Abstract
Objectives
The platelet storage lesion accelerates platelet clearance after transfusion, but the underlying molecular mechanism remains elusive. Although inhibiting sheddase activity hampers clearance of platelets with storage lesion, the target platelet protein responsible for ectodomain shedding-induced clearance is not definitively identified. Monoclonal antibody 5G6 was developed recently to bind specifically human platelet receptor GPIbα and inhibit its shedding but not shedding of other receptors. Here, the role of GPIbα shedding in platelet clearance after transfusion was addressed.
Approach and Results
Both human leukoreduced apheresis-derived platelets and transgenic mouse platelets expressing human GPIbα (hTg) were stored at room temperature in the presence and absence of 5G6 Fab fragment. At various time points aliquots of stored platelets were analyzed and compared. 5G6 Fab inhibited GPIbα shedding in both platelets during storage and preserved higher level of GPIbα on the platelet surface. Compared with age-matched control platelets, 5G6 Fab-stored platelets exhibited similar levels of platelet activation, degranulation, and agonist-induced aggregation. 5G6 Fab-stored hTg platelets exhibited significantly higher post-transfusion recovery and in vivo hemostatic function in recipient mice than control platelets. Consistently 5G6 Fab-stored 8-day-old human platelets produced similar improvement in post-transfusion recovery in immunodeficient mice and in ex vivo thrombus formation over collagen under shear flow.
Conclusions
Specific inhibition of GPIbα shedding in the stored platelets improves post-transfusion platelet recovery and hemostatic function, providing clear evidence for GPIbα shedding as a cause of platelet clearance. These results suggest that specific inhibition of GPIbα shedding may be utilized to optimize platelet storage conditions.
Keywords: Antibody, Platelets, Glycoprotein, Proteolytic enzyme
Introduction
Platelet transfusion is a therapy to treat or prevent hemorrhage in patients with either thrombocytopenia or dysfunctional platelets. Compared with other blood components, platelet products have the shortest storage-life. In the blood bank, platelets can only be stored at room temperature under constant agitation for up to 5 days, mainly because of the risk of bacterial growth and accumulated damage to the platelets1. Pathogen reduction technologies have been developed to inactivate bacteria and viruses in stored platelets and to minimize the risk of contamination and infection, which could potentially extend the platelet shelf life to 7 days2. Recently, platelet storage at 4°C was approved by FDA3, which may reduce the risk of contamination as well. However, largely independent of bacterial growth and pathogen inactivation, during storage platelets undergo progressive and deleterious modifications that collectively are termed the platelet storage lesion. The extent of the platelet storage lesion is strongly associated with a decrease in post-transfusion platelet survival and function1, 4, but the underlying molecular mechanism is not completely understood.
A characteristic of platelet storage lesion is ectodomain shedding of platelet surface receptor glycoprotein (GP)Ibα, as accumulation of glycocalicin, the product of GPIbα shedding, during platelet storage is reported in many studies5–7. As a major part of the GPIb-IX complex, GPIbα is the platelet receptor for von Willebrand factor (VWF) and other ligands present in circulation. ADAM17, a widely expressed metalloprotease, cleaves GPIbα at the Gly464-Val465 peptide bond and releases glycocalicin to the plasma5, 8. Recent reports showed that inhibiting ADAM17 activity using a broad-spectrum metalloprotease inhibitor GM6001 or p38 MAPK inhibitors during storage improved the post-transfusion recovery of stored murine platelets6, 9. These studies suggest that shedding of GPIbα may play a role in fast clearance of platelets with the storage lesion. However, ADAM17 and other metalloproteases have broad substrate specificities. They cleave GPIbα, TNF-α and other protein substrates in platelets5, 8. Thus, studies using the inhibitors of ADAM17 activity could not rule out the possibility that shedding of a platelet receptor other than GPIbα mediates platelet clearance. The definitive evidence linking GPIbα shedding to platelet clearance is still lacking.
