto, 2015). Specifically, Nguyen and colleagues (2014) found that in mice, DXM occasioned significant, dose-dependent increases in locomotor activity, as well as decreases in immobility time in the forced swim test, a commonly used rodent model of depression. In a follow-up study, Nguyen and Matsumoto (2015) additionally found that DXM produced significant, dose-dependent decreases in immobility in mice subjected to the tail suspension test, suggesting an antidepressant effect. A clinical trial of DXM/quinidine for treatment resistant depression is currently underway (clinicaltrials.gov identifier NCT01882829).
Atypical Hallucinogens
For our purposes, the atypical hallucinogens are defined as substances capable of engendering psychedelic-like effects through diverse pharmacological mechanisms in addition to the previously described monoaminergic and glutamatergic mechanisms. The atypical hallucinogens include the indole alkaloid ibogaine, which affects multiple neurotransmitter systems, the kappa opioid receptor (KOR) agonist salvinorin A, and the anticholinergics such as atropine and datura, also known as deliriants, which will not be discussed here. Finally, cannabis is sometimes attributed psychedelic-like properties (Keeler et al., 1971), and has exhibited therapeutic potential for a number of indications, which will be briefly presented.
Ibogaine
Background
Ibogaine is one of several indole alkaloids found in the root and root cortex of the African shrub Tabernathe iboga, native to West Central Africa including Gabon, the Democratic Republic of the Congo, and Angola (Rätsch, 2006). Its ceremonial use has been most extensively documented among the Bwiti religion, dating back to the early 20th century (Fernandez, 1982). Recent scientific and cultural attention has focused on ibogaine’s purported utility as an opioid detoxification agent (Brown, 2013; Popik et al., 1995), a use first suggested by anecdotal reports (Lotsof, 1985, 1995).
In 1991 the National Institute of Drug Abuse (NIDA) initiated its ibogaine research program, including FDA approval for a phase I dose escalation human study in which humans were administered low doses of ibogaine. However all federally funded human trials were discontinued in 1995 due to concerns of neurotoxicity and fatalities (Alper, 2001). Medical ibogaine treatment centers first opened their doors in 1994 in Panama and 1996 in St. Kitts where they continue to function. Underground ibogaine treatment centers and drug scenes eventually appeared both in Europe and in the United States (Alper, 2001). A recent qualitative analysis submits that in the United States alone an estimated 3,414 individuals have taken ibogaine since February 2006, more than four times more than similar estimates reaching back to 2001. Of those 3,414, 68% reported taking it for a substance abuse related disorder and 55% specifically for opioid withdrawal (Alper et al., 2008).
Pharmacology
The neuropharmacology of ibogaine is complex and remains incompletely understood (Koenig & Hilber, 2015; Mash et al., 1998; 2001). Antagonism of the α3β4 nicotinic acetylcholine receptor has been proposed as a potential mechanism of action for ibogaine’s withdrawal attenuating effects (Pace et al., 2004; Taraschenko et al., 2005), as has agonism at the mu opioid receptor for which affinity has been demonstrated in vitro (Sweetnam et al., 1995; Glick et al., 2001). Furthermore, preclinical data suggest that Ibogaine’s anti-addiction effects may be mediated by normalization of accumbal dopamine increases associated with the rewarding effects of morphine, nicotine, and amphetamine (Glick et al., 1993; Maisonneuve et al., 1997). Ibogaine’s properties as a serotonergic agonist are not thought to be relevant to its withdrawal attenuating effects (Wei et al., 1998; Glick et al., 2001), however more research is needed in this area.
The most controversial aspect of clinical ibogaine use is concerns of both neurotoxicity and cardiac toxicity. At dosages of 100 mg / kg several studies have demonstrated degeneration of cerebellar Purkinje cells in rats after a single administration of ibogaine (O’Hearn & Molliver 1993, 1997; O’Hearn et al., 1993). However, at a lower dose of 40 mg / kg, a dose known to be sufficient for reducing both morphine and cocaine self-administration and morphine withdrawal in rats, one study was unable to find any cerebellar toxicity (Molinari et al., 1996).
Concerns over possible cardiac toxicity have come from reports of human fatalities associated with the ingestion of ibogaine (Hoelen et al., 2009). Ibogaine is typically administered at a dosage of 10–25 mg / kg of body weight (Alper et al., 2008). According to a recent medical review of ibogaine fatalities, 19 individuals (15 men and 4 women, all between the ages of 24 and 54) are known to have died within 1.5 and 76 hours of taking ibogaine. Of the 19 total cases, 14 included adequate postmortem data with which to infer cause of death for researchers to determine that preexisting medical comorbidities or concurrent substance abuse contributed to or explained the cause of death (Alper et al., 2012). For additional medical case-reports of human ibogaine consumption see Paling et al. (2012), Papadodima et al. (2013), and Pleskovic et al. (2012).
Preclinical Data
Ibogaine has shown promise as an anti-addictive agent in animal models of drug self-administration and drug withdrawal. Ibogaine has been shown to attenuate signs of opiate withdrawal in rodents (Cappendijk et al., 1994; Dzoljic et al., 1987; Frances et al., 1992; Glick et al., 1992; Leal et al., 2003; Maisonneuve et al., 1991; Parker et al., 2002; Popik et al., 1995a), and primates (Aceto et al., 1992). Ibogaine has also been shown to reduce animal self-administration of morphine (Glick et al., 1991, 1994); cocaine (Glick et al., 1994; Cappendijk & Dzoljic, 1993); amphetamine (Maisonneuve et al., 1992); methamphetamine (Pace et al., 2004); and alcohol (Rezvani et al., 1995), possibly by dampening dopamine efflux in the nucleus accumbens in response to opiates (Maisonneuve et al., 1991; Glick et al., 1994) and nicotine (Benwell et al., 1996; Maisonneuve et al., 1997).
Furthermore, many of these findings have been replicated with a synthetic ibogaine congener, 18-Methoxycoronaridine (18-MC), which was first synthesized in 1996 and has not shown evidence of cerebellar toxicity or tremors in animals (Glick et al., 1996; 1999; 2001). 18-MC has been shown to attenuate acute opiate withdraw in rats (Panchal et al., 2005; Rho & Glick 1998); and decrease self-administration of morphine (Maisonneuve & Glick 1999; Pace et al., 2004); methamphetamine (Glick et al., 2000); alcohol (Rezvani et al., 1997); and nicotine (Glick et al., 1998; 2000). Finally, 18-MC has also been shown to dampen dopamine efflux in the nucleus accumbens in response to opiates and tobacco (Glick et al., 1998, 2000; Taraschenko et al., 2007).
Ibogaine has also shown sex differences in rodent models, with female rats showing higher levels of ibogaine metabolite and greater antagonism of morphine-induced locomotor activity than male rats (Pearl et al., 1997). However, it is not known whether such sex differences generalize to humans.
Clinical Research
To date there are three published case studies of ibogaine treatment. The first characterizes 3 ibogaine treatments including one opioid detoxification (Luciano, 1998). The second describes the use of ibogaine by 33 opioid dependent individuals in the care of a psychiatrist, who reported that withdrawal signs were observed to be markedly decreased in 29 (88%) of the sample (Alper et al., 1999). Finally, an open-label prospective study of ibogaine for opioid detoxification (N = 32) noted both resolution of withdrawal as determined by participant administered rating scales as well as sustained improvements in depression scores one month after treatment (Mash et al., 2001). These results, though preliminary, suggest feasibility of ibogaine as a treatment for opioid dependence. However, concerns over toxicity and adverse events remain.
A recent retrospective analysis reported on 75 alcohol, cannabis, cocaine, and crack cocaine users (72% poly-drug users) who sought treatment at a Brazilian substance abuse clinic using cognitive behavior therapy in combination with ibogaine treatment (Schenberg et al., 2014). In total, the 75 individuals underwent 134 ibogaine sessions, with no fatalities or serious medical adverse events reported. This suggests that ibogaine may be safely administered by experienced professionals in a hospital environment to medically screened volunteers. The data also suggest that ibogaine may be helpful in the treatment of substance abuse. At the time of contact, all women (n=8) were found to be abstinent as well as 51% of men (n=34). Median length of abstinence after only one ibogaine session was 5.5 months, while those treated multiple times remained sober for a median 8.4 months. As a condition of their treatment, all volunteers were required to be abstinent from all drugs for 30–60 days prior to receiving ibogaine. It is unclear from the published report whether this abstinence was biologically verified or self-reported. All participants were given the choice of completing this abstinence period at home or at their in-patient facility. Given the potentially volatile combination between concomitant physiological withdrawal and acute ibogaine exposure (Alper, 2001; Alper et al., 2012) these precautions seem reasonable, however, that participants were able to maintain such long periods of drug abstinence pre-ibogaine treatment calls into question the conclusiveness of post-treatment findings.
Salvia Divinorum
Background
J.B. Johnson, the first anthropologist to observe a traditional mushroom ceremony in 1939, was also the first scientist to write on the indigenous Mazatec use of another sacred plant drug, the leaves of a flowering plant that in 1962 was identified as Salvia divinorum (SD), a new member of the mint family (Johnson, 1939; Wasson 1962). The psychoactive compound contained therein, salvinorin A (SA) was not successfully isolated from the leaves of SD until 1982 (Ortega et al., 1982) and the psychoactive effects of SA were not confirmed until 1994 (Siebert, 1994). SA is active in doses as small as 500 micrograms, putting its potency on par only with that of LSD (Cunningham et al., 2011; Siebert, 1994).
SD is controlled in 20 US states (Drug Enforcement Administration, 2012) and is listed as a “drug of concern” by the US DEA (Perron et al., 2012). SD is legally and commercially available in many states and countries, which likely contributes to its popularity (Khey et al., 2008). SD is most often procured from local “head shops” or the Internet (Baggott et al. 2010, Sumnall et al. 2011) and is usually used either in residential settings with small groups of friends or else at music festivals (Sumnall et al. 2011). Recreational SD use tends to be sporadic, with most users reporting less than 20 lifetime uses (Baggott et al., 2010; Nyi et al., 2010) and the majority of recreational SD users surveyed report less than one use per year (SAMHSA, 2013; Khey et al., 2008). Most recreational use occurs via smoking commercial preparations of SD leaves fortified with additional SA, known as “enhanced leaves” (Baggott et al., 2010). The typical course of effects for inhaled SA is less than 20 minutes, with peak subjective effects achieved approximately two minutes after inhalation (Addy, 2012; Johnson et al., 2011, 2016).
Pharmacology
Unlike many compounds thus far mentioned, SA has no affinity for the 5-HT2AR receptor, and contains no nitrogen (Roth et al., 2002). Both in vivo and in vitro studies suggest SA acts selectively as an agonist at the kappa opioid receptor (KOR) (Roth et al., 2002). Recent work using animal models of drug discrimination have further confirmed these findings (Butelman et al., 2010; Butelman et al., 2004; Killinger et al., 2010). This has been additionally demonstrated in humans in a double-blind placebo-controlled study showing that the nonspecific opioid receptor antagonist naltrexone, but not the selective 5-HT2A antagonist ketanserin, blocked the subjective and physioligcal effects of vaporized SA (Maqueda et al., 2016).
Acute KOR activation by SA leads to decreased synaptic dopamine within the nucleus accumbens and caudate-putamen (Ebner et al., 2010). This effect is opposite to that of drugs of abuse such as cocaine and alcohol, which acutely increase dopamine in this “reward circuitry” and lead to euphoria, conditioned place preference, and compulsive use (Volkow et al., 2002). SA, in contrast, leads to conditioned place aversion and decreased intracranial self-stimulation (Carlezon et al., 2006; Sufka et al., 2014). Dynorphins, the endogenous ligands for the KOR have been implicated in many adverse behaviors including stress-induced drug relapse in animal models (Beardsley et al., 2005) and have been proposed as important therapeutic targets for addiction treatments (Butelman & Kreek, 2015; Chavkin, 2011;). SA may also have downstream effects on the endocannabinoid system (Braida et al., 2007; 2008), but this has not yet been demonstrated in primates. Animal reserarch has shown greater antinociceptive response to KOR agonists in males than females, and sex differences in the ability of KOR agonists like SA to attenuate administration of various drugs of abuse (Rasakham & Liu-Chen, 2011). While these sex differences in SA effects have not yet been observed in human subjects, they require examination in future research. Nevertheless, preclinical evidence suggests some promise for SA as an anti-addiction agent (e.g., Freeman et al., 2014).
Contemporary Research
Several controlled studies have been published recently describing behavioral, subjective, and physiological effects of SA (Addy, 2012; Addy et al., 2015; Johnson et al., 2011; MacLean et al., 2013; Maqueda et al., 2015, 2016; Mendelson et al., 2011; Pichini et al., 2005; Siebert, 1994). None of these studies reported serious adverse events or sustained negative effects. Inhaled SA leads to acute psychotomimetic effects (Ranganathan et al., 2012), intense perceptual alterations (Addy, 2012; Addy et al., 2015; MacLean et al., 2013; Ranganathan et al., 2012), dissociative effects (MacLean et al., 2013; Maqueda et al., 2015), elevated cortisol and prolactin, and decreased resting state EEG spectral power (Ranganathan et al., 2012). Peak plasma levels of inhaled SA were found to occur 2 minutes after inhalation, and to directly correspond with participant and observer ratings of subjective effects (Johnson et al., 2016). Inhaled SA has not been shown to lead to anxiogenic effects (MacLean et al., 2013), or significant changes in physiology such as heart rate or blood pressure (Addy, 2012; Johnson et al., 2011; Ranganathan et al., 2012). Auditory hallucinations and the sensed presence of other entities in the room have also been observed in human SA laboratory research (Addy et al., 2015; MacLean et al., 2013).
Given the effects of SA on reward circuitry, it is perhaps no surprise that recreational SD use is not associated with dependence. One Internet-based survey assessed 155 SD users with the Severity of Dependence Scale (SDS). No respondents met criteria for SD use disorders based on self-report (Sumnall et al., 2011). Another Internet-based survey assessed 500 SD users for DSM-IV criteria for substance abuse and dependence. Nine respondents (1.8%) endorsed such criteria, most commonly endorsing craving to use, but no respondents qualified for DSM substance misuse disorders (Baggott et al., 2010). Consistent with these data, research subjects given SA in the laboratory do not report experiencing euphoria or craving to use, and do not seek out SD subsequent to experimental SA exposure (Addy, 2012; Ranganathan et al., 2012).
Consistent with indigenous SD use for religious purposes, laboratory data indicate that SA administration can lead to experiences with mystical or spiritual qualities as measured by the Hood Mysticism Scale and States of Consciousness Questionnaire (MacLean et al., 2013). Several surveys of recreational SD users report “spiritual purposes” as a common reason for use (Baggott et al. 2010; Nygård, 2007; Sumnall et al. 2011). The most commonly reported spiritual experiences included (a) “shamanic experiences” such as “out of body travel” (Nygård 2007, p. 133) and “contact with other entities” (p. 134), and (b) perception of a greater or more accurate understanding of reality. Many of the alterations of consciousness attributed to SD including contact with entities and interoceptive disturbances have been characterized by users as unique to SD (Addy et al., 2015), and represent an important area for further research on the functions of the KOR system and its role in mediating subjective awareness.
Cannabinoids
Background
Cannabis sativa (cannabis) is among the oldest of cultivated plants known to mankind, with the earliest evidence for cultivated use going back to northeast China over 5,000 years ago where it was used medicinally, as a food source, but most importantly as a source of fiber (Li, 1973). The oldest sample of paper known to archeology dates to the early Han dynasty of China approximately 100 BC and is made of hemp, a non-psychoactive member of the cannabis genus (Li, 1974). Throughout the ages, cannabis has held a prominent role in the medicine of cultures as diverse as ancient China, ancient Greece and Victorian England (Clendinning 1843; Mikuriya, 1973; Russo, 1998). Cannabis as a psychoactive drug has played a prominent role in the religious and ceremonial life of many peoples both past and present, including recognition as a sacred plant in Hindu scripture dating back more than 3,000 years (Touw, 1981), and religious use in Jamaican Rastafari since the 1930s (Semaj, 1980). While a comprehensive discussion of cannabis’ risks and therapeutic potentials is outside the scope of this paper, a brief review will highlight some key points here.
Pharmacology
The plant genus Cannabis is commonly divided in two species, Cannabis sativa and Cannabis indica, with some distinguishing a third species as Cannabis ruderalis (Hillig, 2004). Cannabis is commonly smoked, or prepared in food or beverage form (Vandrey et al., 2015), and cannabinoids can also be extracted from the plant or synthesized in the laboratory (Hazekamp et al., 2013). In Cannabis sativa the number of cannabinoids identified now exceeds 100 with more continually being cataloged (ElSohly & Gul, 2014). The cannabinoid primarily responsible for the psychoactive effects of the drug is Δ9-tetra-hydrocannabinol (THC), was first isolated in 1963 (Gaoni & Mechoulam, 1964). Additionally, cannabidiol (CBD) a non-psychoactive cannabinoid, has emerged as a potential therapeutic agent of interest (Mechoulam et al., 2002). In the human organism there are two cannabinoid receptors (CB1 and CB2) and two endogenous cannabinoids N-arachidonoylethanolamin (anandamide) and 2-arachidonoylglycerol (2AG; Battista et al., 2012). The CB1 receptor is most commonly found within the central and peripheral nervous system and is one of the most commonly expressed G-Protein coupled receptors (GPCRs) in the mammalian nervous system (Devane et al., 1988), while CB2 preponderates in the immune system and other peripheral organs (Klein & Cabral, 2006; Mackie, 2008).