A monoclonal antibody, designated 5G6, was recently developed to specifically bind the shedding cleavage site of human GPIbα and thus limit its access to sheddases10, 11. Like the full-length antibody, 5G6 Fab fragment inhibited shedding of only GPIbα, but not other receptors, in platelets without inducing platelet activation10. Injection of 5G6 does not cause thrombocytopenia in mice12. In this study, we report that 5G6 Fab-mediated inhibition of GPIbα shedding during prolonged storage of both human leukoreduced apheresis-derived platelets (LR-ADP) and hTg murine platelets significantly improves post-transfusion recovery of stored platelets and markedly enhances their hemostatic function in vivo. This study demonstrates the cause-effect relation between GPIbα shedding and platelet clearance, providing the foundation for further mechanistic investigation of platelet clearance and future development of better platelet storage conditions.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Two models were utilized to ascertain the effects of 5G6 Fab on stored platelets. In the first model, aliquots of human LR-ADP were stored with either 5G6 Fab, mouse IgG Fab (Ctrl Fab) or saline for up to 8 days under standard blood-banking conditions, and analyzed periodically for platelet activity (Fig. 1A). The clearance of human LR-ADP was assessed in SCID mice13. In the second model, hTg murine platelets that express only human GPIbα were utilized because 5G6 recognizes human but not murine GPIbα10, 14. Pooled hTg murine PRP was stored with 5G6 Fab, Ctrl Fab or saline at room temperature under agitation conditions for up to 16 hours, and analyzed periodically during storage (Fig. 1E). The post-transfusion survival and hemostatic function of stored hTg platelets was assessed in mice.
Figure 1. 5G6 Fab inhibits GPIbα shedding of stored platelets.
(A) Overview of the LR-ADP storage study. (B) Binding of Fab to stored LR-ADP. Binding was detected by flow cytometry using FITC-conjugated goat anti-mouse IgG, and quantitated by mean fluorescence intensity (MFI). (C) The release of glycocalicin (GC) during storage. The GC level in the supernatant of LR-ADP was detected by Western blot using WM23. The blot shown in the top panel is a representative of 5 independent experiments. The intensities of GC bands in the same blot were quantified and plotted as the fold change over the GC level in the 2-day-old LR-ADP before storage. (D) The surface expression level of GPIbα in LR-ADP during storage was assessed by flow cytometry using biotinylated WM23 and FITC-conjugated streptavidin. The measured MFI was normalized with the level of GPIbα in 2-day-old LR-ADP as 100%. (E) Overview of the hTg platelet storage study. (F) Binding of Fab to stored hTg platelets. (G, H) GPIbα and GPVI surface expression level in stored hTg platelets were quantified using WM23 or JAQ-1 antibody respectively. Results are shown as mean ± SEM (n = 5). **, P < 0.01; *, P < 0.05 (t test). Note: in some case the curve of saline was partially obscured by that of Ctrl Fab.
5G6 Fab inhibited GPIbα shedding during platelet storage
Over the course of storage the level of 5G6 binding changed little in both human LR-ADP and murine hTg platelets (Fig. 1B,F). Consistently, treatment of 5G6 Fab, but not saline or Ctrl Fab, inhibited the release of glycocalicin into the plasma and prevented down-regulation of GPIbα surface expression (Fig. 1C,D,G). It should be noted that GPIbα surface expression in platelet samples treated with 5G6 Fab increased after prolonged storage (Fig. 1D,G). This is likely due to the redistribution of membranes, and GPIbα therein, from the platelet open canalicular system to the plasma membrane7, 15, and also possibly new synthesis of GPIbα16. Likewise, GPVI surface expression in hTg platelets increased slightly during storage and was not affected by 5G6 Fab (Fig. 1H). Overall, these results demonstrated that 5G6 Fab inhibited GPIbα shedding in both LR-ADP and hTg platelets during prolonged storage.
Treatment of 5G6 Fab during storage did not affect the activation state of platelets
Periodically during storage LR-ADP and hTg platelets were evaluated for phosphatidylserine (PS) exposure, integrin αIIbβ3 activation and P-selectin expression, all of which are considered markers of platelet activation. As shown in Supplement Figure 1, 5G6 Fab-treated LR-ADP or hTg platelets displayed the same levels of PS exposure, αIIbβ3 activation and P-selectin expression as saline- or Ctrl Fab-treated platelets, suggesting that 5G6 Fab did not alter the activation and functional state of stored platelets.
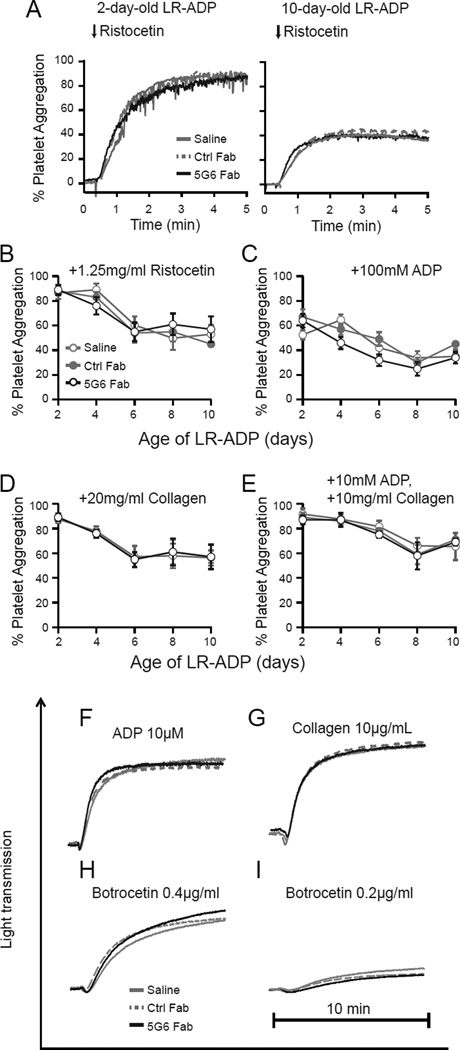
To determine if treatment of 5G6 Fab could modulate the function of stored platelets, we performed agonist-induced platelet aggregation assays. Since LR-ADP contains high concentration of ACD-A, agonists at doses higher than those typically used for washed platelets were used to induce platelet aggregation17–19. Throughout the storage of LR-ADP 5G6 Fab exhibited little effect on ristocetin-, ADP-, or collagen-mediated aggregation (Fig. 2A–E). Similarly, after storage 5G6 Fab-treated hTg murine platelets displayed the same aggregation activity as saline- or Ctrl Fab-treated ones in response to ADP, collagen and botrocetin (Fig. 2F–H). Consistently, αIIbβ3 activation and P-selectin expression of stored hTg platelets were unaltered upon collagen stimulation (Supplement Figure 2).
Figure 2. 5G6 Fab does not alter the function of stored platelets.
LR-ADP and hTg PRP were stored with saline, Ctrl Fab or 5G6 Fab, then were stimulated with different agonists, and aggregation was measured. (A) LR-ADP aggregation traces are shown. (B-E) The extents of maximal aggregation are plotted versus the age of stored LR-ADP. Stored LR-ADP were stimulated by 1.25 mg/ml ristocetin (B), 100 mM ADP (C), 20 mg/ml collagen (D) or 10 mM ADP + 10 mg/ml Collagen (E). (F–I) The aggregation trace of stored hTg PRP was recorded. After storage for 16 hours, hTg PRP were stimulated with different agonists 10 µM ADP (F), 10 µg/ml collagen (G), 0.4 µg/ml (H) or 0.2 µg/ml (I) botrocetin, and light transmission was recorded. Data are shown as mean ± SEM (n=4).
Treatment of 5G6 Fab during prolonged storage improved the post-transfusion recovery of LR-ADP and hTg platelets in vivo
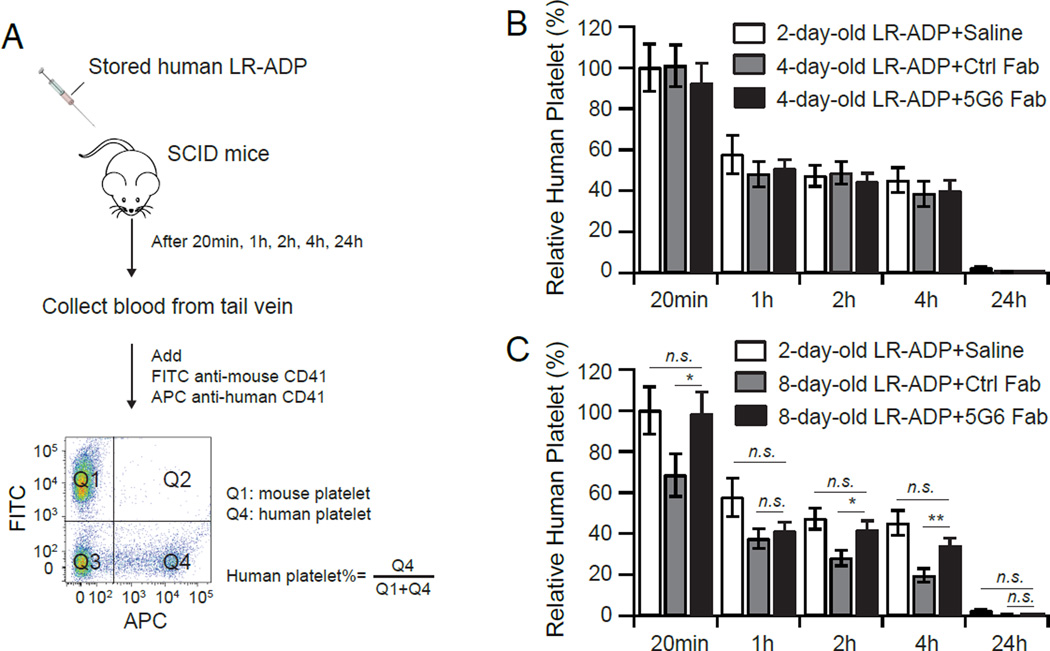
Next we evaluated post-transfusion recovery of stored LR-ADP in severe combined immunodeficient (SCID) mice13. SCID mice are capable of identifying lesions imparted to human platelets by prolonged storage as identified by their increased clearance from circulation13. We chose to compare 4-day-old LR-ADP, a model for platelets stored within the 5-day shelf life, and 8-day-old LR-ADP, a model for platelets with prolonged storage. The saline-treated 2-day-old LR-ADP, also included in the study, was considered fresh platelets, and the 20-min recovery of this platelet sample in SCID mice was used as the 100% recovery for comparison for all other