Cannabis has demonstrated some significant sex differences in preclinical and human research, including greater sensitivity to the rewarding effects of cannabis, and greater vulnerability to cannabis dependence in females (Fattore, 2013). In humans, similar trends have been observed, with women reporting significantly greater abuse-related drug effects such as “good” and “take again” compared to men, although both sexes reported comparable levels of intoxication (Cooper & Haney, 2014). These differences will be important to take into account in considering both medical and recreational cannabis use, and in implementing appropriate gender-tailored treatments for cannabis use disorders (Fattore, 2013).
Contemporary Research
The medical conditions for which cannabis has been used, both historically and presently is diverse, including neuropathic pain, cancer related pain, headache, epilepsy and multiple sclerosis (Baron, 2015; Friedman & Devinsky, 2015; Whiting et al., 2015). Some cannabinoids have been made available as approved medications, including dronabinol (synthetic THC) and nabilone (synthetic cannabinoid mimicking THC) as capsules, and nabiximols (synthetic THC and CBD) as oromucosal spray (Hazekamp et al., 2013). A recent meta-analysis of 79 randomized clinical trials (total N = 6462), found a significant therapeutic effect of cannabinoids in treating nausea and vomiting due to chemotherapy, chronic neuropathic or cancer pain, and spasticity due to multiple sclerosis, with potential benefits for weight gain in HIV patients, Tourette syndrome, and treatment of sleep disorders (Whiting et al., 2015). The review also found an increased risk of short-term adverse effects including balance problems, confusion, dizziness, euphoria, and drowsiness (Whiting et al., 2015).
Several other therapeutic applications of cannabinoids have garnered interest and demonstrated some preliminary or preclinical evidence of feasibility, including as an anticancer agent (Velasco et al., 2016), for epilepsy (Alexander, 2016), mood disorders (Bambico et al., 2007), and PTSD (Greer et al., 2014). Additionally, medical access to cannabis in the US has been linked to a significant 24.8% decrease in average opioid overdose mortalities from 1999 to 2010 as compared to states without medical cannabis access, consistent with data on efficacy of cannabinoids for treating pain, and suggesting a potential role for cannabis as a means of reducing opioid abuse (Bachhuber et al., 2015).
Regarding cancer, animal models have shown that cannabinoids can reduce tumor growth, potentially through inhibition of tumor angiogenesis and metastasis, and induction of cancer cell death (Galve-Roperh, 2000; Guzman, 2003; Velasco et al., 2016). However, clinical research applying cannabinoids as a cancer treatment is still lacking.
Preclinical evidence, case reports, and preliminary human data indicate that cannabinoids, and particularly CBD, may be useful in reducing seizures in epilepsy with limited adverse effects (Cunha et al., 1980; Devinsky et al., 2014; Maa & Figi, 2014). Effects of CBD on the G-protein-coupled receptor GPR55, serotonin 1A receptor, α3 and α1 glycine receptors, vanilloid type-I Channel, and equilibrative nucleoside transporter are among the hypothesized mechanisms by which CBD may exert acute anticonvulsant effects (Devinsky et al., 2014), though data from well controlled trials are still forthcoming.
Cannabis and the endocannabinoid system have also been implicated in the neurobiology of anxiety and depression (Viveros et al., 2005; Witkin et al 2005). Synthetic CB1 agonists have shown powerful antidepressant effects in rodent models (Bambico et al., 2007; Hill & Gorzalka, 2005), as have enzyme inhibitors that prevent the normal breakdown of anandamide (Bortolato et al., 2007). The CB1 receptor has also demonstrated a mediating relationship with anxious behavior (Haller et al 2002, 2004; Urigüen et al 2004). Several earlier studies suggested the potential of smoked cannabis for the management of depressive symptoms in cancer patients (Regelson et al., 1976; Gruber et al., 1996), though this remains to be tested in larger controlled trials.
Similarly, individuals with PTSD often self-medicate with cannabis (Akirav, 2013; Bonn-Miller et al., 2011; Cougle et al., 2011; Passie et al., 2012; Potter et al., 2011), and have reported decreased PTSD symptoms as a result (Greer et al., 2014). Recent human neuroimaging studies have shown pronounced differences in CB1 receptor densities and anandamide signaling in sufferers of PTSD as compared to healthy controls and traumatized individuals without PTSD (Hauer et al., 2013; Neumeister et al., 2013). Cannabinoids have also been shown to reduce traumatic nightmares in sufferers of PTSD (Fraser, 2009). One small study of 10 chronic PTSD patients showed that twice a day supplementation with THC led to significant improvements in sleep quality and nightmares (Shalev et al., 2013).
These data offer a compelling glimpse of some of the diverse therapeutic potentials of cannabis that will likely be explored in more depth over the coming years and decades, especially as greater access to medical cannabis, and development of cannabinoid-based treatments continue to proliferate. Despite such broad potential, further clinical research with cannabinoids are necessary before definite conclusions can be drawn, with the current lack of evidence attributable in part to cannabis’ current Schedule I status (Grinspoon & Bakalar, 1998; Nutt et al., 2013).
Discussion
The data presented here provide a review of potential therapeutic applications of hallucinogenic drugs, many of which have been dismissed as drugs of abuse with no clinical utility. The psychedelics, including LSD, psilocybin, mescaline, DMT and the DMT containing admixture ayahuasca, have shown promise in treating a range of psychological disorders for which currently available treatments are often insufficient, such as mood, substance use, and anxiety disorders (Table 2). These studies have mostly been conducted in small, relatively homogeneous samples, limiting the generalizability of their findings. However, safety and feasibility of psychedelic-facilitated treatment models have been established by these initial studies, paving the way for further investigation in larger, more diverse samples, using randomized controlled designs.
This also holds true for the entactogens, specifically MDMA, which has shown promise as a treatment for PTSD (Table 3), and whose other therapeutic potentials are beginning to be systematically investigated for conditions such as social anxiety in indiviuals with autism spectrum disorder (Danforth et al., 2015), and psychological distress associated with life-threatening illness. While some concerns may remain about possible neurotoxicity or adverse cognitive effects associated with chronic abuse of ‘Ecstasy’ (Parrott, 2013, 2014), such effects have not been observed in individuals undergoing limited exposure to pure MDMA in controlled settings (Doblin et al., 2014). Nevertheless, ongoing clinical MDMA research should continue to address these concerns through rigorous monitoring of acute and persisting adverse effects as assessed in previous studies (Mithoefer et al., 2011). It should also be noted that at low to moderate doses, classic psychedelics such as LSD or psilocybin may serve as reasonable alternatives to MDMA, with similar mood elevating and pro-social effects (e.g., Schmid et al., 2015). Although classic psychedelics have not been examined in clinical trials for PTSD, preclinical evidence (Catlow et al., 2013), and neuroscience findings (Kraehenmann et al., 2014), indicate feasibility.
The dissociatives have been classified less restrictively than the psychedelics and entactogens. As such, they have been studied more thoroughly over the past several decades. In particular, ketamine’s antidepressant properties have been well-established (Abdallah et al., 2015a, 2015b). Ketamine has also shown considerable promise as an anti-addictive agent in conjunction with psychotherapy (Krupitsky et al., 1992, 2002, 2007). However, translation to clinical practice has not been widely adopted, in part due to concerns about durability of treatment effects, and potential adverse effects of repeated, chronic administration (Schatzberg, 2014). Other dissociatives like DXM (Doody et al., 2015) and nitrous oxide (Nagele et al., 2015) are currently being revisited as potential treatments for mood disorders (Table 4), an area that will likely see further research in coming years.
Finally, the atypical hallucinogens discussed here have shown varying degrees of clinical potential. Ibogaine has demonstrated good preclinical evidence as an anti-addictive agent (Glick et al., 1991; 1994), as well as anecdotal reports and preliminary clinical findings showing promise as an aid in opioid detoxification and substance use disorder treatment (Alper et al., 1999; Lotsof, 1995; Schenberg et al., 2014). However, concerns remain about safety and toxicity of ibogaine, tempering the widespread implementation of ibogaine research and treatment for the time being (Alper et al., 2012; Hoelen et al., 2009). Preclinical data and basic human research on Salvinorin A (SA) suggest an important role for the Kappa opioid receptor system in modulating addiction, mood, and consciousness (Addy et al., 2012; 2015; Freeman et al., 2014; Johnson et al., 2011; 2016); though clinical research has yet to provide evidence for therapeutic use of SA, which remains an important direction for future research (Butelman & Kreek, 2015; Chavkin, 2011). Cannabis and the cannabinoids have shown therapeutic value as a treatment for chronic pain, spasticity in multiple sclerosis, and chemotherapy induced nausea (Whiting et al., 2015), with a number of other conditions such as cancer, epilepsy, sleep disorders, and PTSD implicated as possible targets for cannabis-based treatments. Given the growing interest in cannabis-based treatments and expanding access to medicinal cannabis, these areas will more than likely see significant growth in the years ahead (Russo et al., 2015).
With the advent of the medical cannabis movement, which has become increasingly widespread over the past two decades, citizens and lawmakers alike are beginning to reconsider the therapeutic potentials of a variety of once taboo illicit drugs. At the state level, medical cannabis is now legal in 25 states, as well as Washington D.C., and recreational cannabis use has been legalized in Colorado, Oregon, Alaska, and Washington state. Nevertheless, cannabis remains a Schedule I substance at the federal level, and thus poses an ongoing conundrum regarding the ultimate legality and safety of both medical and recreational use. Additionally, with the ever-growing availability of novel hallucinogenic drugs, such as the synthetic phenethylamines (2-CB, etc.), and synthetic cannabinoids (e.g., ‘Spice’; Spaderna et al. 2013), the potential risks and benefits of such substances pose a very real and timely public health issue that warrants serious consideration.
The convergence of these historic events have brought us to the verge of a new scientific framework that is presently taking shape within which to consider all drugs, both scheduled and unscheduled. This new approach ought to take into account not only the adverse effects of various drugs, but should also acknowledge their possible beneficial uses (Werb et al., 2016). This conceptual shift is further accompanied by the realization that the legal status of a drug does not always accurately reflect its actual harm to the user or to society (Nutt et al., 2007, 2010, 2013; Van Amsterdam et al., 2010, 2011). Current legal classification of cannabis, psychedelics, and entactogens place them in the most restricted class of drugs (Schedule I in the U.S.), indicating a high potential for abuse, no currently accepted medical use, and a lack of accepted safety for use under medical supervision (Nutt et al., 2013). Based on the evidence presented here, preliminary data indicate safety and feasibility of hallucinogen-facilitated treatments when conducted under appropriately structured conditions (Johnson et al., 2008). Furthermore, a growing body of research suggests a wide range of medical uses for these drugs, particularly psilocybin, LSD, MDMA, and cannabis, thereby challenging their current legal classification.
Population data from 2010 indicate that in the US alone, approximately 32 million individuals have used a psychedelic in their lifetime (Krebs & Johansen, 2013a), and a recent series of epidemiological analyses using data from the National Survey of Drug Use and Health (NSDUH) offered compelling results regarding the associations between psychedelic use and public health (Hendricks et al., 2015; Johansen & Krebs, 2015; Krebs & Johansen, 2013b). One such epidemiological study pooled NSDUH data from 2001–2004 (N = 130,152), revealing that lifetime psychedelic use was not associated with mental health problems of any kind, and that lifetime use of LSD or psilocybin was associated with lower rates of mental health treatment and psychiatric medication prescriptions (Krebs & Johansen, 2013b). Another study examining NSDUH data from 2008–2011 (N = 135,095) found no significant association between lifetime psychedelic use and psychological distress, depression, anxiety, or suicidality (Johansen & Krebs, 2015).
A related study using pooled NSDUH data from 2008–2012 provided a larger sample and thus more statistical power (N = 191,382), finding that lifetime psychedelic use was associated with significantly lower rates of past month psychological distress, and past year suicidality, while illicit use of other drug classes such as sedatives was generally associated with increased risk of psychological distress and suicidality (Hendricks et al., 2015). While these findings cannot demonstrate causation, they do indicate that in nationally representative samples psychedelics do not pose enough of a public health concern to justify current Schedule I status, and may even offer some protective benefits that warrant further investigation, consistent with the fact that many psychedelic drugs have been used in religious contexts for thousands of years (Ott et al., 1993; Baker, 2005; Schultes et al., 2001). Currently, in the US two religious organizations, the Native American Church and O Centro Espirita Beneficente Uniao do Vegetal (UDV) have won the right to use Schedule I psychedelics (peyote and ayahuasca, respectively) as a tenet of their religious life (Bullis, 2008; de Vergas, 1974).
No class of drugs has undergone a more critical reappraisal in the past decade than the hallucinogens discussed in this manuscript. During the the latter half of the 1960s, when prohibition of psychedelics was becoming more prominent, Pahnke and Richards offered this prescient evaluation of the situation:
We are confronted by the very real possibility that the known and unknown uses of these drugs that could prove to be legitimate and beneficial for individual persons and society may be suppressed until some future century when investigation will be permitted to proceed unhampered by popular hysteria and over-restrictive legislation. In the United States, interested and capable scientists are hesitating to investigate this field because of the abundance of unfavorable publicity and the threat of condemnation by identification with irresponsible researchers. Even among those who are willing to risk their reputations, some are finding it difficult to obtain the governmental approval now prerequisite for the legal acquisition of these drugs for research purposes. Paradoxically, a significant danger confronting our society may lie in losing out on the values that the responsible use of these drugs may offer. (1966, p. 176)
Ultimately, at this time it is critical to take a level-headed assessment of the current state of the field, and in the scientific domain, to continue examining the harms and clinical potentials of these substances in a thorough and balanced manner. In the popular press and media, sensational reports can abound from either side, expounding on the “miraculous” healing qualities of certain compounds, or conversely demonizing the potential physical and psychological damages that they can entail. A judicious assessment however, paints a much more nuanced and complex picture. The hallucinogens discussed here exhibit a collection of remarkable properties, some of which can elicit harm, and others which when applied skillfully, can promote healing or wellbeing. This is similar to many other classes of drugs that can be used therapeutically, or abused in destructive ways. It should also be noted that many of the hallucinogen-facilitated treatments presented here are designed as a combination of drug administration plus psychotherapy within a structured treatment context. Thus, variability in set and setting, as well as therapeutic rapport with treatment providers will undeniably affect observed outcomes, and must be taken into account when designing and conducting clinical research with hallucinogens (Johnson et al., 2008). Furthermore, sex differences in drug effects will have to be investigated more thoroughly to elucidate ramifications for hallucinogen-facilitated treatments (Fattore, 2013; Liechti et al., 2001).
It seems reasonable to assert that while individuals continue to suffer the burden of illnesses such as addiction and post-traumatic stress disorder, for which current treatment models often fall short, it is the moral responsibility of biomedical researchers and clinicians to explore every available treatment option. Public policy should be beholden to the scientific data on these substances, not vice versa. As stated by Nutt and colleagues, “It is surprising that the scientific community, particularly neuroscientists, has not protested against the effective ban of research on drugs that could offer so many insights into human brain function and such great opportunities for new treatments” (Nutt et al., 2013, p. 583). In collecting and summarizing the evidence presented here, this paper represents a tangible step toward making the case for additional basic, translational, and clinical research with hallucinogens, as well as a thorough reappraisal of their current legal status worldwide.
Public Health Significance.
Although most hallucinogens are currently highly restricted, some of these substances may have therapeutic applications for a variety of difficult to treat conditions, such as substance use, anxiety, and mood disorders. This review presents data on several classes of hallucinogens with a particular focus on psychedelics, entactogens, and dissociatives, for which clinical utility has been most extensively documented. Findings presented here suggest several hallucinogens have a favorable safety profile when administered under carefully controlled conditions, and warrant reconsideration as tools for clinical treatment.
Footnotes
By spiritual we mean here that the experience held some quality perceived by the experiencer to be related to a higher power, divinity, or a transcendent dimension of existence.
This study administered psilocybin by intravenous infusion specifically to examine onset of psychedelic effects. All other studies in this section administered psilocybin capsules orally.
Contributor Information
Brennan Kersgaard, Email: brennankersgaard@gmail.com.
Peter H. Addy, Email: peter.addy@yale.edu.