LR-ADP samples and time points13. Since the mouse platelet count does not change significantly throughout the experiment, human platelets were tracked by its relative abundance in the total population of CD41+ platelets (Fig. 3A). As shown in Figure 3B, the recovery and survival of both Ctrl Fab- and 5G6 Fab-stored 4-day-old LR-ADP was essentially the same as that of 2-day-old sample, suggesting that treatment of 5G6 Fab did not have a significant impact on clearance of platelets within the 5-day shelf life. Consistent with earlier reports13, the recovery of Ctrl Fab-stored 8-day-old LR-ADP was markedly lower than that of 2-day-old LR-ADP. In comparison, 5G6 Fab-stored 8-day-old LR-ADP produced a significantly improved post-transfusion recovery than its Ctrl Fab-stored counterpart (Fig. 3C). Moreover, its recovery was similar to that of 2-day-old LR-ADP.
Figure 3. Treatment of 5G6 Fab during prolonged storage improved the post-transfusion recovery of LR-ADP in vivo.
(A) Overview of the study. (B) Survival plots of 2-day-old LR-ADP stored with saline (white bar), 4-day-old LR-ADP stored with control Fab (grey), and that with 5G6 Fab (black). (C) Survival plots of 2-day-old LR-ADP stored with saline (white), 8-day-old LR-ADP stored with control Fab (grey), and that with 5G6 Fab (black). The relative abundance of human platelets at a time point was normalized with that of 2-day-old LR-ADP at 20 min post-transfusion being 100%. Data are shown as mean ± SEM (n = 12). **, P < 0.01; *, P < 0.05; n.s., not significant (t test).
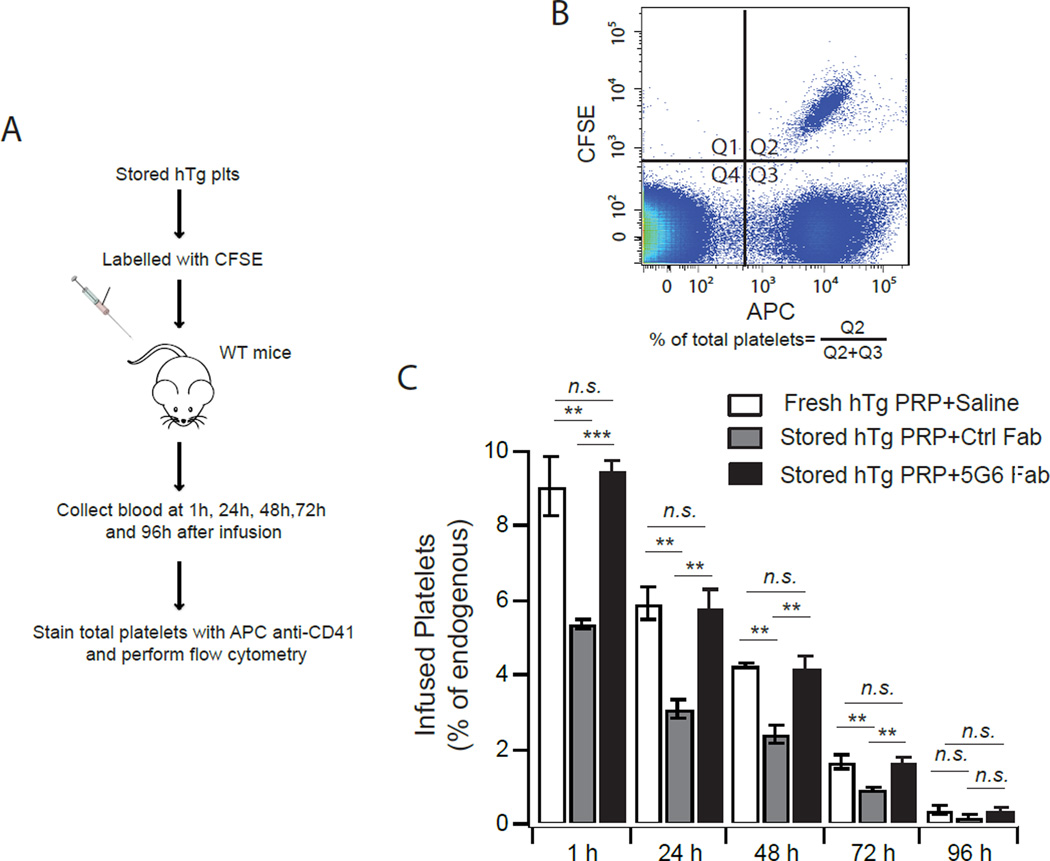
Although immune-mediated rapid clearance of human platelets was significantly diminished in SCID mice20, it is notable that LR-ADP, regardless of its age, survived in SCID mice for less than 24 hours. This may be due to the fact that the innate immunity remains intact in SCID mice20. To address this limitation, post-transfusion recovery and survival of stored hTg murine platelets in WT mice were measured. After storage of 16 hours, hTg platelets were labeled with fluorescent dye CFSE and transfused into WT mice. At various time points after transfusion, small volume of blood was drawn from recipient mice and the proportion of infused CFSE+ platelets in total platelets was quantified by flow cytometry (Fig. 4A,B). Consistent with earlier studies of WT platelets6, 9, Ctrl Fab-stored hTg platelets exhibited much lower recovery than fresh platelets, with a significant portion (~35%) being cleared within an hour of transfusion (Fig. 4C). In contrast, 5G6 Fab-stored hTg platelets exhibited the same recovery as fresh hTg platelets (Fig. 4C). After the first hour, transfused platelets showed similar survival rates as the endogenous ones, suggesting that 5G6 Fab treatment does not affect post-transfusion survival of the platelets. Together these findings demonstrate that 5G6 Fab during extended storage improved the in vivo recovery of LR-ADP and hTg platelets.
Figure 4. Treatment of 5G6 Fab during storage improved the post-transfusion recovery of hTg platelets in vivo.
(A) The strategy of post transfusion recovery study of stored hTg platelets. (B) The relative amount of infused platelets was measured by flow cytometry. (C) Compared to Ctrl Fab, 5G6 Fab enhances stored hTg platelet post transfusion recovery. Data are shown as mean ± SEM (n = 6). **, P < 0.01; *, P < 0.05; n.s., not significant (t test).
Treatment of 5G6 Fab during prolonged storage preserved adhesive function of LR-ADP
To address whether 5G6 Fab affects the adhesive function of stored platelets, the ex vivo adhesion of LR-ADP in reconstituted whole blood on collagen fibrils was assessed in the perfusion chamber as described6. After 2 min of perfusion, saline-, Ctrl Fab- and 5G6 Fab-treated 2-day-old LR-ADP adhered to collagen equally well (Fig. 5). They all covered about 20% of the collagen surface area, which was comparable to that covered by fresh whole blood as reported before21. In accordance with the progression of platelet lesion over the course of prolonged storage, the surface area covered by saline- and control Fab-stored LR-ADP steadily deceased with the platelet age. Although storage with 5G6 Fab did not alter the overall trend of decrease in adhesive capacity, it visibly delayed such decrease for several days, as the surface area covered by 5G6 Fab-stored 4-day-old LR-ADP was the same as the 8-day-old. Consequently, 5G6 Fab-stored 8-day-old LR-ADP visibly covered significantly larger surface area than its control Fab-stored counterpart (10.1±2.1% vs 3.5±1.2%, Fig. 5). These results indicate that incubation with 5G6 Fab during prolonged storage preserved the adhesive function of LR-ADP.
Figure 5. Treatment of 5G6 Fab during prolonged storage improved ex vivo thrombus formation of LR-ADP.