References
- Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid- acting antidepressants. Annals of the New York Academy of Sciences. 2015a;1344(1):66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annual review of medicine. 2015b;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson HA, Rolo A. Comparison of LSD with methysergide and psilocybin on test subjects. In: Abramson H, editor. The Use of LSD in Psychotherapy and Alcoholism. Indianapolis, IN: Bobbs-Merrill; 1967. pp. 53–73. [Google Scholar]
- Aceto MD, Bowman ER, Harris LS, May EL. Dependence studies of new compounds in the rhesus monkey and mouse (1991) NIDA research monograph. 1992;119:513. [PubMed] [Google Scholar]
- Addy PH. Facilitating transpersonal experiences with dextromethorphan: potential, cautions, and caveats. Journal of Transpersonal Psychology. 2007;39(1):1–22. [Google Scholar]
- Addy PH. Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology (Berl) 2012;220(1):195–204. doi: 10.1007/s00213-011-2470-6. [DOI] [PubMed] [Google Scholar]
- Addy PH, Garcia-Romeu A, Metzger M, Wade J. The subjective experience of acute, experimentally-induced Salvia divinorum inebriation. Journal of Psychopharmacology. 2015;29(4):426–435. doi: 10.1177/0269881115570081. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Akirav I. Targeting the endocannabinoid system to treat haunting traumatic memories. Frontiers in behavioral neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh BJ, Anderson PO. Peyote in the treatment of alcoholism among American Indians. American Journal of Psychiatry. 1974;131(11):1247–1250. doi: 10.1176/ajp.131.11.1247. [DOI] [PubMed] [Google Scholar]
- Alexander SP. Therapeutic potential of cannabis-related drugs. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2016;64:157–166. doi: 10.1016/j.pnpbp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Alonso JF, Romero S, Mañanas MÀ, Riba J. Serotonergic Psychedelics Temporarily Modify Information Transfer in Humans. International Journal of Neuropsychopharmacology. 2015;18(8):1–9. doi: 10.1093/ijnp/pyv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper KR. Ibogaine: a review. The Alkaloids: Chemistry and Biology. 2001;56:1–38. doi: 10.1016/s0099-9598(01)56005-8. [DOI] [PubMed] [Google Scholar]
- Alper KR, Lotsof HS, Frenken G, Luciano DJ, Bastiaans J. Treatment of acute opioid withdrawal with ibogaine. The American Journal on Addictions. 1999;8(3):234–242. doi: 10.1080/105504999305848. [DOI] [PubMed] [Google Scholar]
- Alper KR, Lotsof HS, Kaplan CD. The ibogaine medical subculture. Journal of ethnopharmacology. 2008;115(1):9–24. doi: 10.1016/j.jep.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Alper KR, Stajić M, Gill JR. Fatalities temporally associated with the ingestion of ibogaine. Journal of forensic sciences. 2012;57(2):398–412. doi: 10.1111/j.1556-4029.2011.02008.x. [DOI] [PubMed] [Google Scholar]
- Aricioglu F, Altunbas H. Harmane Induces Anxiolysis and Antidepressant- Like Effects in Rats. Annals of the New York Academy of Sciences. 2003;1009(1):196–201. doi: 10.1196/annals.1304.024. [DOI] [PubMed] [Google Scholar]
- Arria AM, McLellan AT. Evolution of concept, but not action, in addiction treatment. Substance use & misuse. 2012;47(8–9):1041–1048. doi: 10.3109/10826084.2012.663273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, Yacoubian GS, Jr, Fost E, Wish ED. Ecstasy use among club rave attendees. Archives of Pediatrics & Adolescent Medicine. 2002;156(3):295–296. doi: 10.1001/archpedi.156.3.295. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Rugowski JA. Lysergic acid diethylamide: effect on embryos. Science. 1967;157(3794):1325–1326. doi: 10.1126/science.157.3794.1325. [DOI] [PubMed] [Google Scholar]
- Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA internal medicine. 2014;174(10):1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott MJ, Coyle JR, Erowid E, Erowid F, Robertson LC. Abnormal visual experiences in individuals with histories of hallucinogen use: A web-based questionnaire. Drug and alcohol dependence. 2011;114(1):61–67. doi: 10.1016/j.drugalcdep.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Galloway GP, Mendelson J. Use patterns and self-reported effects of Salvia divinorum: An internet-based survey. Drug and alcohol dependence. 2010;111(3):250–256. doi: 10.1016/j.drugalcdep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Baggott MK, Mendelson J. Does MDMA cause brain damage? In: Holland J, editor. Ecstasy, A Complete Guide. Rochester VT: Inner Traditions; 2001. pp. 110–145. [Google Scholar]
- Baggott MJ, Kirkpatrick MG, Bedi G, de Wit H. Intimate insight: MDMA changes how people talk about significant others. Journal of Psychopharmacology. 2015;29(6):669–677. doi: 10.1177/0269881115581962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JR. Psychedelic sacraments. Journal of psychoactive drugs. 2005;37(2):179–187. doi: 10.1080/02791072.2005.10399799. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. The Journal of Neuroscience. 2007;27(43):11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanoj MJ, Riba J, Clos S, Gimenez S, Grasa E, Romero S. Daytime ayahuasca administration modulates REM and slow-wave sleep in healthy volunteers. Psychopharmacology. 2008;196(2):315–326. doi: 10.1007/S00213-007-0963-0. [DOI] [PubMed] [Google Scholar]
- Barbosa PCR, Cazorla IM, Giglio JS, Strassman R. A six-month prospective evaluation of personality traits, psychiatric symptoms and quality of life in ayahuasca-naïve subjects. Journal of psychoactive drugs. 2009;41(3):205–212. doi: 10.1080/02791072.2009.10400530. [DOI] [PubMed] [Google Scholar]
- Barbosa PCR, Giglio JS, Dalgalarrondo P. Altered states of consciousness and short-term psychological after-effects induced by the first time ritual use of ayahuasca in an urban context in Brazil. Journal of Psychoactive Drugs. 2005;37(2):193–201. doi: 10.1080/02791072.2005.10399801. [DOI] [PubMed] [Google Scholar]
- Barbosa PCR, Mizumoto S, Bogenschutz MP, Strassman RJ. Health status of ayahuasca users. Drug testing and analysis. 2012;4(7–8):601–609. doi: 10.1002/dta.1383. [DOI] [PubMed] [Google Scholar]
- Barclay J. Interviews with people who just smoked DMT. [accessed 25 September 2015];Vice Magazine Online. 2012 Retrieved from: http://www.vice.com/read/interviews-with-people-who-just-smoked-dmt.
- Barker SA, Borjigin J, Lomnicka I, Strassman R. LC/MS/MS analysis of the endogenous dimethyltryptamine hallucinogens, their precursors, and major metabolites in rat pineal gland microdialysate. Biomedical Chromatography. 2013;27(12):1690–1700. doi: 10.1002/bmc.2981. [DOI] [PubMed] [Google Scholar]
- Barker SA, McIlhenny EH, Strassman R. A critical review of reports of endogenous psychedelic N, N- dimethyltryptamines in humans: 1955 2010. Drug testing and analysis. 2012;4(7–8):617–635. doi: 10.1002/dta.422. [DOI] [PubMed] [Google Scholar]
- Barker SA, Monti JA, Christian ST. Metabolism of the hallucinogen N, N-dimethyltryptamine in rat brain homogenates. Biochemical Pharmacology. 1980;29(7):1049–1057. doi: 10.1016/0006-2952(80)90169-0. [DOI] [PubMed] [Google Scholar]
- Baron EP. Comprehensive review of medicinal marijuana, cannabinoids, and therapeutic implications in medicine and headache: What a long strange trip it’s been. Headache. 2015;55(6):885–916. doi: 10.1111/head.12570. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. Journal of Psychopharmacology. 2015;29(11):1182–1190. doi: 10.1177/0269881115609019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Frontiers in behavioral neuroscience. 2012;6 doi: 10.3389/fnbeh.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3, 4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology. 2007;189(4):407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183(1):118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology. 2009;207(1):73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of±3, 4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biological psychiatry. 2010;68(12):1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem JL, Peck R. Dextromethorphan. Drug Safety. 1992;7(3):190–199. doi: 10.2165/00002018-199207030-00004. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Holtom PE, Moran RJ, Balfour DJ. Neurochemical and behavioural interactions between ibogaine and nicotine in the rat. British journal of pharmacology. 1996;117(4):743–749. doi: 10.1111/j.1476-5381.1996.tb15253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RL. Navajo peyote use: Its apparent safety. American Journal of Psychiatry. 1971;128(6):695–699. doi: 10.1176/ajp.128.6.695. [DOI] [PubMed] [Google Scholar]
- Bergquist L. Look (1 de septiembre de 1959) 1959. The Curious Story Behind the New Cary Grant. [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, … Pittenger C. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biological psychiatry. 2012;72(11):964–970. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PCR, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology. 2015;29(3):289–299. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Pommy JM. Therapeutic mechanisms of classic hallucinogens in the treatment of addictions: From indirect evidence to testable hypotheses. Drug Testing & Analysis. 2012;4(7–8):543–555. doi: 10.1002/dta.1376. [DOI] [PubMed] [Google Scholar]
- Bokor G, Anderson PD. Ketamine An Update on Its Abuse. Journal of pharmacy practice. 2014;27(6):582–586. doi: 10.1177/0897190014525754. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychology of Addictive Behaviors. 2011;25(3):485. doi: 10.1037/a0021945. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, … Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biological psychiatry. 2007;62(10):1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Böszörmenyi Z, Der P, Nagy T. Observations on the psychotogenic effect of NN diethyltryptamine, a new tryptamine derivative. The British Journal of Psychiatry. 1959;105(438):171–181. doi: 10.1192/bjp.105.438.171. [DOI] [PubMed] [Google Scholar]
- Bouso JC, Doblin R, Farré M, Alcázar MÁ, Gómez-Jarabo G. MDMA-assisted psychotherapy using low doses in a small sample of women with chronic posttraumatic stress disorder. Journal of psychoactive drugs. 2008;40(3):225–236. doi: 10.1080/02791072.2008.10400637. [DOI] [PubMed] [Google Scholar]
- Bouso JC, González D, Fondevila S, Cutchet M, Fernández X, Barbosa PCR, … Riba J. Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: A longitudinal study. PloS one. 2012;7(8):e42421. doi: 10.1371/journal.pone.0042421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouso JC, Palhano-Fontes F, Rodríguez-Fornells A, Ribeiro S, Sanches R, Crippa JAS, … Riba J. Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. European Neuropsychopharmacology. 2015;25(4):483–492. doi: 10.1016/j.euroneuro.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Bouso JC, Riba J. Ayahuasca and the treatment of drug addiction. In: Labate BC, Cavnar C, editors. The Therapeutic Use of Ayahuasca. Heidelberg: Springer; 2014. pp. 95–109. [Google Scholar]
- Bowdle AT, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers relationship to steady-state plasma concentrations. The Journal of the American Society of Anesthesiologists. 1998;88(1):82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, … Sala M. Involvement of κ-opioid and endocannabinoid system on Salvinorin A-induced reward. Biological psychiatry. 2008;63(3):286–292. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Pegorini S, Zani A, Guerini-Rocco C, Gori E, Sala M. Hallucinatory and rewarding effect of salvinorin A in zebrafish: κ-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology. 2007;190(4):441–448. doi: 10.1007/s00213-006-0639-1. [DOI] [PubMed] [Google Scholar]
- Brodie BB, Shore PA. A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Annals of the New York Academy of Sciences. 1957;66(3):631–642. doi: 10.1111/j.1749-6632.1957.tb40753.x. [DOI] [PubMed] [Google Scholar]
- Brown TK. Ibogaine in the treatment of substance dependence. Current drug abuse reviews. 2013;6(1):3–16. doi: 10.2174/15672050113109990001. [DOI] [PubMed] [Google Scholar]
- Bruhn JG, De Smet PAGM, El-Seedi HR, Beck O. Mescaline use for 5700 years. The Lancet. 2002;359(9320):1866. doi: 10.1016/S0140-6736(02)08701-9. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. The Journal of Clinical Endocrinology & Metabolism. 2005;90(5):3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Bullis RK. The “vine of the soul” vs. the Controlled Substances Act: implications of the hoasca case. Journal of psychoactive drugs. 2008;40(2):193–199. doi: 10.1080/02791072.2008.10400630. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang GE, Quigley DI, … Grandy DK. Amphetamine, 3, 4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Molecular Pharmacology. 2001;60(6):1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Progress in neurobiology. 2006;79(5):223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. The plant-derived hallucinogen, salvinorin A, produces kappa-opioid agonist-like discriminative effects in rhesus monkeys. Psychopharmacology (Berl) 2004;172(2):220–4. doi: 10.1007/s00213-003-1638-0. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Kreek MJ. Salvinorin A, a kappa-opioid receptor agonist hallucinogen: pharmacology and potential template for novel pharmacotherapeutic agents in neuropsychiatric disorders. Frontiers in Pharmacology. 2015;6:190. doi: 10.3389/fphar.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Rus S, Prisinzano TE, Kreek MJ. The discriminative effects of the κ-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects. Psychopharmacology. 2010;210(2):253–262. doi: 10.1007/s00213-009-1771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakic V, Potkonyak J, Marshall A. Dimethyltryptamine (DMT): Subjective effects and patterns of use among Australian recreational users. Drug and alcohol dependence. 2010;111(1):30–37. doi: 10.1016/j.drugalcdep.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Callaway JC. A proposed mechanism for the visions of dream sleep. Medical hypotheses. 1988;26(2):119–124. doi: 10.1016/0306-9877(88)90064-3. [DOI] [PubMed] [Google Scholar]
- Callaway JC, Airaksinen MM, McKenna DJ, Brito GS, Grob CS. Platelet serotonin uptake sites increased in drinkers of ayahuasca. Psychopharmacology. 1994;116(3):385–387. doi: 10.1007/BF02245347. [DOI] [PubMed] [Google Scholar]
- Callaway JC, McKenna DJ, Grob CS, Brito GS, Raymon LP, Poland RE, … Mash DC. Pharmacokinetics of Hoasca alkaloids in healthy humans. Journal of ethnopharmacology. 1999;65(3):243–256. doi: 10.1016/s0378-8741(98)00168-8. [DOI] [PubMed] [Google Scholar]
- Callaway JC, Raymon LP, Hearn WL, McKenna DJ, Grob CS, Brito GS, Mash DC. Quantitation of N, N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. Journal of Analytical Toxicology. 1996;20(6):492–497. doi: 10.1093/jat/20.6.492. [DOI] [PubMed] [Google Scholar]
- Cappendijk SL, Dzoljic MR. Inhibitory effects of ibogaine on cocaine self-administration in rats. European journal of pharmacology. 1993;241(2):261–265. doi: 10.1016/0014-2999(93)90212-z. [DOI] [PubMed] [Google Scholar]
- Cappendijk SL, Fekkes D, Dzoljic MR. The inhibitory effect of norharman on morphine withdrawal syndrome in rats: comparison with ibogaine. Behavioural brain research. 1994;65(1):117–119. doi: 10.1016/0166-4328(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB. The role of 5-HT2A, 5-HT2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N, N-dimethyltryptamine and N, N-diisopropyltryptamine in rats and mice. Psychopharmacology. 2014:1–10. doi: 10.1007/s00213-014-3658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016 doi: 10.1016/S2215-0366(16)30065-7. published online May 17 http://dx.doi.org/10.1016/S2215-0366(16)30065-7. [DOI] [PubMed]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, … Nutt DJ. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proceedings of the National Academy of Sciences. 2012;109(6):2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Whalley MG, Bolstridge M, Feilding A, Nutt DJ. LSD enhances suggestibility in healthy volunteers. Psychopharmacology. 2014a:1–10. doi: 10.1007/s00213-014-3714-z. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, … Nutt D. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Frontiers in human neuroscience. 2014b;8 doi: 10.3389/fnhum.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ. Experienced Drug Users Assess the Relative Harms and Benefits of Drugs: A Web-Based Survey. Journal of psychoactive drugs. 2013;45(4):322–328. doi: 10.1080/02791072.2013.825034. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, … Cohen BM. Depressive-like effects of the κ-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. Journal of Pharmacology and Experimental Therapeutics. 2006;316(1):440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Carter LP, Reissig CJ, Johnson MW, Klinedinst MA, Griffiths RR, Mintzer MZ. Acute cognitive effects of high doses of dextromethorphan relative to triazolam in humans. Drug and alcohol dependence. 2013;128(3):206–213. doi: 10.1016/j.drugalcdep.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudevilla-Gálligo F, Riba J, Ventura M, González D, Farré M, Barbanoj MJ, Bouso JC. 4-Bromo-2, 5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects. Journal of Psychopharmacology. 2012;26(7):1026–1035. doi: 10.1177/0269881111431752. [DOI] [PubMed] [Google Scholar]
- Chadwick IS, Curry PD, Linsley A, Freemont AJ, Doran B. Ecstasy, 3-4 methylenedioxymethamphetamine (MDMA), a fatality associated with coagulopathy and hyperthermia. Journal of the Royal Society of Medicine. 1991;84(6):371. doi: 10.1177/014107689108400622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C. The therapeutic potential of κ-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36(1):369. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian ST, Harrison R, Quayle E, Pagel J, Monti J. The in vitro identification of dimethyltryptamine (DMT) in mammalian brain and its characterization as a possible endogenous neuroregulatory agent. Biochemical medicine. 1977;18(2):164–183. doi: 10.1016/0006-2944(77)90088-6. [DOI] [PubMed] [Google Scholar]
- Chwelos N, Blewett DB, Smith CM, Hoffer A. Use of d-lysergic acid diethylamide in the treatment of alcoholism. Quarterly Journal of Studies on Alcohol. 1959;20:577–590. [PubMed] [Google Scholar]