LR-ADP samples stored with saline, Ctrl Fab or 5G6 Fab were dyed at 37°C, and mixed with freshly prepared human platelet-poor whole blood. Reconstituted blood was perfused over the collagen surface at a shear rate of 1,000/s. Two minutes after perfusion, platelet adhesion was visualized by microscope. The percentage of the surface area covered by fluorescent platelets was calculated. (A) The extent of thrombus formation was plotted versus the age of stored LR-ADP. Data are shown as mean ± SEM (n=4). *, P < 0.05 (t test). (B) Representative images of thrombi formed on the collagen surface. All the images were taken at the same magnification scale and shown at the same contrast (scale bar: 100 µm).
Treatment of 5G6 Fab during extended storage protect hemostatic function of hTg platelets
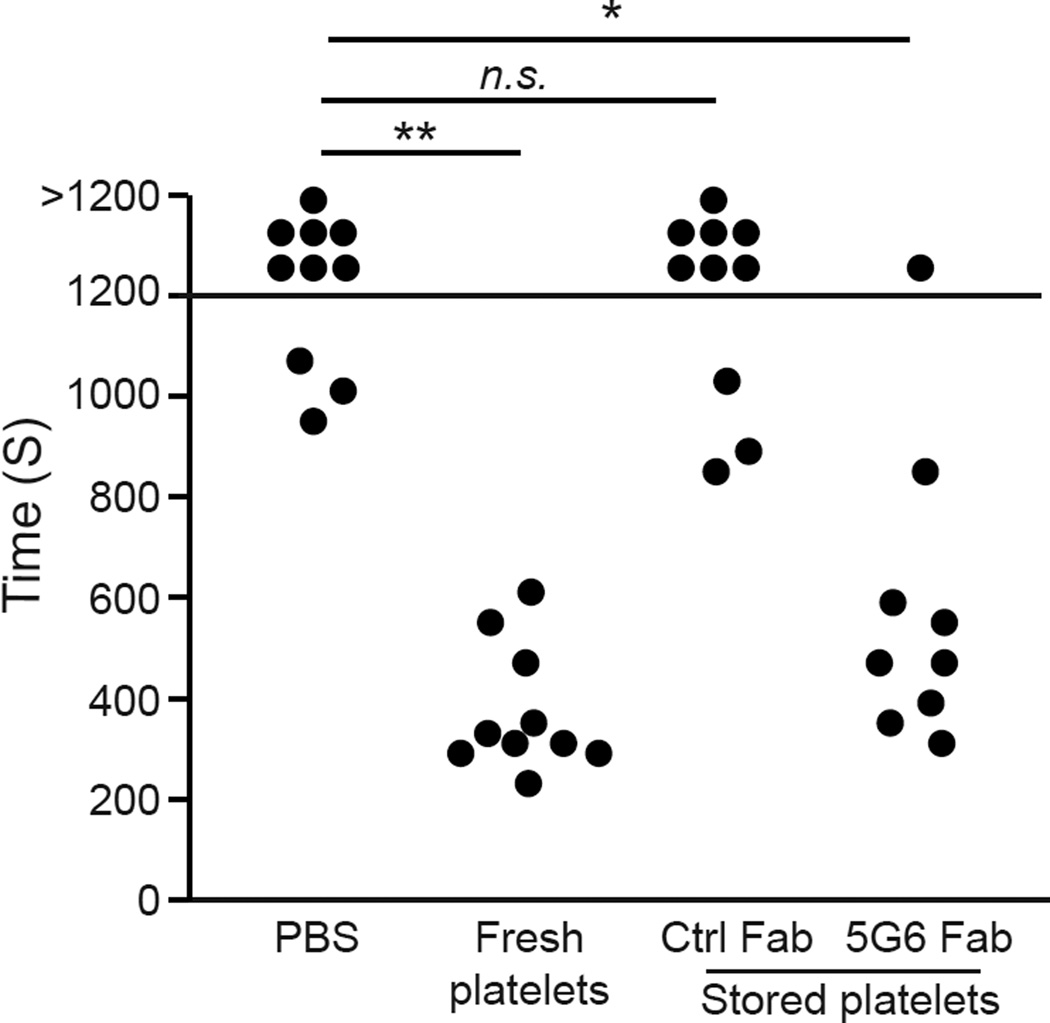
To investigate whether storage with 5G6 Fab influences hemostatic function of hTg platelets, a tail bleeding time assay was performed using IL4R-IbαTg mice. In IL4R-IbαTg platelets, the extracellular domain of GPIbα was replaced with that of interleukin-4 receptor22. As a result, IL4R-IbαTg platelets do not bind VWF and other GPIbα ligands, and hemostasis in IL4R-IbαTg mice is severely impaired22, 23. IL4R-IbαTg mice are also moderately thrombocytopenic22 (Deng et al. manuscript submitted). In this study, fresh or stored hTg platelets were infused into IL4R-IbαTg mice (~1×108 platelets per mouse). One hour after infusion, 2 mm of the mouse tail tip was amputated and the time to cessation of bleeding was recorded. In accordance with previous reports9, 22, IL4R-IbαTg mice could not stop tail bleeding within 20 min, but transfusion of fresh hTg platelets stopped tail bleeding in most IL4R-IbαTg mice within 500 s (Fig. 6). Transfusing Ctrl Fab-stored hTg platelets did not protect IL4R-IbαTg mice from tail bleeding. In comparison, transfusing 5G6 Fab-stored hTg platelets significantly shortened the tail bleeding time in IL4R-IbαTg mice, approaching those transfused with fresh hTg platelets (Fig. 6). These results demonstrate that 5G6 treatment during storage helped to preserve the hemostatic function of stored platelets.
Figure 6. 5G6 Fab protects platelet hemostatic function after storage.
IL4Tg mice were transfused with PBS, fresh hTg platelets, control Fab-stored hTg platelets, or 5G6 Fab-stored hTg platelets. One hour after transfusion, 1 mm segment of the mouse tail was cut off. Blood drops were absorbed every 20s using a filter paper until bleeding ceased. Each symbol represents the bleeding time of one mouse. **, P < 0.01; *, P < 0.05; n.s., not significant (Two-tailed Fisher’s test)
Discussion
Platelets after prolonged storage are cleared rapidly upon transfusion, but the underlying molecular mechanism remains elusive. During storage platelets undergo a variety of morphological and biochemical changes, making it difficult to pinpoint the molecular events that critically accelerates platelet clearance. Adding metalloprotease or p38 MAPK inhibitors that blocked ADAM17 activity improved the recovery of stored murine platelets6, 9, establishing a close link between ectodomain shedding and platelet clearance. This is an important finding that narrowed the scope of investigation. However, as many receptors can be cleaved by ADAM17 or related metalloproteases and these receptors often elicit different signals, it is still unclear which platelet receptor, upon its shedding, can lead to platelet clearance. Shedding of GPIbα was suggested because GPIbα is one of the most abundant platelet receptors undergoing shedding and it is implicated in platelet apoptosis and clearance8, 24, 25. Moreover, a correlation between GPIbα shedding and the extent of storage lesion has been reported5–7. However, it remains unclear whether this is an epiphenomenon or a cause-effect relationship. To address this question, monoclonal antibody 5G6 was recently developed to bind directly to the shedding cleavage site of human GPIbα10. 5G6 does not bind WT murine platelets but only hTg platelets10. The crystal structure of 5G6 Fab in complex with its GPIbα-derived epitope peptide and related mutagenesis analysis identified several residues in the epitope peptide as required for the tight binding with the antibody11. Many of these residues are polar residues and unique to human GPIbα11, illustrating nicely why 5G6 does not bind murine GPIbα or any other platelet receptors. Consistent with its binding specificity to human GPIbα, we showed earlier that 5G6 inhibits induced shedding of GPIbα but not that of GPVI or GPV in fresh platelets10. In the present study we verified that treatment of 5G6 Fab during storage inhibited GPIbα shedding without affecting the GPVI expression level and the activities of stored human and hTg murine platelets (Fig. 1,2). Furthermore, 5G6 Fab significantly improved post-transfusion recovery of stored platelets in mice (Fig. 3,4). It is noteworthy that post-transfusion recovery of 5G6 Fab-stored outdated platelets is similar to that of fresh platelets. These results, together with those demonstrating the binding and inhibitory specificity of 5G6 Fab10, 11, provide the first direct evidence supporting the idea that shedding of GPIbα leads to clearance of stored platelets. It remains to be determined whether shedding of another receptor also leads to platelet clearance. With the caveat that platelet clearance in mice may not reflect entirely that in human, our findings suggest that specific inhibition of GPIbα shedding may be potentially utilized to improve the recovery of stored platelets.