- Clendinning J. Observations on the medicinal properties of the cannabis sativa of India. Medico-chirurgical transactions. 1843;26:188. doi: 10.1177/095952874302600116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Marinello MJ, Back N. Chromosomal damage in human leukocytes induced by lysergic acid diethylamide. Science. 1967;155:1417–1419. doi: 10.1126/science.155.3768.1417. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Shiloh Y. Genetic toxicology of lysergic acid diethylamide (LSD-25) Mutation Research/Reviews in Genetic Toxicology. 1977;47(3–4):183–209. doi: 10.1016/0165-1110(77)90003-3. [DOI] [PubMed] [Google Scholar]
- Cohen S. The therapeutic potential of LSD-25. In: Featherstone RM, Simon A, editors. A Pharmacologic Approach to the Study of the Mind. Springfield, IL: Thomas; 1959. [Google Scholar]
- Cohen S. Lysergic acid diethylamide: side effects and complications. The Journal of nervous and mental disease. 1960;130(1):30–40. doi: 10.1097/00005053-196001000-00005. [DOI] [PubMed] [Google Scholar]
- Cole JC, Bailey M, Sumnall HR, Wagstaff GF, King LA. The content of ecstasy tablets: implications for the study of their long- term effects. Addiction. 2002;97(12):1531–1536. doi: 10.1046/j.1360-0443.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and alcohol dependence. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazza O, Assi S, Schifano F. From “Special K” to “Special M”: the evolution of the recreational use of ketamine and methoxetamine. CNS neuroscience & therapeutics. 2013;19(6):454–460. doi: 10.1111/cns.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biological psychiatry. 2012;72(7):555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, Hawkins KA. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychology of Addictive Behaviors. 2011;25(3):554. doi: 10.1037/a0023076. [DOI] [PubMed] [Google Scholar]
- Cozzi NV. Psychedelic Breakthroughs in Neuroscience: How Psychedelic Drugs Influenced the Growth and Development of Psychopharmacology. MAPS Bulletin. 2013;23(1):16–19. [Google Scholar]
- Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, … Siffert J. Effect of Dextromethorphan-Quinidine on Agitation in Patients With Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA. 2015;314(12):1242–1254. doi: 10.1001/jama.2015.10214. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, … Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- Cunningham CW, Rothman RB, Prisinzano TE. Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A. Pharmacol Rev. 2011;63(2):316–47. doi: 10.1124/pr.110.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silveira DX, Grob CS, de Rios MD, Lopez E, Alonso LK, Tacla C, Doering-Silveira E. Ayahuasca in adolescence: a preliminary psychiatric assessment. Journal of psychoactive drugs. 2005;37(2):129–133. doi: 10.1080/02791072.2005.10399792. [DOI] [PubMed] [Google Scholar]
- Dakwar E, Anerella C, Hart CL, Levin FR, Mathew SJ, Nunes EV. Therapeutic infusions of ketamine: Do the psychoactive effects matter? Drug and alcohol dependence. 2014;136:153–157. doi: 10.1016/j.drugalcdep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E, Levin F, Foltin RW, Nunes EV, Hart CL. The Effects of Subanesthetic Ketamine Infusions on Motivation to Quit and Cue-Induced Craving in Cocaine-Dependent Research Volunteers. Biological psychiatry. 2013;76(1):40–46. doi: 10.1016/j.biopsych.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth AL, Struble CM, Klosinski B, Grob CS. MDMA-assisted therapy: A new treatment paradigm for autistic adults with social anxiety. Progress in neuro-psychopharmacology & biological psychiatry. 2015;64:237–249. doi: 10.1016/j.pnpbp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Daumann J, Heekeren K, Neukirch A, Thiel CM, Möller-Hartmann W, Gouzoulis-Mayfrank E. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology. 2008;200(4):573–583. doi: 10.1007/s00213-008-1237-1. [DOI] [PubMed] [Google Scholar]
- Daumann J, Wagner D, Heekeren K, Neukirch A, Thiel CM, Gouzoulis-Mayfrank E. Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. Journal of Psychopharmacology. 2010;24(10):1515–1524. doi: 10.1177/0269881109103227. [DOI] [PubMed] [Google Scholar]
- De la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, … Camí J. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Therapeutic drug monitoring. 2004;26(2):137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- de Lima Osório F, de Macedo LRH, de Sousa JPM, Pinto JP, Quevedo J, de Souza Crippa JA, Hallak JEC. The therapeutic potential of harmine and ayahuasca in depression: Evidence from exploratory animal and human studies. The ethnopharmacology of ayahuasca. 2011 May;:75–85. [Google Scholar]
- Deniker P. Biological changes in man following intravenous administration of mescaline. The Journal of nervous and mental disease. 1957;125(3):427–431. doi: 10.1097/00005053-195707000-00013. [DOI] [PubMed] [Google Scholar]
- de Verges G. Constitutional Law: Freedom of Religion: Peyote and the Native American Church. American Indian Law Review. 1974;2(2):71–79. [Google Scholar]
- Devane WA, Dysarz F3, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Molecular pharmacology. 1988;34(5):605–613. [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, … Martinez-Orgado J. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim L, Brutsche N, Ameli R, Henter ID, Luckenbaugh DA, … Zarate CA., Jr Rapid resolution of suicidal ideation after a single infusion of an NMDA antagonist in patients with treatment-resistant major depressive disorder. The Journal of clinical psychiatry. 2010a;71(12):1605. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, … Zarate CA. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of general psychiatry. 2010b;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishotsky NI, Loughman WD, Mogar RE, Lipscomb WR. LSD and genetic damage. Science. 1971;172:431–447. doi: 10.1126/science.172.3982.431. [DOI] [PubMed] [Google Scholar]
- Dittrich A. The Standardized Psychometric Assessment of Altered States of Consciousness (ASCs) in Humans. Pharmacopsychiatry. 1998;31(S 2):80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- Doblin R. Pahnke’s Good Friday experiment: a long-term followup and methodological critique. Journal of Transpersonal Psychology. 1991;23(1):1–28. [Google Scholar]
- Doblin R. Dr. Leary’s concord prison experiment: A 34-year follow-up study. Journal of Psychoactive Drugs. 1998;30(4):419–426. doi: 10.1080/02791072.1998.10399715. [DOI] [PubMed] [Google Scholar]
- Doblin R, Greer G, Holland J, Jerome L, Mithoefer MC, Sessa B. A reconsideration and response to Parrott AC (2013)“Human psychobiology of MDMA or ‘Ecstasy’: an overview of 25 years of empirical research”. Human Psychopharmacology: Clinical and Experimental. 2014;29(2):105–108. doi: 10.1002/hup.2389. [DOI] [PubMed] [Google Scholar]
- Doering-Silveira E, Grob CS, de Rios MD, Lopez E, Alonso LK, Tacla C, Da Silveira DX. Report on psychoactive drug use among adolescents using ayahuasca within a religious context. Journal of Psychoactive Drugs. 2005a;37(2):141–144. doi: 10.1080/02791072.2005.10399794. [DOI] [PubMed] [Google Scholar]
- Doering-Silveira E, Lopez E, Grob CS, de Rios MD, Alonso LK, Tacla C, … Da Silveira DX. Ayahuasca in adolescence: a neuropsychological assessment. Journal of psychoactive drugs. 2005b;37(2):123–128. doi: 10.1080/02791072.2005.10399791. [DOI] [PubMed] [Google Scholar]
- Doody RS, D’Amico S, Cutler AJ, Davis CS, Shin P, Ledon F, … Siffert J. An open-label study to assess safety, tolerability, and effectiveness of dextromethorphan/ quinidine for pseudobulbar affect in dementia: PRISM II results. CNS spectrums. 2015:1–10. doi: 10.1017/S1092852915000620. [DOI] [PubMed] [Google Scholar]
- Dos Santos RG, Grasa E, Valle M, Ballester MR, Bouso JC, Nomdedéu JF, … Riba J. Pharmacology of ayahuasca administered in two repeated doses. Psychopharmacology. 2012;219(4):1039–1053. doi: 10.1007/s00213-011-2434-x. [DOI] [PubMed] [Google Scholar]
- Dos Santos RG, Landeira-Fernandez J, Strassman RJ, Motta V, Cruz APM. Effects of ayahuasca on psychometric measures of anxiety, panic-like and hopelessness in Santo Daime members. Journal of Ethnopharmacology. 2007;112(3):507–513. doi: 10.1016/J.Jep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Dos Santos RG, Valle M, Bouso JC, Nomdedéu JF, Rodríguez-Espinosa J, McIlhenny EH, … Riba J. Autonomic, neuroendocrine, and immunological effects of ayahuasca: a comparative study with d-amphetamine. Journal of clinical psychopharmacology. 2011;31(6):717–726. doi: 10.1097/JCP.0b013e31823607f6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration. Drug & Chemical Evaluation Section. Office of Diversion Control, Drug Enforcement Administration; 2012. [Accessed October 26]. Salvia divinorum and salvinorin A. Last Modified October 2013. http://www.deadiversion.usdoj.gov/drug_chem_info/salvia_d.pdf. [Google Scholar]
- Dumont GJH, Sweep FCGJ, Van der Steen R, Hermsen R, Donders ART, Touw DJ, … Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3, 4-methylenedioxymethamphetamine) administration. Social neuroscience. 2009;4(4):359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Dumont GJH, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. Journal of Psychopharmacology. 2006;20(2):176–187. doi: 10.1177/0269881106063271. [DOI] [PubMed] [Google Scholar]
- Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Canadian Journal of Psychiatry. 2005;50:381–388. doi: 10.1177/070674370505000703. [DOI] [PubMed] [Google Scholar]
- Dzoljic ED, Kaplan CD, Dzoljic MR. Effect of ibogaine on naloxone-precipitated withdrawal syndrome in chronic morphine-dependent rats. Archives internationales de pharmacodynamie et de thérapie. 1987;294:64–70. [PubMed] [Google Scholar]
- Easton N, Marsden CA. Ecstasy: are animal data consistent between species and can they translate to humans? Journal of Psychopharmacology. 2006;20(2):194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharm. 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egozcue J, Irwin S, Maruffo CA. Chromosomal damage in LSD users. JAMA. 1968;204(3):214–218. doi: 10.1001/jama.204.3.214. [DOI] [PubMed] [Google Scholar]
- Eisner BG, Cohen S. Psychotherapy with lysergic acid diethylamide. The Journal of nervous and mental disease. 1958;127(6):528–539. doi: 10.1097/00005053-195812000-00006. [DOI] [PubMed] [Google Scholar]
- El-Seedi HR, De Smet PA, Beck O, Possnert G, Bruhn JG. Prehistoric peyote use: alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas. J Ethnopharmacol. 2005;101:238–42. doi: 10.1016/j.jep.2005.04.022. [DOI] [PubMed] [Google Scholar]
- ElSohly M, Gul W. Constituents of Cannabis sativa. Handbook of Cannabis. 2014:3. [Google Scholar]
- Evarts EV. A review of the neurophysiological effects of lysergic acid diethylamide (LSD) and other psychotomimetic agents. Annals of the New York Academy of Sciences. 1957;66(3):479–495. doi: 10.1111/j.1749-6632.1957.tb40744.x. [DOI] [PubMed] [Google Scholar]
- Fábregas JM, González D, Fondevila S, Cutchet M, Fernández X, Barbosa PCR, … Bouso JC. Assessment of addiction severity among ritual users of ayahuasca. Drug and alcohol dependence. 2010;111(3):257–261. doi: 10.1016/j.drugalcdep.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Fadiman J. The Psychedelic Explorer’s Guide: Safe, Therapeutic, and Sacred Journeys. Inner Traditions/Bear & Co; 2011. [DOI] [PubMed] [Google Scholar]
- Faillace LA, Snyder SH, Weingartner H. 2, 5-dimethoxy-4-methylamphetamine: clinical evaluation of a new hallucinogenic drug. The Journal of nervous and mental disease. 1970;150(2):119–126. [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochemical pharmacology. 2008;75(1):17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, Winger G. Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behavioural pharmacology. 2004;15(2):149–157. doi: 10.1097/00008877-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Farzin D, Mansouri N. Antidepressant-like effect of harmane and other β-carbolines in the mouse forced swim test. European neuropsychopharmacology. 2006;16(5):324–328. doi: 10.1016/j.euroneuro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fattore L. Considering gender in cannabinoid research: a step towards personalized treatment of marijuana addicts. Drug testing and analysis. 2013;5(1):57–61. doi: 10.1002/dta.1401. [DOI] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, … Charney DS. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA psychiatry. 2014;71(6):681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- Fernandez JW. Bwiti: An ethnography of the religious imagination in Africa. Princeton: Princeton University Press; 1982. [Google Scholar]
- Fernández X, dos Santos RG, Cutchet M, Fondevila S, González D, Alcázar MÁ, … Fábregas JM. Assessment of the psychotherapeutic effects of ritual ayahuasca use on drug dependency: A pilot study. In: Labate BC, Cavnar C, editors. The Therapeutic Use of Ayahuasca. Springer; Heidelberg: 2014. pp. 183–196. [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N, N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323(5916):934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort J, Metzner R. LSD, chromosomes and sensationalism. Psychedelic Review. 1968;(10):47–51. [Google Scholar]
- Fortunato JJ, Réus GZ, Kirsch TR, Stringari RB, Fries GR, Kapczinski F, … Quevedo J. Effects of β-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: Further evidence of antidepressant properties. Brain research bulletin. 2010;81(4):491–496. doi: 10.1016/j.brainresbull.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Fortunato JJ, Réus GZ, Kirsch TR, Stringari RB, Stertz L, Kapczinski F, … Quevedo J. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(8):1425–1430. doi: 10.1016/j.pnpbp.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Frances B, Gout R, Cros J, Zajac JM. Effects of ibogaine on naloxone- precipitated withdrawal in morphine- dependent mice. Fundamental & clinical pharmacology. 1992;6(8–9):327–332. doi: 10.1111/j.1472-8206.1992.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Franzen F, Gross H. Tryptamine, N, N-dimethyltryptamine, N, N-dimethyl-5-hydroxytryptamine and 5-methoxytryptamine in human blood and urine. 1965 doi: 10.1038/2061052a0. [DOI] [PubMed] [Google Scholar]
- Fraser GA. The use of a synthetic cannabinoid in the management of Treatment- Resistant nightmares in posttraumatic stress disorder (PTSD) CNS neuroscience & therapeutics. 2009;15(1):84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frecska E, Szabo A, Winkelman MJ, Luna LE, McKenna DJ. A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. Journal of Neural Transmission. 2013;120(9):1295–1303. doi: 10.1007/s00702-013-1024-y. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Johanson CE, Tancer ME. Thermoregulatory effects of 3, 4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2005;183(2):248–256. doi: 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL. Assessment of the kappa opioid agonist, salvinorin A, as a punisher of drug self-administration in monkeys. Psychopharmacology. 2014;231(14):2751–2758. doi: 10.1007/s00213-014-3436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenmann RW, Öxler F, Bernschneider-Reif S. The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents*. Addiction. 2006;101(9):1241–1245. doi: 10.1111/j.1360-0443.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Devinsky O. Cannabinoids in the Treatment of Epilepsy. New England Journal of Medicine. 2015;373(11):1048–1058. doi: 10.1056/NEJMra1407304. [DOI] [PubMed] [Google Scholar]
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24–34. doi: 10.1111/j.1360-0443.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Sánchez C, Cortés ML, del Pulgar TG, Izquierdo M, Guzmán M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nature medicine. 2000;6(3):313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of the American chemical society. 1964;86(8):1646–1647. [Google Scholar]
- Garcia-Romeu A, Griffiths RR, Johnson WM. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Current drug abuse reviews. 2014;7(3):157–164. doi: 10.2174/1874473708666150107121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity JF. Jesus, peyote, and the holy people: alcohol abuse and the ethos of power in Navajo healing. Med Anthropol Q. 2000;14:521–542. doi: 10.1525/maq.2000.14.4.521. [DOI] [PubMed] [Google Scholar]
- Gasser P. Psycholytic Therapy with MDMA and LSD in Switzerland. MAPS Newsletter. 1994;5(3):3–7. [Google Scholar]
- Gasser P, Holstein D, Michel Y, Doblin R, Berra Yazar-Klosinski P, Passie T, Brenneisen R. Safety and Efficacy of Lysergic Acid Diethylamide-Assisted Psychotherapy for Anxiety Associated With Life-threatening Diseases. The Journal of nervous and mental disease. 2014a;202(7):513. doi: 10.1097/NMD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Kirchner K, Passie T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: A qualitative study of acute and sustained subjective effects. Journal of psychopharmacology (Oxford, England) 2014b;29(1):57–68. doi: 10.1177/0269881114555249. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Lysergic Acid Diethylamide and Psilocybin Revisited. Biological Psychiatry. 2015;78(8):516–518. doi: 10.1016/j.biopsych.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Kaplan J, Stillman R, Wyatt RJ. The psychedelic model of schizophrenia: the case of N, N-dimethyltryptamine. Am J Psychiatry. 1976;133(2):203–208. doi: 10.1176/ajp.133.2.203. [DOI] [PubMed] [Google Scholar]
- Glass GS, Bowers MB. Chronic psychosis associated with long-term psychotomimetic drug abuse. Archives of general psychiatry. 1970;23(2):97–103. doi: 10.1001/archpsyc.1970.01750020001001. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT 2 antagonists. European journal of pharmacology. 1983;91(2):189–196. doi: 10.1016/0014-2999(83)90464-8. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain research. 1996;719(1):29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW, Carlson JN. Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum. Brain research. 1994;657(1):14–22. doi: 10.1016/0006-8993(94)90948-2. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18- MC reduces methamphetamine and nicotine self- administration in rats. Neuroreport. 2000;11(9):2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Hough LB, Kuehne ME, Bandarage UK. (±)- 18- Methoxycoronaridine: A Novel Iboga Alkaloid Congener Having Potential Anti- Addictive Efficacy. CNS Drug Reviews. 1999;5(1):27–42. [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. Mechanisms of action of ibogaine: relevance to putative therapeutic effects and development of a safer iboga alkaloid congener. The Alkaloids: Chemistry and Biology. 2001;56:39–53. doi: 10.1016/s0099-9598(01)56006-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Visker KE, Fritz KA, Bandarage UK, Kuehne ME. 18-Methoxycoronardine attenuates nicotine-induced dopamine release and nicotine preferences in rats. Psychopharmacology. 1998;139(3):274–280. doi: 10.1007/s002130050716. [DOI] [PubMed] [Google Scholar]
- Glick SD, Rossman K, Rao NC, Maisonneuve IM, Carlson JN. Effects of ibogaine on acute signs of morphine withdrawal in rats: independence from tremor. Neuropharmacology. 1992;31(5):497–500. doi: 10.1016/0028-3908(92)90089-8. [DOI] [PubMed] [Google Scholar]
- Glick SD, Rossman K, Steindorf S, Maisonneuve IM, Carlson JN. Effects and aftereffects of ibogaine on morphine self-administration in rats. European journal of pharmacology. 1991;195(3):341–345. doi: 10.1016/0014-2999(91)90474-5. [DOI] [PubMed] [Google Scholar]
- Glick SD, Rossman K, Wang S, Dong N, Keller RW. Local effects of ibogaine on extracellular levels of dopamine and its metabolites in nucleus accumbens and striatum: interactions withd-amphetamine. Brain research. 1993;628(1):201–208. doi: 10.1016/0006-8993(93)90956-n. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Batel P, Adès J, Hamon M, Boni C. Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biological psychiatry. 2000;48(4):259–264. doi: 10.1016/s0006-3223(00)00840-4. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar KA. Psychological effects of (S)-ketamine and N, N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38(6):301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. Journal of psychoactive drugs. 2014;46(1):73–77. doi: 10.1080/02791072.2013.873843. [DOI] [PubMed] [Google Scholar]
- Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. Journal of Psychoactive Drugs. 1986;18(4):319–327. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- Greer GR, Tolbert R. The therapeutic use of MDMA. In: Peroutka SJ, editor. Ecstasy: The Clinical, Pharmacological and Neurotoxicological Effects of the Drug MDMA. Springer; US: 1990. pp. 21–35. [Google Scholar]
- Greer GR, Tolbert R. A method of conducting therapeutic sessions with MDMA. Journal of psychoactive drugs. 1998;30(4):371–379. doi: 10.1080/02791072.1998.10399713. [DOI] [PubMed] [Google Scholar]
- Greiner T, Burch NR, Edelberg R. Psychopathology and psychophysiology of minimal LSD-25 dosage: a preliminary dosage-response spectrum. Archives of Neurology and Psychiatry. 1958;79(2):208–210. doi: 10.1001/archneurpsyc.1958.02340020088016. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. Psychedelic drugs reconsidered. 2. New York, NY: Lindesmith Center; 1997. [Google Scholar]
- Grinspoon L, Bakalar JB. The use of cannabis as a mood stabilizer in bipolar disorder: anecdotal evidence and the need for clinical research. Journal of Psychoactive Drugs. 1998;30(2):171–177. doi: 10.1080/02791072.1998.10399687. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology. 2011;218(4):649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, McCann UD, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. Journal of psychopharmacology. 2008 doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187(3):268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of general psychiatry. 2011;68(1):71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Grob CS, McKenna DJ, Callaway JC, Brito GS, Neves ES, Oberlaender G, … Boone KB. Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. The Journal of nervous and mental disease. 1996;184(2):86–94. doi: 10.1097/00005053-199602000-00004. [DOI] [PubMed] [Google Scholar]
- Grof S. The effects of LSD on chromosomes, genetic mutation, fetal development and malignancy. In: Grof S, editor. LSD psychotherapy. Pomoma, CA: Hunter House; 1980. pp. 320–347. [Google Scholar]
- Grof S, Goodman LE, Richards WA, Kurland AA. LSD-assisted psychotherapy in patients with terminal cancer. International pharmacopsychiatry. 1972;8(3):129–144. doi: 10.1159/000467984. [DOI] [PubMed] [Google Scholar]
- Grof S, Soskin RA, Richards WA, Kurland AA. DPT as an adjunct in psychotherapy of alcoholics. International pharmacopsychiatry. 1973;8(1):104. doi: 10.1159/000467979. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG, Brown ME. Do patients use marijuana as an antidepressant? Depression. 1996;4(2):77–80. doi: 10.1002/(SICI)1522-7162(1996)4:2<77::AID-DEPR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Guerra-Doce E. Psychoactive Substances in Prehistoric Times: Examining the Archaeological Evidence. Time and Mind. 2015;8(1):1–22. [Google Scholar]
- Gutstein HB, Akil H. Opioid analgesics. In: Hardman JG, Limbird LE, Gilman A, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 10. New York, NY: McGraw-Hill; 2001. pp. 569–619. [Google Scholar]
- Guzman M. Cannabinoids: potential anticancer agents. Nature Reviews Cancer. 2003;3(10):745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, … Mathew SJ. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. International Journal of Neuropsychopharmacology. 2014;17(2):331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL. Recent Advances in the Neuropsychopharmacology of Serotonergic Hallucinogens. Behavioural Brain Research. 2014;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. European Journal of Neuroscience. 2002;16(7):1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context- dependent effects of CB1 cannabinoid gene disruption on anxiety- like and social behaviour in mice. European journal of neuroscience. 2004;19(7):1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynänen OP, … Tiihonen J. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Molecular psychiatry. 1999;4(4):385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- Halpern JH, Pope HG., Jr Hallucinogen persisting perception disorder: what do we know after 50 years? Drug and alcohol dependence. 2003;69(2):109–119. doi: 10.1016/s0376-8716(02)00306-x. [DOI] [PubMed] [Google Scholar]
- Halpern JH, Pope HG, Sherwood AR, Barry S, Hudson JI, Yurgelun-Todd D. Residual neuropsychological effects of illicit 3, 4-methylenedioxymethamphetamine (MDMA) in individuals with minimal exposure to other drugs. Drug and alcohol dependence. 2004;75(2):135–147. doi: 10.1016/j.drugalcdep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Halpern JH, Sherwood AR, Hudson JI, Yurgelun-Todd D, Pope HG., Jr Psychological and cognitive effects of long-term peyote use among Native Americans. Biological Psychiatry. 2005;58(8):624–631. doi: 10.1016/j.biopsych.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Halpern JH, Sherwood AR, Passie T, Blackwell KC, Ruttenber AJ. Evidence of health and safety in American members of a religion who use a hallucinogenic sacrament. Med Sci Monit. 2008;14(8):22. [PubMed] [Google Scholar]
- Harman WW, McKim RH, Mogar RE, Fadiman J, Stolaroff MJ. Psychedelic agents in creative problem-solving: A pilot study. Monograph Supplement 2-V19. Psychological Reports. 1966;19(1):211–227. doi: 10.2466/pr0.1966.19.1.211. [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3, 4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162(4):396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Harris R, Gurel L. A study of ayahuasca use in North America. Journal of psychoactive drugs. 2012;44(3):209–215. doi: 10.1080/02791072.2012.703100. [DOI] [PubMed] [Google Scholar]
- Hauer D, Schelling G, Gola H, Campolongo P, Morath J, Roozendaal B, … Kolassa IT. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. 2013 doi: 10.1371/journal.pone.0062741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazekamp A, Ware MA, Muller-Vahl KR, Abrams D, Grotenhermen F. The medicinal use of cannabis and cannabinoids an international cross-sectional survey on administration forms. Journal of psychoactive drugs. 2013;45(3):199–210. doi: 10.1080/02791072.2013.805976. [DOI] [PubMed] [Google Scholar]
- Heekeren K, Neukirch A, Daumann J, Stoll M, Obradovic M, Kovar KA, … Gouzoulis-Mayfrank E. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N, N-dimethyltryptamine (DMT) models of psychosis. Journal of Psychopharmacology. 2007;21(3):312–320. doi: 10.1177/0269881107077734. [DOI] [PubMed] [Google Scholar]
- Heffter A. Ueber Cacteenalkaloi de. Ber Dtsch Chem Ges. 1896;29:216–27. [Google Scholar]
- Hendricks PS, Clark CB, Johnson MW, Fontaine KR, Cropsey KL. Hallucinogen use predicts reduced recidivism among substance-involved offenders under community corrections supervision. Journal of Psychopharmacology. 2014;28(1):62–66. doi: 10.1177/0269881113513851. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Thorne CB, Clark CB, Coombs DW, Johnson MW. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. Journal of Psychopharmacology. 2015;29(3):280–288. doi: 10.1177/0269881114565653. [DOI] [PubMed] [Google Scholar]
- Henry JA, Rella JG. Medical risks associated with MDMA use. In: Holland J, editor. Ecstasy, A Complete Guide. Rochester VT: Inner Traditions; 2001. pp. 71–86. [Google Scholar]
- Hermle L, Fünfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, … Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biological psychiatry. 1992;32(11):976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Hermle L, Gouzoulis-Mayfrank E, Spitzer M. Blood flow and cerebral laterality in the mescaline model of psychosis. Pharmacopsychiatry. 1998;31:85–91. doi: 10.1055/s-2007-979352. [DOI] [PubMed] [Google Scholar]
- Hernandez SC, Bertolino M, Xiao Y, Pringle KE, Caruso FS, Kellar KJ. Dextromethorphan and its metabolite dextrorphan block a3b4 neuronal nicotinic receptors. J Pharmacol Exp Ther. 2000;293(3):962–967. [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB 1 receptor activity elicits an antidepressant-like response in the rat forced swim test. European Neuropsychopharmacology. 2005;15(6):593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hillig KW. A chemotaxonomic analysis of terpenoid variation in Cannabis. Biochemical systematics and ecology. 2004;32(10):875–891. [Google Scholar]
- Hoelen DW, Spiering W, Valk GD. Long-QT syndrome induced by the antiaddiction drug ibogaine. New England journal of medicine. 2009;360(3):308–309. doi: 10.1056/NEJMc0804248. [DOI] [PubMed] [Google Scholar]
- Hofmann A. How LSD originated. Journal of Psychoactive Drugs. 1979;11(1–2):53–60. doi: 10.1080/02791072.1979.10472092. [DOI] [PubMed] [Google Scholar]
- Hofmann A. LSD: my problem child. Oxford, UK: Oxford University Press; 2013. [Google Scholar]
- Hofmann A, Heim R, Brack A, Kobel H. Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen Rauschpilz Psilocybe mexicana Heim. Cellular and Molecular Life Sciences. 1958;14(3):107–109. doi: 10.1007/BF02159243. [DOI] [PubMed] [Google Scholar]
- Hoffmeister F, Wuttke W. Psychotropic drugs as negative reinforcers. Pharmacological reviews. 1975;27(3):419–428. [PubMed] [Google Scholar]
- Holland J, editor. Ecstasy: the complete guide: a comprehensive look at the risks and benefits of MDMA. Rochester, VT: Inner Traditions/Bear & Co; 2001. [Google Scholar]
- Hollister LE, Hartman AM. Mescaline, lysergic acid diethylamide and psilocybin: comparison of clinical syndromes, effects on color perception and biochemical measures. Comprehensive Psychiatry. 1962;3(4):235–241. doi: 10.1016/s0010-440x(62)80024-8. [DOI] [PubMed] [Google Scholar]
- Hood RW., Jr The construction and preliminary validation of a measure of reported mystical experience. Journal for the scientific study of religion. 1975:29–41. [Google Scholar]
- Hoshi R, Mullins K, Boundy C, Brignell C, Piccini P, Curran HV. Neurocognitive function in current and ex-users of ecstasy in comparison to both matched polydrug-using controls and drug-naive controls. Psychopharmacology. 2007;194(3):371–379. doi: 10.1007/s00213-007-0837-5. [DOI] [PubMed] [Google Scholar]
- Huxley A. The Doors of Perception. London: Chatto and Windus; 1954. [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology. 2012;222(2):293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, … Liechti ME. MDMA enhances emotional empathy and prosocial behavior. Social cognitive and affective neuroscience. 2013;9(11):1645–52. doi: 10.1093/scan/nst161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Cultura. Declaracio n Patrimonio Cultural de la nacio n a los conocimientos y usos tradicionales de la ayahuasca practicados por comunidades nativas amazo nicas. Lima, Peru: 2008. Resolucio n Directoral Nacional, no. 836. [Google Scholar]
- Isbell H. Comparison of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacologia. 1959;1(1):29–38. doi: 10.1007/BF00408109. [DOI] [PubMed] [Google Scholar]
- Isbell H, Belleville RE, Fraser HF, Wikler A, Logan CR. Studies on lysergic acid diethylamide (LSD-25): 1. Effects in former morphine addicts and development of tolerance during chronic intoxication. AMA Archives of Neurology & Psychiatry. 1956;76(5):468–478. [PubMed] [Google Scholar]
- Jacob MS, Presti DE. Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine. Medical hypotheses. 2005;64(5):930–937. doi: 10.1016/j.mehy.2004.11.005. [DOI] [PubMed] [Google Scholar]
- James W. The varieties of religious experience. Cambridge, MA: Harvard University Press; 1902. Quotation pages are from the 1994 Modern Library Edition: James, W. (1994). The varieties of religious experience: A study in human nature. New York: Modern Library. [Google Scholar]
- Jansen KL. A review of the nonmedical use of ketamine: use, users and consequences. Journal of Psychoactive Drugs. 2000;32(4):419–433. doi: 10.1080/02791072.2000.10400244. [DOI] [PubMed] [Google Scholar]
- Johansen PØ, Krebs TS. How could MDMA (ecstasy) help anxiety disorders? A neurobiological rationale. Journal of Psychopharmacology. 2009;23(4):389–391. doi: 10.1177/0269881109102787. [DOI] [PubMed] [Google Scholar]
- Johansen PØ, Krebs TS. Psychedelics not linked to mental health problems or suicidal behavior: a population study. Journal of Psychopharmacology. 2015;29:270–279. doi: 10.1177/0269881114568039. [DOI] [PubMed] [Google Scholar]
- Johnson JB. The elements of Mazatec witchcraft. Goteborg Ethnografiska Museum Ethnologiska Studier. 1939;9:119–149. [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology. 2014 doi: 10.1177/0269881114548296. 0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. Journal of Psychopharmacology. 2008 doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Caspers MJ, Prisinzano TE, Griffiths RR. Time course of pharmacokinetic and hormonal effects of inhaled high-dose salvinorin A in humans. Journal of psychopharmacology (Oxford, England) 2016;30(4):323–329. doi: 10.1177/0269881116629125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug and alcohol dependence. 2011;115(1):150–155. doi: 10.1016/j.drugalcdep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Sewell RA, Griffiths RR. Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug and alcohol dependence. 2012;123(1):132–140. doi: 10.1016/j.drugalcdep.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, Barrett FS, Roseman L, Lorenz R, Family N, Bolstridge M, … Carhart-Harris RL. LSD enhances the emotional response to music. Psychopharmacology. 2015;232(19):3607–3614. doi: 10.1007/s00213-015-4014-y. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Kilford EJ, Minchin S, Moss A, Lawn W, Das RK, … Freeman TP. Recreational 3, 4-methylenedioxy-N-methylamphetamine (MDMA) or ‘ecstasy’and self-focused compassion: Preliminary steps in the development of a therapeutic psychopharmacology of contemplative practices. Journal of Psychopharmacology. 2015 doi: 10.1177/0269881115587143. 0269881115587143. [DOI] [PubMed] [Google Scholar]
- Kamilar-Britt P, Bedi G. The Prosocial Effects of 3, 4-methylenedioxymethamphetamine (MDMA): Controlled Studies in Humans and Laboratory Animals. Neuroscience & Biobehavioral Reviews. 2015 doi: 10.1016/j.neubiorev.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch SB. A historical review of MDMA. Open Forensic Science Journal. 2011;4:20–24. [Google Scholar]
- Kast EC, Collins VJ. Study of lysergic acid diethylamide as an analgesic agent. Anesthesia & Analgesia. 1964;43(3):285–291. [PubMed] [Google Scholar]
- Katz S. My 12 Hours As A Madman. Maclean’s. 1953;1:9–11. [Google Scholar]
- Keeler MH, Ewing JA, Rouse BA. Hallucinogenic effects of marijuana as currently used. American Journal of Psychiatry. 1971;128(2):213–216. doi: 10.1176/ajp.128.2.213. [DOI] [PubMed] [Google Scholar]
- Khey DN, Miller BL, Griffin OH. Salvia divinorum use among a college student sample. Journal of Drug Education. 2009;38(3):297–306. doi: 10.2190/DE.38.3.g. [DOI] [PubMed] [Google Scholar]
- Khorramzadeh E, Lofty A. The use of ketamine in psychiatry. Psychosomatics. 1973;14:344–346. doi: 10.1016/S0033-3182(73)71306-2. [DOI] [PubMed] [Google Scholar]
- Killinger BA, Peet MM, Baker LE. Salvinorin A fails to substitute for the discriminative stimulus effects of LSD or ketamine in Sprague Dawley rats. Pharmacology Biochemistry and Behavior. 2010;96(3):260–265. doi: 10.1016/j.pbb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Kindlundh-Högberg AM, Blomqvist A, Malki R, Schiöth HB. Extensive neuroadaptive changes in cortical gene-transcript expressions of the glutamate system in response to repeated intermittent MDMA administration in adolescent rats. BMC neuroscience. 2008;9(1):39. doi: 10.1186/1471-2202-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Baggott MJ, Mendelson JE, Galloway GP, Liechti ME, Hysek CM, de Wit H. MDMA effects consistent across laboratories. Psychopharmacology. 2014a;231(19):3899–3905. doi: 10.1007/s00213-014-3528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Delton AW, de Wit H, Robertson TE. Prosocial effects of MDMA: A measure of generosity. Journal of Psychopharmacology. 2015 doi: 10.1177/0269881115573806. 0269881115573806. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology. 2014b;39(7):1654–1663. doi: 10.1038/npp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H. MDMA: a social drug in a social context. Psychopharmacology. 2015;232(6):1155–1163. doi: 10.1007/s00213-014-3752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Low CB. Suggestion in the treatment of depression. American Journal of Clinical Hypnosis. 2013;55(3):221–229. doi: 10.1080/00029157.2012.738613. [DOI] [PubMed] [Google Scholar]
- Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. Journal of Neuroimmune Pharmacology. 2006;1(1):50–64. doi: 10.1007/s11481-005-9007-x. [DOI] [PubMed] [Google Scholar]
- Koenig X, Hilber K. The Anti-Addiction Drug Ibogaine and the Heart: A Delicate Relation. Molecules. 2015;20(2):2208–2228. doi: 10.3390/molecules20022208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncuoğlu H. Drug addiction and AIDS. Springer; Vienna: 1991. The treatment with dextromethorphan of heroin addicts; pp. 320–329. [Google Scholar]
- Koyuncuoğlu H, Saydam B. The treatment of heroin addicts with dextromethorphan: a double-blind comparison of dextromethorphan with chlorpromazine. International journal of clinical pharmacology, therapy, and toxicology. 1990;28(4):147–152. [PubMed] [Google Scholar]
- Kraehenmann R, Preller KH, Scheidegger M, Pokorny T, Bosch OG, Seifritz E, Vollenweider FX. Psilocybin-Induced Decrease in Amygdala Reactivity Correlates with Enhanced Positive Mood in Healthy Volunteers. Biological psychiatry. 2014;78(8):572–581. doi: 10.1016/j.biopsych.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Krebs TS, Johansen PØ. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. Journal of Psychopharmacology. 2012;26(7):994–1002. doi: 10.1177/0269881112439253. [DOI] [PubMed] [Google Scholar]
- Krebs TS, Johansen PØ. Over 30 million psychedelic users in the United States. F1000Research. 2013a;2:98. doi: 10.12688/f1000research.2-98.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs TS, Johansen PØ. Psychedelics and Mental Health: A Population Study. PLoS ONE. 2013b;8(8):1–9. doi: 10.1371/journal.pone.0063972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Dunaevsky IV, Romanova TN, Slavina TY, Grinenko AY. Single versus repeated sessions of ketamine-assisted psychotherapy for people with heroin dependence. Journal of psychoactive drugs. 2007;39(1):13–19. doi: 10.1080/02791072.2007.10399860. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Burakov A, Romanova T, Dunaevsky I, Strassman R, Grinenko A. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. Journal of substance abuse treatment. 2002;23(4):273–283. doi: 10.1016/s0740-5472(02)00275-1. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. Journal of psychoactive drugs. 1997;29(2):165–183. doi: 10.1080/02791072.1997.10400185. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Grinenko AY. Ten year study of ketamine psychedelic therapy (KPT) of alcohol dependence. The Heffter Review of Psychedelic Research. 1998;1:56–61. [Google Scholar]
- Krupitsky EM, Grineko AY, Berkaliev TN, Paley AI, Tetrov UN, Mushkov KA, Borodikin YS. The combination of psychedelic and aversive approaches in alcoholism treatment: the affective contra-attribution method. Alcoholism Treatment Quarterly. 1992;9(1):99–105. [Google Scholar]
- Labate BC, De Rose IS, dos Santos RG. Ayahuasca religions: A comprehensive bibliography and critical essays. MAPS (Multidisciplinary Association for Psychedelic Studies); 2008. [Google Scholar]
- Labate BC, Santos RG, Anderson B, Mercante M, Barbosa PCR. The treatment and handling of substance dependence with ayahuasca: Reflections on current and future research. In: Labate BC, MacRae E, editors. Ayahuasca, ritual and religion in Brazil. London: Equinox; 2010. pp. 205–227. [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. International Journal of Neuropsychopharmacology. 2011;14(8):1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC. Dextromethorphan as a potential rapid-acting antidepressant. Medical hypotheses. 2011;76(5):717–719. doi: 10.1016/j.mehy.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC. An extension of hypotheses regarding rapid-acting, treatment-refractory, and conventional antidepressant activity of dextromethorphan and dextrorphan. Medical hypotheses. 2012;78(6):693–702. doi: 10.1016/j.mehy.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta- analytic update. Human Psychopharmacology: Clinical and Experimental. 2007;22(6):381–388. doi: 10.1002/hup.857. [DOI] [PubMed] [Google Scholar]
- Leal MB, Michelin K, Souza DO, Elisabetsky E. Ibogaine attenuation of morphine withdrawal in mice: role of glutamate N-methyl-D-aspartate receptors. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(5):781–785. doi: 10.1016/S0278-5846(03)00109-X. [DOI] [PubMed] [Google Scholar]
- Leary T. The effects of consciousness-expanding drugs on prisoner rehabilitation. Psychedelic Review. 1969;10:29–44. [Google Scholar]
- Leary T, Litwin GH, Metzner R. Reactions to Psilocybjn Administered in A Supportive Environment. The Journal of nervous and mental disease. 1963;137(6):561–573. doi: 10.1097/00005053-196312000-00007. [DOI] [PubMed] [Google Scholar]
- Leary T, Metzner R, Presnell M, Weil G, Schwitzgebel R, Kinne S. A new behavior change pattern using psilocybin. Psychotherapy: Theory, Research and Practice. 1965;2(2):61–72. [Google Scholar]
- Lee MA, Shlain B. Acid dreams: The complete social history of LSD: The CIA, the sixties, and beyond. New York, NY: Grove Press; 1992. [Google Scholar]
- Lenz W, Pfeiffer RA, Kosenow W, Hayman DJ. Thalidomide and congenital abnormalities. The Lancet. 1962;279(7219):45–46. [Google Scholar]
- Lewin L. In: Phantastica: Narcotic and Stimulating Drugs: Their Use and Abuse. Wirth PHA, translator. New York: E. P. Dutton & Co; 1931. [Google Scholar]
- Li HL. An archaeological and historical account of cannabis in China. Economic Botany. 1973;28(4):437–448. [Google Scholar]
- Li HL. The origin and use of Cannabis in eastern Asia linguistic-cultural implications. Economic Botany. 1974;28(3):293–301. [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, … Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology. 2001;154(2):161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23(4):396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Lotsof HS. 4,499,096. Washington, DC: U.S. Patent and Trademark Office; US Patent No. 1985
- Lotsof HS. Ibogaine in the treatment of chemical dependence disorders: Clinical perspectives. MAPS Bulletin. 1995;3:16–27. [Google Scholar]
- Luciano D. Observations on treatment with ibogaine. The American Journal on Addictions. 1998;7(1):89. doi: 10.1111/j.1521-0391.1998.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, … Zarate CA. Do the dissociative side effects of ketamine mediate its antidepressant effects? Journal of affective disorders. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna LE. Apéndices. América indígena. 1986a;46(1):247–251. [Google Scholar]
- Luna LE. Acta Universitatis Stockolmiensis, Stockolm Studies in Comparative religion. 1986b. Vegetalismo: Shamanism among the mestizo population of the peruvian amazon; p. 151p. [Google Scholar]
- Lyvers M, Hasking P. Have Halpern et al.(2004) detected ‘residual neuropsychological effects’ of MDMA? Not likely. Drug & Alcohol Dependence. 2004;75(2):149–152. doi: 10.1016/j.drugalcdep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783–786. doi: 10.1111/epi.12610. [DOI] [PubMed] [Google Scholar]
- Mabit J. Blending Traditions-Using Indigenous Medicinal Knowledge to Treat Drug Adiction. MAPS Bulletin. 2002;7(2):25–32. [Google Scholar]
- Mabit J. Ayahuasca in the treatment of addictions. Psychedelic medicine: New evidence for hallucinogenic substances as treatments. 2007;2:87–105. [Google Scholar]
- Mackie K. Signaling via CNS cannabinoid receptors. Molecular and cellular endocrinology. 2008;286(1):S60–S65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology. 2011;25(11):1453–1461. doi: 10.1177/0269881111420188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR. Dose-related effects of salvinorin A in humans: dissociative, hallucinogenic, and memory effects. Psychopharmacology. 2013;226(2):381–392. doi: 10.1007/s00213-012-2912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Leoutsakos JMS, Johnson MW, Griffiths RR. Factor analysis of the mystical experience questionnaire: A study of experiences occasioned by the hallucinogen psilocybin. Journal for the scientific study of religion. 2012;51(4):721–737. doi: 10.1111/j.1468-5906.2012.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae E. Guided by the moon Shamanism and the ritual use of ayahuasca in the Santo Daime religion in Brazil. São Paulo: Editora Brasiliense. 1992 Ebook retrieved June 30, 2012. [Google Scholar]
- Maisonneuve IM, Glick SD. Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. European journal of pharmacology. 1999;383(1):15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD, Mann GL, Deibel CR. Ibogaine and the dopaminergic response to nicotine. Psychopharmacology. 1997;129(3):249–256. doi: 10.1007/s002130050187. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Keller RW, Glick SD. Interactions between ibogaine, a potential anti-addictive agent, and morphine: an in vivo microdialysis study. European journal of pharmacology. 1991;199(1):35–42. doi: 10.1016/0014-2999(91)90634-3. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Keller RW, Glick SD. Interaction of ibogaine and d-amphetamine: in vivo microdialysis and motor behavior in rats. Brain research. 1992;579(1):87–92. doi: 10.1016/0006-8993(92)90745-u. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Mann GL, Deibel CR, Glick SD. Ibogaine and the dopaminergic response to nicotine. Psychopharmacology. 1997;129(3):249–256. doi: 10.1007/s002130050187. [DOI] [PubMed] [Google Scholar]
- Malitz S, Esecover H, Wilkens B, Hoch PH. Some observations on psilocybin, a new hallucinogen, in volunteer subjects. Comprehensive psychiatry. 1960;1(1):8–17. doi: 10.1016/s0010-440x(60)80045-4. [DOI] [PubMed] [Google Scholar]
- Maqueda AE, Valle M, Addy PH, Antonijoan RM, Puntes M, Coimbra J, … Barker S. Salvinorin-A induces intense dissociative effects, blocking external sensory perception and modulating interoception and sense of body ownership in humans. International Journal of Neuropsychopharmacology. 2015 Jun 5;18(12) doi: 10.1093/ijnp/pyv065. pii: pyv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueda AE, Valle M, Addy PH, Antonijoan RM, Puntes M, Coimbra J, … Barker S. Naltrexone but Not Ketanserin Antagonizes the Subjective, Cardiovascular, and Neuroendocrine Effects of Salvinorin-A in Humans. International Journal of Neuropsychopharmacology. 2016:pyw016. doi: 10.1093/ijnp/pyw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Kovera CA, Buck BE, Norenberg MD, Shapshak P, Hearn W, Sanchez-Ramos J. Medication Development of Ibogaine as a Pharmacotherapy for Drug Dependencea. Annals of the New York Academy of Sciences. 1998;844(1):274–292. doi: 10.1111/j.1749-6632.1998.tb08242.x. [DOI] [PubMed] [Google Scholar]
- Mash DC, Kovera CA, Pablo J, Tyndale R, Ervin FR, Kamlet JD, Lee Hearn W. Ibogaine in the treatment of heroin withdrawal. The Alkaloids: Chemistry and Biology. 2001;56:155–171. doi: 10.1016/s0099-9598(01)56012-5. [DOI] [PubMed] [Google Scholar]
- Mashour GA. From LSD to the IRB: Henry Beecher’s psychedelic research and the foundation of clinical ethics. International anesthesiology clinics. 2007;45(4):105–111. doi: 10.1097/AIA.0b013e31811f4613. [DOI] [PubMed] [Google Scholar]
- Masters R, Houston J. The varieties of psychedelic experience: The classic guide to the effects of LSD on the human psyche. Inner Traditions/Bear & Co; 2000. [Google Scholar]
- Mangini M. Treatment of alcoholism using psychedelic drugs: a review of the program of research. J Psychoactive Drugs. 1998;30(4):381–418. doi: 10.1080/02791072.1998.10399714. [DOI] [PubMed] [Google Scholar]
- McDowell DM, Kleber HD. MDMA: its history and pharmacology. Psychiatric Annals. 1994;24(3):127–130. [Google Scholar]
- McGlothlin WH, Arnold DO. LSD revisited: A ten-year follow-up of medical LSD use. Archives of General Psychiatry. 1971;24(1):35–49. doi: 10.1001/archpsyc.1971.01750070037005. [DOI] [PubMed] [Google Scholar]
- McGlothlin W, Cohen S, McGlothlin MS. Long lasting effects of LSD on normals. Archives of General Psychiatry. 1967;17(5):521–532. doi: 10.1001/archpsyc.1967.01730290009002. [DOI] [PubMed] [Google Scholar]
- McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacology & therapeutics. 2004;102(2):111–129. doi: 10.1016/j.pharmthera.2004.03.002. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Callaway JC, Grob CS. The scientific investigation of Ayahuasca: a review of past and current research. The Heffter Review of Psychedelic Research. 1998;1:65–77. [Google Scholar]
- McKenna DJ, Peroutka SJ. Neurochemistry and Neurotoxicity of 3, 4-Methylenedioxymethamphetamine (MDMA,“Ecstasy”) Journal of neurochemistry. 1990;54(1):14–22. doi: 10.1111/j.1471-4159.1990.tb13277.x. [DOI] [PubMed] [Google Scholar]
- McKenna D, Riba J. New World Tryptamine Hallucinogens and the Neuroscience of Ayahuasca. In: Geyer MA, Ellenbrook BA, Marsden CA, Barnes ThRE, editors. Current Topics in Behavioral Neuroscience. Heidelberg: Springer; 2015. pp. 1–27. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Towers GHN. Biochemistry and Pharmacology of Tryptamines and β-Carbolines A Minireview. Journal of Psychoactive Drugs. 1984;16(4):347–358. doi: 10.1080/02791072.1984.10472305. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Towers GHN, Abbott FS. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and β-carboline constituents of ayahuasca. J Ethnopharmacol. 1984a;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Towers GHN, Abbott FS. Monoamine oxidase inhibitors in South American hallucinogenic plants part 2: constituents of orally-active myristicaceous hallucinogens. Journal of Ethnopharmacology. 1984b;12(2):179–211. doi: 10.1016/0378-8741(84)90048-5. [DOI] [PubMed] [Google Scholar]
- McKinney CH, Antoni MH, Kumar M, Tims FC, McCabe PM. Effects of guided imagery and music (GIM) therapy on mood and cortisol in healthy adults. Health Psychology. 1997;16(4):390–400. doi: 10.1037//0278-6133.16.4.390. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. The Journal of Clinical Pharmacology. 2002;42(S1):11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Mendelson JE, Coyle JR, Lopez JC, Baggott MJ, Flower K, Everhart ET, … Cohen BM. Lack of effect of sublingual salvinorin A, a naturally occurring kappa opioid, in humans: a placebo-controlled trial. Psychopharmacology. 2011;214(4):933–939. doi: 10.1007/s00213-010-2103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner R. Teonanácatl: Sacred mushroom of visions. El Verano, CA: Four Trees Press; 2004. [Google Scholar]
- Mikuriya T. Marijuana Medical Papers. Medi-Comp Press; 1973. [Google Scholar]
- Miller AC, Jamin CT, Elamin EM. Continuous intravenous infusion of ketamine for maintenance sedation. Minerva Anestesiol. 2011;77(8):812–820. [PubMed] [Google Scholar]
- Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings) CNS neuroscience & therapeutics. 2013;19(6):370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of±3, 4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. Journal of Psychopharmacology. 2011;25(4):439–452. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, … Doblin R. Durability of improvement in posttraumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3, 4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. Journal of Psychopharmacology. 2012 doi: 10.1177/0269881112456611. 0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari HH, Maisonneuve IM, Glick SD. Ibogaine neurotoxicity: a reevaluation. Brain research. 1996;737(1):255–262. doi: 10.1016/0006-8993(96)00739-1. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. Journal of Clinical Psychiatry. 2006;67(11):1735–1740. doi: 10.4088/jcp.v67n1110. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neuroscience letters. 2011;493(3):76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Ketamine use: a review. Addiction. 2012;107(1):27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Monaghan L, Curran HV. Beyond the K- hole: a 3- year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction. 2004;99(11):1450–1461. doi: 10.1111/j.1360-0443.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self- administration upon neurocognitive function and psychological wellbeing: a 1- year longitudinal study. Addiction. 2010;105(1):121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- Morley KC, McGregor IS. (±)−3, 4-Methylenedioxymethamphetamine (MDMA,‘Ecstasy’) increases social interaction in rats. European journal of pharmacology. 2000;408(1):41–49. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- Morris H, Wallach J. From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs. Drug testing and analysis. 2014;6(7–8):614–632. doi: 10.1002/dta.1620. [DOI] [PubMed] [Google Scholar]
- Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJA, Curran HV. Journey through the K-hole: phenomenological aspects of ketamine use. Drug and alcohol dependence. 2008;95(3):219–229. doi: 10.1016/j.drugalcdep.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, … Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. American Journal of Psychiatry. 2014;170:1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, … Conway CR. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biological psychiatry. 2015;78(1):10–18. doi: 10.1016/j.biopsych.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Naranjo P. Hallucinogenic plant use and related indigenous belief systems in the Ecuadorian Amazon. Journal of Ethnopharmacology. 1979;1(2):121–145. doi: 10.1016/0378-8741(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Nau F, Jr, Yu B, Martin D, Nichols CD. Serotonin 5-HT 2A receptor activation blocks TNF-α mediated inflammation in vivo. PloS one. 2013;8(10):e75426. doi: 10.1371/journal.pone.0075426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau F, Miller J, Saravia J, Ahlert T, Yu B, Happel KI, … Nichols CD. Serotonin 5-HT2 receptor activation prevents allergic asthma in a mouse model. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;308(2):L191–L198. doi: 10.1152/ajplung.00138.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, … Huang Y. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Molecular psychiatry. 2013;18(9):1034–1040. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Matsumoto RR. Involvement of AMPA receptors in the antidepressant-like effects of dextromethorphan in mice. Behavioural brain research. 2015;295:26–34. doi: 10.1016/j.bbr.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PloS one. 2014;9(2):e89985. doi: 10.1371/journal.pone.0089985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. Journal of psychoactive drugs. 1986;18(4):305–313. doi: 10.1080/02791072.1986.10472362. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Medical chemistry and structure-activity relationships. In: Cho AK, Segal DS, editors. Amphetamine and its analogs. Pharmacology, toxicology and abuse. Academic Press; San Diego, CA: 1994. pp. 3–41. [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacology & therapeutics. 2004;101(2):131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Psychedelics. Pharmacological reviews. 2016;68(2):264–355. doi: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Oberlender R. Structure-activity relationships of MDMA-like substances. NIDA Res Monogr. 1989;94:1–29. [PubMed] [Google Scholar]
- Nichols DE, Oberlender R. Structure-activity relationships of MDMA and related compounds: a new class of psychoactive agents? In: Peroutka SJ, editor. Ecstasy: The Clinical, Pharmacological and Neurotoxicological Effects of The Drug MDMA. Norwell, MA: Springer US; 1990. pp. 105–131. [Google Scholar]
- Nulsen CE, Fox AM, Hammond GR. Differential effects of ecstasy on short-term and working memory: a meta-analysis. Neuropsychology review. 2010;20(1):21–32. doi: 10.1007/s11065-009-9124-z. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Equasy An Overlooked Addiction. Journal of Psychopharmacology. 2009;23(1):3–5. doi: 10.1177/0269881108099672. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Mind- altering drugs and research: from presumptive prejudice to a Neuroscientific Enlightenment? EMBO reports. 2014;15(3):208–211. doi: 10.1002/embr.201338282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nature Reviews Neuroscience. 2013;14(8):577–585. doi: 10.1038/nrn3530. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. The Lancet. 2010;376(9752):1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. The Lancet. 2007;369(9566):1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- Nygård EA. Doctoral dissertation. Institute of Transpersonal Psychology; Palo Alto, CA: 2007. Listening to the sage: The experience of learning from the Salvia divinorum altered state. [Google Scholar]
- Nyi PP, Lai EP, Lee DY, Biglete SA, Torrecer GI, Anderson IB. Influence of age on Salvia divinorum use: results of an Internet survey. Journal of Psychoactive Drugs. 2010;42(3):385–392. doi: 10.1080/02791072.2010.10400701. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. The Journal of neuroscience. 1988;8(8):2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn E, Long DB, Molliver ME. Ibogaine induces glial activation in parasagittal zones of the cerebellum. Neuroreport. 1993;4(3):299–302. doi: 10.1097/00001756-199303000-00018. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55(2):303–310. doi: 10.1016/0306-4522(93)90500-f. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. The Journal of neuroscience. 1997;17(22):8828–8841. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, Schnyder U. A randomized, controlled pilot study of MDMA (±3, 4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD) Journal of Psychopharmacology. 2013;27(1):40–52. doi: 10.1177/0269881112464827. [DOI] [PubMed] [Google Scholar]
- Ortega A, Blount JF, Manchand PS. Salvinorin, a new trans-neoclerodane diterpene from Salvia divinorum (Labiatae) Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry. 1982:2505–2508. [Google Scholar]
- Osmond H. A review of the clinical effects of psychotomimetic agents. Annals of the New York Academy of Sciences. 1957;66(3):418–434. doi: 10.1111/j.1749-6632.1957.tb40738.x. [DOI] [PubMed] [Google Scholar]
- Osório FDL, Sanches RF, Macedo LR, dos Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, … Hallak JE. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Revista Brasileira de Psiquiatria. 2015;37(1):13–20. doi: 10.1590/1516-4446-2014-1496. [DOI] [PubMed] [Google Scholar]
- Ott J. Their Plant Sources and History. Kennewick, Washington: Natural Products Co; 1993. Pharmacotheon: Entheogenic Drugs. [Google Scholar]
- Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. European journal of pharmacology. 2004;492(2):159–167. doi: 10.1016/j.ejphar.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Pahnke WN. Unpublished doctoral dissertation. Harvard University; Cambridge, MA: 1963. Drugs and mysticism: An analysis of the relationship between psychedelic drugs and the mystical consciousness. [Google Scholar]
- Pahnke WN. Psychedelic drugs and mystical experience. International psychiatry clinics. 1969;5(4):149–162. [PubMed] [Google Scholar]
- Pahnke WN, Richards WA. Implications of LSD and experimental mysticism. Journal of Religion and Health. 1966;5(3):175–208. doi: 10.1007/BF01532646. [DOI] [PubMed] [Google Scholar]
- Palhano-Fontes F, Alchieri JC, Oliveira JPM, Soares BL, Hallak JE, Galvao-Coelho N, de Araujo DB. The therapeutic potentials of ayahuasca in the treatment of depression. In: Labate BC, Cavnar C, editors. The Therapeutic Use of Ayahuasca. Heidelberg: Springer; 2014. pp. 23–39. [Google Scholar]
- Palhano-Fontes F, Andrade KC, Tofoli LF, Santos AC, Crippa JAS, Hallak JE, … de Araujo DB. The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PloS one. 2015;10:e0118143. doi: 10.1371/journal.pone.0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paling FP, Andrews LM, Valk GD, Blom HJ. Life-threatening complications of ibogaine: three case reports. Drugs. 2012;1:2. [PubMed] [Google Scholar]
- Panchal V, Taraschenko OD, Maisonneuve IM, Glick SD. Attenuation of morphine withdrawal signs by intracerebral administration of 18-methoxycoronaridine. European journal of pharmacology. 2005;525(1):98–104. doi: 10.1016/j.ejphar.2005.09.060. [DOI] [PubMed] [Google Scholar]
- Papadodima SA, Dona A, Evaggelakos CI, Goutas N, Athanaselis SA. Ibogaine related sudden death: a case report. Journal of forensic and legal medicine. 2013;20(7):809–811. doi: 10.1016/j.jflm.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Parker LA, Burton P, McDonald RV, Kim JA, Siegel S. Ibogaine interferes with motivational and somatic effects of naloxone-precipitated withdrawal from acutely administered morphine. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26(2):293–297. doi: 10.1016/s0278-5846(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Recreational Ecstasy/MDMA, the serotonin syndrome, and serotonergic neurotoxicity. Pharmacology Biochemistry and Behavior. 2002;71(4):837–844. doi: 10.1016/s0091-3057(01)00711-0. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Chronic tolerance to recreational MDMA (3, 4-methylenedioxymethamphetamine) or Ecstasy. Journal of Psychopharmacology. 2005;19(1):71–83. doi: 10.1177/0269881105048900. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA and temperature: a review of the thermal effects of ‘Ecstasy’ in humans. Drug and alcohol dependence. 2012;121(1):1–9. doi: 10.1016/j.drugalcdep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Human psychobiology of MDMA or ‘Ecstasy’: an overview of 25 years of empirical research. Human Psychopharmacology: Clinical and Experimental. 2013;28(4):289–307. doi: 10.1002/hup.2318. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Lock J, Adnum L, Thome J. MDMA can increase cortisol levels by 800% in dance clubbers. Journal of Psychopharmacology. 2013;27(1):113–114. doi: 10.1177/0269881112454231. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA is certainly damaging after 25 years of empirical research: a reply and refutation of Doblin et al. (2014) Human Psychopharmacology: Clinical and Experimental. 2014;29(2):109–119. doi: 10.1002/hup.2390. [DOI] [PubMed] [Google Scholar]
- Passie T, Emrich HM, Karst M, Brandt SD, Halpern JH. Mitigation of post-traumatic stress symptoms by Cannabis resin: A review of the clinical and neurobiological evidence. Drug testing and analysis. 2012;4(7–8):649–659. doi: 10.1002/dta.1377. [DOI] [PubMed] [Google Scholar]
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A. The pharmacology of lysergic acid diethylamide: a review. CNS Neuroscience & Therapeutics. 2008;14(4):295–314. doi: 10.1111/j.1755-5949.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passie T, Seifert J, Schneider U, Emrich HM. The pharmacology of psilocybin. Addiction Biology. 2002;7(4):357–364. doi: 10.1080/1355621021000005937. [DOI] [PubMed] [Google Scholar]
- Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychological bulletin. 2003;129(4):495–521. doi: 10.1037/0033-2909.129.4.495. [DOI] [PubMed] [Google Scholar]
- Pearl SM, Hough LB, Boyd DL, Glick SD. Sex differences in ibogaine antagonism of morphine-induced locomotor activity and in ibogaine brain levels and metabolism. Pharmacology Biochemistry and Behavior. 1997;57(4):809–815. doi: 10.1016/s0091-3057(96)00383-8. [DOI] [PubMed] [Google Scholar]
- Pentney AR. An exploration of the history and controversies surrounding MDMA and MDA. Journal of psychoactive drugs. 2001;33(3):213–221. doi: 10.1080/02791072.2001.10400568. [DOI] [PubMed] [Google Scholar]
- Petri G, Expert P, Turkheimer F, Carhart-Harris R, Nutt D, Hellyer PJ, Vaccarino F. Homological scaffolds of brain functional networks. Journal of The Royal Society Interface. 2014;11(101):20140873. doi: 10.1098/rsif.2014.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron BE, Ahmedani BK, Vaughn MG, Glass JE, Abdon A, Wu LT. Use of Salvia divinorum in a nationally representative sample. The American journal of drug and alcohol abuse. 2012;38(1):108–113. doi: 10.3109/00952990.2011.600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichini S, Abanades S, Farré M, Pellegrini M, Marchei E, Pacifici R, … Zuccaro P. Quantification of the plant-derived hallucinogen salvinorin A in conventional and non-conventional biological fluids by gas chromatography/mass spectrometry after Salvia divinorum smoking. Rapid communications in mass spectrometry. 2005;19(12):1649–1656. doi: 10.1002/rcm.1970. [DOI] [PubMed] [Google Scholar]
- Pleskovic A, Gorjup V, Brvar M, Kozelj G. Ibogaine-associated ventricular tachyarrhythmias. Clinical Toxicology. 2012;50(2):157–157. doi: 10.3109/15563650.2011.647031. [DOI] [PubMed] [Google Scholar]
- Poling A, Bryceland J. Voluntary drug self-administration by nonhumans: a review. Journal of Psychedelic Drugs. 1979;11:185–90. doi: 10.1080/02791072.1979.10472103. [DOI] [PubMed] [Google Scholar]
- Popik P, Layer RT, Fossom LH, Benveniste M, Geter-Douglass B, Witkin JM, Skolnick P. NMDA antagonist properties of the putative antiaddictive drug, ibogaine. Journal of Pharmacology and experimental therapeutics. 1995a;275(2):753–760. [PubMed] [Google Scholar]
- Popik P, Layer RT, Skolnick P. 100 years of ibogaine: neurochemical and pharmacological actions of a putative anti-addictive drug. Pharmacological Reviews. 1995b;47(2):235–254. [PubMed] [Google Scholar]
- Potter CM, Vujanovic AA, Marshall-Berenz EC, Bernstein A, Bonn-Miller MO. Posttraumatic stress and marijuana use coping motives: the mediating role of distress tolerance. Journal of anxiety disorders. 2011;25(3):437–443. doi: 10.1016/j.janxdis.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri L, Pieri M, Haefely W. LSD as an agonist of dopamine receptors in the striatum. Nature. 1974;252:586–588. doi: 10.1038/252586a0. [DOI] [PubMed] [Google Scholar]
- Presti DE, Nichols DE. Biochemistry and neuropharmacology of psilocybin mushrooms. In: Metzner R, Darling DC, editors. Teonanácatl: Sacred mushroom of visions. Four Trees; El Verano, CA: 2004. pp. 89–108. [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, … Mathew SJ. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depression and anxiety. 2014;31(4):335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological psychiatry. 2009;66(5):522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett JI, Liester MB. Hypotheses Regarding Ayahuasca’s Potential Mechanisms of Action in the Treatment of Addiction. In: Labate BC, Cavnar C, editors. The Therapeutic Use of Ayahuasca. Heidelberg: Springer; 2014. pp. 111–132. [Google Scholar]
- Prue B. Indigenous supports for recovery from alcoholism and drug abuse: The Native American Church. Journal of Ethnic And Cultural Diversity in Social Work. 2013;22(3–4):271–287. [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist salvinorin A in humans. Biological psychiatry. 2012;72(10):871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasakham K, Liu-Chen LY. Sex differences in kappa opioid pharmacology. Life sciences. 2011;88(1):2–16. doi: 10.1016/j.lfs.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rätsch C. The encyclopedia of psychoactive plants: ethnopharmacology and its applications. Rochester, VT: Inner Traditions/Bear & Co; 2005. [Google Scholar]
- Ray TS. Psychedelics and the human receptorome. PLoS One. 2010;5(2):e9019. doi: 10.1371/journal.pone.0009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regelson W, Butler JR, Schulz J, Kirk T, Peek L, Green ML, Zalis MO. Delta-9-tetrahydrocannabinol as an effective antidepressant and appetite-stimulating agent in advanced cancer patients. Pharmacology of marihuana. 1976;2:763–776. [Google Scholar]
- Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology. 2012;223(1):1–15. doi: 10.1007/s00213-012-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Leef YW. Attenuation of alcohol intake by ibogaine in three strains of alcohol-preferring rats. Pharmacology Biochemistry and Behavior. 1995;52(3):615–620. doi: 10.1016/0091-3057(95)00152-m. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacology Biochemistry and Behavior. 1997;58(2):615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Rhead JC, Soskin RA, Turek I, Richards WA, Yensen R, Kurland AA, Ota KY. Psychedelic drug (DPT)-assisted psychotherapy with alcoholics: A controlled study. Journal of Psychoactive Drugs. 1977;9(4):287–300. [Google Scholar]
- Rho B, Glick SD. Effects of 18-methoxycoronaridine on acute signs of morphine withdrawal in rats. Neuroreport. 1998;9(7):1283–1285. doi: 10.1097/00001756-199805110-00004. [DOI] [PubMed] [Google Scholar]
- Riba J, Barbanoj MJ. Bringing ayahuasca to the clinical research laboratory. Journal of psychoactive drugs. 2005;37(2):219–230. doi: 10.1080/02791072.2005.10399804. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Urbano G, Morte A, Antonijoan R, Montero M, … Barbanoj MJ. Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology. 2001;154(1):85–95. doi: 10.1007/s002130000606. [DOI] [PubMed] [Google Scholar]
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. Journal of Pharmacology and Experimental Therapeutics. 2003;306(1):73–83. doi: 10.1124/jpet.103.049882. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, McCann UD. (+/−) 3, 4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology. 1999;42(1):5–10. doi: 10.1159/000026664. [DOI] [PubMed] [Google Scholar]
- Richards WA. Mystical and archetypal experiences of terminal patients in DPT-assisted psychotherapy. Journal of religion and health. 1978;17(2):117–126. doi: 10.1007/BF01532413. [DOI] [PubMed] [Google Scholar]
- Richards WA, Rhead JC, DiLeo FB, Yensen R, Kurland AA. The peak experience variable in DPT-assisted psychotherapy with cancer patients. Journal of Psychoactive Drugs. 1977;9(1):1–10. [Google Scholar]
- Richards WA, Rhead JC, Grof S, Goodman LE, Di Leo F, Rush L. DPT as an adjunct in brief psychotherapy with cancer patients. OMEGA-Journal of Death and Dying. 1980;10(1):9–26. [Google Scholar]
- Riedlinger JE. The scheduling of MDMA: A pharmacist’s perspective. Journal of psychoactive drugs. 1985;17(3):167–171. doi: 10.1080/02791072.1985.10472337. [DOI] [PubMed] [Google Scholar]
- Rinkel M. Pharmacodynamics of LSD and mescaline. The Journal of nervous and mental disease. 1957;125(3):424–426. doi: 10.1097/00005053-195707000-00012. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, … Simpson HB. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, … Somerville M. The harmful health effects of recreational ecstasy: a systematic review of observational evidence. Health Technology Assessment. 2009;13(6):xii–338. doi: 10.3310/hta13050. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Rogers RD, Sahakian BJ. Neuropsychological function in ecstasy users: a study controlling for polydrug use. Psychopharmacology. 2007;189(4):505–516. doi: 10.1007/s00213-005-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, Leech R, Feilding A, Nutt DJ, Carhart-Harris RL. The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Frontiers in human neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. Serotonergic hallucinogens and emerging targets for addiction pharmacotherapies. Psychiatric Clinics of North America. 2012;35(2):357–374. doi: 10.1016/j.psc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, … Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous κ opioid selective agonist. Proceedings of the National Academy of Sciences. 2002;99(18):11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruck CA, Bigwood J, Staples D, Ott J, Wasson RG. Entheogens. Journal of Psychoactive Drugs. 1979;11(1–2):145–146. doi: 10.1080/02791072.1979.10472098. [DOI] [PubMed] [Google Scholar]
- Russo E. Cannabis for migraine treatment: the once and future prescription? An historical and scientific review. Pain. 1998;76(1):3–8. doi: 10.1016/s0304-3959(98)00033-5. [DOI] [PubMed] [Google Scholar]
- Russo EB, Mead AP, Sulak D. Current Status and Future of Cannabis Research. Clinical Researcher. 2015;29(2):58–63. [Google Scholar]
- SAMHSA. National Survey on Drug Use and Health, 2006–2013. SAMHSA; 2013. [Google Scholar]
- Sandison RA, Spencer AM, Whitelaw JDA. The therapeutic value of lysergic acid diethylamide in mental illness. The British Journal of Psychiatry. 1954;100(419):491–507. doi: 10.1192/bjp.100.419.491. [DOI] [PubMed] [Google Scholar]
- Savage C, McCabe OL. Residential psychedelic (LSD) therapy for the narcotic addict: a controlled study. Archives of General Psychiatry. 1973;28(6):808–814. doi: 10.1001/archpsyc.1973.01750360040005. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Lipe GW, Ali SF, Holson RR, Frith CH, Slikker W., Jr Neuropathological evaluation by combined immunohistochemistry and degeneration-specific methods: application to methylenedioxymethamphetamine. Neurotoxicology. 1987;9(3):529–537. [PubMed] [Google Scholar]
- Schatzberg AF. A word to the wise about ketamine. The American journal of psychiatry. 2014;171(3):262–264. doi: 10.1176/appi.ajp.2014.13101434. [DOI] [PubMed] [Google Scholar]
- Schenberg EE, de Castro Comis MA, Chaves BR, da Silveira DX. Treating drug dependence with the aid of ibogaine: A retrospective study. Journal of Psychopharmacology. 2014;28(11):993–1000. doi: 10.1177/0269881114552713. [DOI] [PubMed] [Google Scholar]
- Schindler EA, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA. Indoleamine Hallucinogens in Cluster Headache: Results of the Clusterbusters Medication Use Survey. Journal of psychoactive drugs. 2015;47(5):372–381. doi: 10.1080/02791072.2015.1107664. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, … Liechti ME. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biological psychiatry. 2015;78(8):544–553. doi: 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Schüle C. Neuroendocrinological mechanisms of actions of antidepressant drugs. Journal of neuroendocrinology. 2007;19(3):213–226. doi: 10.1111/j.1365-2826.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- Schultes RE. Teonanácatl: The Narcotic Mushroom of the Aztecs. American Anthropologist. 1940;42(3):429–443. [Google Scholar]
- Schultes RE, Hofmann A, Rätsch C. Plants of the gods: their sacred, healing, and hallucinogenic powers. Rochester, VT: Healing Arts Press; 2001. pp. 124–135. [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annual review of pharmacology. 1969;9(1):483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Semaj LT. Rastafari: From religion to social theory. Caribbean Quarterly. 1980:22–23. [Google Scholar]
- Sewell RA, Halpern JH, Pope HG. Response of cluster headache to psilocybin and LSD. Neurology. 2006;66(12):1920–1922. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- Shahar B, Britton WB, Sbarra DA, Figueredo AJ, Bootzin RR. Mechanisms of change in mindfulness-based cognitive therapy for depression: Preliminary evidence from a randomized controlled trial. International Journal of Cognitive Therapy. 2010;3(4):402–418. [Google Scholar]
- Shalev A, Roitman P, Mechoulam R. Pilot open label trial on oral absorbable Δ9–THC for chronic PTSD. Israel Society for Biological Psychiatry abstract book 2013 [Google Scholar]
- Sherlock K, Wolff K, Hay AW, Conner M. Analysis of illicit ecstasy tablets: implications for clinical management in the accident and emergency department. Journal of accident & emergency medicine. 1999;16(3):194–197. doi: 10.1136/emj.16.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin AT. Mescaline: the chemistry and pharmacology of its analogs. Lloydia. 1973;36(1–4):46–58. [PubMed] [Google Scholar]
- Shulgin AT, Nichols DE. Characterization of three new psychotomimetics. The psychopharmacology of hallucinogens. 1978:74–83. [Google Scholar]
- Shulgin A, Shulgin A. PIHKAL: phenethylamines I have known and loved: A chemical love story. Berkeley, CA: Transform Press; 1995. [Google Scholar]
- Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. Journal of ethnopharmacology. 1994;43(1):53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Siu A, Drachtman R. Dextromethorphan: A Review of N-methyl-d-aspartate Receptor Antagonist in the Management of Pain. CNS drug reviews. 2007;13(1):96–106. doi: 10.1111/j.1527-3458.2007.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart RG, Storm T. The Efficacy of LSD in the Treatment of Alcoholism. Quarterly Journal on the Study of Alcohol. 1964;25:333–38. [PubMed] [Google Scholar]
- Smart RG, Storm T, Baker EFW, Solursh L. A controlled study of lysergide in the treatment of alcoholism: The effects on drinking behavior. Quarterly Journal on the Study of Alcohol. 1966;27(3):469–482. [PubMed] [Google Scholar]
- Soler J, Elices M, Franquesa A, Barker S, Friedlander P, Feilding A, … Riba J. Exploring the therapeutic potential of Ayahuasca: acute intake increases mindfulness-related capacities. Psychopharmacology. 2015:1–7. doi: 10.1007/s00213-015-4162-0. [DOI] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol Lett. 2013;34(4):101–107. [PubMed] [Google Scholar]
- Soskin RA, Grof S, Richards WA. Low doses of dipropyltryptamine in psychotherapy. Archives of general psychiatry. 1973;28(6):817. doi: 10.1001/archpsyc.1973.01750360047006. [DOI] [PubMed] [Google Scholar]
- Spaderna M, Addy PH, D’Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology. 2013;228(4):525–540. doi: 10.1007/s00213-013-3188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce R. On some remarkable narcotics of the Amazon Valley and Orinoco. Ocean Highways Geographical Magazine. 1873;1:184–193. [Google Scholar]
- Stamets P. Psilocybin mushrooms of the world: an identification guide. Ten Speed; Berkeley, CA: 1996. [Google Scholar]
- Stolaroff MJ. The secret chief: Conversations with a pioneer of the underground psychedelic therapy movement. Multidisciplinary Association for Psychedelic Studies; 1997. [Google Scholar]
- Stoll WA. Lysergsa urc-dia thylamid, ein PhantasticucnausderMutter-korngruppe.(Lysergic acid diethylamide, a phantasticon of the ergot group) Schweizer Arch Neurol und Psychiat. 1947;60:1. [Google Scholar]
- Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. The Journal of nervous and mental disease. 1984;172(10):577–595. doi: 10.1097/00005053-198410000-00001. [DOI] [PubMed] [Google Scholar]
- Strassman RJ. Hallucinogenic drugs in psychiatric research and treatment perspectives and prospects. The Journal of nervous and mental disease. 1995a;183(3):127–138. doi: 10.1097/00005053-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Strassman RJ. Human psychopharmacology of N, N-dimethyltryptamine. Behavioural brain research. 1995b;73(1):121–124. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR. Dose-response study of N, N-dimethyltryptamine in humans: I. Neuroendocrine, autonomic, and cardiovascular effects. Archives of General Psychiatry. 1994a;51(2):85–97. doi: 10.1001/archpsyc.1994.03950020009001. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N, N-dimethyltryptamine in humans. Biological Psychiatry. 1996;39(9):784–795. doi: 10.1016/0006-3223(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N, N-dimethyltryptamine in humans: II. Subjective effects and preliminary results of a new rating scale. Archives of General Psychiatry. 1994b;51(2):98–08. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PLoS One. 2012;7(2):e30800. doi: 10.1371/journal.pone.0030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. Journal of psychopharmacology. 2011;25(11):1434–1452. doi: 10.1177/0269881110382466. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Vaupel DB. When the endogenous hallucinogenic trace amine N, N-dimethyltryptamine meets the sigma-1 receptor. Science signaling. 2009;2(61):pe12. doi: 10.1126/scisignal.261pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufka KJ, Loria MJ, Lewellyn K, Zjawiony JK, Ali Z, Abe N, Khan IA. The effect of Salvia divinorum and Mitragyna speciosa extracts, fraction and major constituents on place aversion and place preference in rats. Journal of ethnopharmacology. 2014;151(1):361–364. doi: 10.1016/j.jep.2013.10.059. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Measham F, Brandt SD, Cole JC. Salvia divinorum use and phenomenology: results from an online survey. Journal of Psychopharmacology. 2011;25(11):1496–1507. doi: 10.1177/0269881110385596. [DOI] [PubMed] [Google Scholar]
- Sweetnam PM, Lancaster J, Snowman A, Collins JL, Perschke S, Bauer C, Ferkany J. Receptor binding profile suggests multiple mechanisms of action are responsible for ibogaine’s putative anti-addictive activity. Psychopharmacology. 1995;118(4):369–376. doi: 10.1007/BF02245936. [DOI] [PubMed] [Google Scholar]
- Szabo A. Psychedelics and immunomodulation: novel approaches and therapeutic opportunities. Frontiers in Immunology. 2015;6(358):1–11. doi: 10.3389/fimmu.2015.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szara SS. Dimethyltryptamin: Its metabolism in man; the relation to its psychotic effect to the serotonin metabolism. Experientia. 1956;12:441–442. doi: 10.1007/BF02157378. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D, Chialvo DR. Enhanced repertoire of brain dynamical states during the psychedelic experience. Human brain mapping. 2014;35(11):5442–5456. doi: 10.1002/hbm.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschenko OD, Panchal V, Maisonneuve IM, Glick SD. Is antagonism of α3β4 nicotinic receptors a strategy to reduce morphine dependence? European journal of pharmacology. 2005;513(3):207–218. doi: 10.1016/j.ejphar.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD. 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse. 2007;61(7):547–560. doi: 10.1002/syn.20396. [DOI] [PubMed] [Google Scholar]
- Terracciano A, McCrae RR, Brant LJ, Costa PT., Jr Hierarchical linear modeling analyses of the NEO-PI-R scales in the Baltimore Longitudinal Study of Aging. Psychology and aging. 2005;20(3):493. doi: 10.1037/0882-7974.20.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Lucas P, Capler NR, Tupper KW, Martin G. Ayahuasca-assisted therapy for addiction: results from a preliminary observational study in Canada. Curr Drug Abuse Rev. 2013;6(1):30–42. doi: 10.2174/15733998113099990003. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Moon E, Kim UJ, Xu J, Siciliano MJ, Weinshilboum RM. Human indolethylamine N-methyltransferase: cDNA cloning and expression, gene cloning, and chromosomal localization. Genomics. 1999;61(3):285–297. doi: 10.1006/geno.1999.5960. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT1A receptors in the prosocial effects of 3, 4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146(2):509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka JT, Bergström KA, Karhu J, Viinamäki H, Lehtonen J, … Hakola P. Single-photon emission tomography imaging of monoamine transporters in impulsive violent behaviour. European journal of nuclear medicine. 1997;24(10):1253–1260. doi: 10.1007/s002590050149. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology. 1988;94(2):213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug and alcohol dependence. 1999;55(1):105–115. doi: 10.1016/s0376-8716(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Touw M. The religious and medicinal uses of Cannabis in China, India and Tibet. Journal of psychoactive drugs. 1981;13(1):23–34. doi: 10.1080/02791072.1981.10471447. [DOI] [PubMed] [Google Scholar]
- Trichter S, Klimo J, Krippner S. Changes in spirituality among ayahuasca ceremony novice participants. Journal of Psychoactive Drugs. 2009;41(2):121–134. doi: 10.1080/02791072.2009.10399905. [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. The Journal of clinical psychiatry. 2005;66(10):1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Tupper KW, Wood E, Yensen R, Johnson MW. Psychedelic medicine: a re-emerging therapeutic paradigm. CMAJ: Canadian Medical Association journal= journal de l’Association medicale canadienne. 2015;187(14):1054. doi: 10.1503/cmaj.141124. https://dx.doi.org/10.1503/cmaj.141124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urigüen L, Pérez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB 1 receptors. Neuropharmacology. 2004;46(7):966–973. doi: 10.1016/j.neuropharm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, … Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1 H]-MRS. Psychiatry Research: Neuroimaging. 2011;191(2):122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491–2493. doi: 10.1001/jama.2015.6613. [DOI] [PubMed] [Google Scholar]
- Van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regulatory Toxicology and Pharmacology. 2011;59(3):423–429. doi: 10.1016/j.yrtph.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Van Amsterdam J, Opperhuizen A, Koeter M, van den Brink W. Ranking the harm of alcohol, tobacco and illicit drugs for the individual and the population. European Addiction Research. 2010;16(4):202–207. doi: 10.1159/000317249. [DOI] [PubMed] [Google Scholar]
- Velasco G, Hernández-Tiedra S, Dávila D, Lorente M. The use of cannabinoids as anticancer agents. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2016;64:259–266. doi: 10.1016/j.pnpbp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacology Biochemistry and Behavior. 2005;81(2):331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of learning and memory. 2002;78(3):610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nature Reviews Neuroscience. 2010;11(9):642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9(17):3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Hell D, Leenders KL. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man—a PET study with [11C] raclopride. Neuropsychopharmacology. 1999;20(5):424–433. doi: 10.1016/S0893-133X(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Wallach JV. Endogenous hallucinogens as ligands of the trace amine receptors: a possible role in sensory perception. Medical hypotheses. 2009;72(1):91–94. doi: 10.1016/j.mehy.2008.07.052. [DOI] [PubMed] [Google Scholar]
- Wardle MC, de Wit H. MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacology. 2014;231(21):4219–4229. doi: 10.1007/s00213-014-3570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Kirkpatrick MG, de Wit H. ‘Ecstasy’ as a social drug: MDMA preferentially affects responses to emotional stimuli with social content. Social cognitive and affective neuroscience. 2014;9(8):1076–1081. doi: 10.1093/scan/nsu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson RG. A new Mexican psychotropic drug from the mint family. Botanical Museum Leaflets, Harvard University. 1962;20(3):77–84. [Google Scholar]
- Wasson RG. The wondrous mushroom: mycolatry in Meso-america. New York: McGraw-Hill; 1980. [Google Scholar]
- Wei D, Maisonneuve IM, Kuehne ME, Glick SD. Acute iboga alkaloid effects on extracellular serotonin (5-HT) levels in nucleus accumbens and striatum in rats. Brain research. 1998;800(2):260–268. doi: 10.1016/s0006-8993(98)00527-7. [DOI] [PubMed] [Google Scholar]
- Weinbroum AA, Rudick V, Paret G, Ben-Abraham R. The role of dextromethorphan in pain control. Canadian Journal of Anesthesia. 2000;47(6):585–596. doi: 10.1007/BF03018952. [DOI] [PubMed] [Google Scholar]
- Werb D, Kazatchkine M, Kerr T, Nutt D, Strathdee S, Hankins C, … Wood E. A call to reprioritise metrics to evaluate illicit drug policy. The Lancet. 2016;387(10026):1371. doi: 10.1016/S0140-6736(16)30074-5. [DOI] [PubMed] [Google Scholar]
- Werling LL, Keller A, Frank JG, Nuwayhid SJ. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Experimental neurology. 2007;207(2):248–257. doi: 10.1016/j.expneurol.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. The discovery of serotonin and its role in neuroscience. Neuropsychopharmacology. 1999;21:2S–8S. doi: 10.1016/S0893-133X(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, … Kleijnen J. Cannabinoids for medical use: a systematic review and meta-analysis. Jama. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Kaar S, Borschmann R. Dimethyltryptamine (DMT): Prevalence, user characteristics and abuse liability in a large global sample. Journal of Psychopharmacology. 2013;28(1):49–54. doi: 10.1177/0269881113513852. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behavioural pharmacology. 2005;16(5–6):315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Wolbach AB, Miner EJ, Isbell H. Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacology. 1962;3(3):219–223. doi: 10.1007/BF00412109. [DOI] [PubMed] [Google Scholar]
- Wolff K, Tsapakis EM, Pariante CM, Kerwin RW, Forsling ML, Aitchison KJ. Pharmacogenetic studies of change in cortisol on ecstasy (MDMA) consumption. Journal of Psychopharmacology. 2012;26(3):419–428. doi: 10.1177/0269881111415737. [DOI] [PubMed] [Google Scholar]
- Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, Aitchison KJ. Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. Journal of Psychopharmacology. 2006;20(3):400–410. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- Wolff K, Winstock AR. Ketamine. CNS drugs. 2006;20(3):199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proceedings of the National Academy of Sciences of the United States of America. 1954a;40(4):228. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. Some neurophysiological aspects of serotonin. British medical journal. 1954b;2(4880):122–126. doi: 10.1136/bmj.2.4880.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. Evidence for the participation of serotonin in mental processes. Annals of the New York Academy of Sciences. 1957;66(3):649–667. doi: 10.1111/j.1749-6632.1957.tb40755.x. [DOI] [PubMed] [Google Scholar]
- Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. Journal of Pharmacology and Experimental Therapeutics. 2008;327(2):316–323. doi: 10.1124/jpet.108.143461. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, … Luckenbaugh DA. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biological psychiatry. 2012;71(11):939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, … Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zeng MC, Niciu MJ, Luckenbaugh DA, Ionescu DF, Mathews DC, Richards EM, … Zarate CA. Acute stress symptoms do not worsen in posttraumatic stress disorder and abuse with a single subanesthetic dose of ketamine. Biological psychiatry. 2013;73(12):e37–e38. doi: 10.1016/j.biopsych.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Zhou GZ, Musacchio J. Computer-assisted modeling of multiple dextromethorphan and sigma binding sites in guinea pig brain. European Journal of Pharmacology: Molecular Pharmacology. 1991;206(4):261–269. doi: 10.1016/0922-4106(91)90108-t. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. European Psychiatry. 2014;29(7):419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]