Upon transfusion a portion of outdated platelets was cleared quickly, and the remaining ones were cleared at a rate similar to that of endogenous platelets. It is noteworthy that the effect of 5G6 treatment was primarily manifested in the improved recovery of stored hTg platelets, as the survival rate of 5G6 Fab-stored hTg platelets was similar to that of Ctrl Fab-stored ones (Fig. 4C). Although the survival of LR-ADP in SCID mice was difficult to assess as the infused platelets were cleared within 24 hours, the beneficial effect of 5G6 storage was clear for the recovery of 8-day-old LR-ADP (Fig. 3C). These results suggest that multiple processes participate in the clearance of platelets, and GPIbα shedding may affect a distinct phase of clearance. That same effects of 5G6 treatment were observed in both human and murine platelets suggests that the two species may share a similar molecular mechanistic link between GPIbα shedding and platelet clearance.
An important physiological function of platelets is to form a plug at the injured vessel wall to prevent blood loss. GPIbα is a critical receptor mediating the interaction of platelets with subendothelial matrices lining the vessel wall26. Collagen, a major component of the subendothelial matrix that becomes exposed to the blood upon vessel injury, binds VWF in the plasma and also serves as a ligand for platelet receptors. At high shear rate (>800/s), the collagen-bound VWF captures platelets to the injury site through GPIbα27. Platelets of Bernard-Soulier syndrome patients, which expressed little GPIbα, fail to adhere to collagen under flow conditions28. Consistently, GPIbα-dependent thrombus formation is absent in IL4R-IbαTg mice22, 23. Furthermore, GPIbα may mediate thrombus formation through binding of matrix proteins other than VWF to collagen29 and also potentially modulating GPVI-mediated signaling responses30. Platelets during storage continuously shed GPIbα from the surface and therefore progressively lose their aggregative and adhesive capacity in response to collagen4. In this study, utilizing the coated collagen surface in the flow chamber to mimic the physiological scenario of the injured vessel31, we made similar observations that the adhesive capacity of untreated LR-ADP decreased with the age of stored platelets (Fig. 5). Incubation with 5G6 Fab during blood bank storage preserved the expression level of GPIbα in the LR-ADP and visibly delayed platelet storage lesion-mediated decrease in adhesive capacity (Fig. 5). In agreement with the in vitro and ex vivo findings, in vivo study further confirmed the potential effect of 5G6 Fab on protecting the hemostatic function of stored platelets. The transfusion of 5G6 Fab-stored hTg platelets into IL4R-IbαTg mice, which exhibits severe bleeding disorder9, 22, significantly shortened the tail bleeding time, while transfusion of Ctrl Fab-stored platelets did not ameliorate bleeding (Fig. 6). These results confirm the importance of GPIbα in mediating platelet aggregation and adhesion to collagen, and suggest that maintaining the GPIbα level during prolonged storage of platelets may help to preserve the hemostatic function of outdated platelets in addition to impeding their clearance.
The molecular mechanism by which GPIbα shedding leads to platelet clearance remains to be elucidated. Recent reports have suggested that exposure of β-galactose and/or N-acetyl-glucosamine on the to-be-cleared platelet, as a result of deglycosylation, can mediate platelet clearance32–35. Particularly, the change of glycans in the extracellular domain of GPIbα has been implicated32, 34. Since GPIbα shedding removes the GPIbα extracellular domain from the platelet surface, the released GPIbα extracellular domain, including the glycans therein, should not mediate clearance of platelets. One possibility is that GPIbα shedding leads to the exposure of a new N-terminal end of GPIbα at residue Val465, which may become a ligand for recognition by a clearing receptor. Nicastrin as a part of the γ-secretase complex can recognize the newly exposed N-terminus of a shedding product36, but there have been no reports of further cleavage of GPIbα by γ-secretase following the initial shedding. Another possibility is that GPIbα shedding transmits a signal through GPIb-IX into the platelet, leading to presentation of a clear-me sign on the platelet surface. Consistently, a peptidomimetic inhibitor of intracellular GPIb-IX signaling can inhibit lipopolysaccharide-induced thrombocytopenia37. GPIbα contains a juxtamembrane mechanosensory domain (MSD), and shear-induced unfolding of MSD, particularly the juxtamembrane Trigger sequence therein, induces GPIb-IX signaling38 (Deng et al, manuscript submitted). Since the shedding cleavage site of GPIbα is located in the MSD38, it is conceivable that after GPIbα shedding the remaining residues from the MSD, which contains the Trigger sequence, become unfolded, thereby transmitting a signal into the platelet that leads to its clearance. Consistent with the idea that GPIbα shedding may induce platelet signaling that leads to its clearance, we observed here that the extent of GPIbα shedding during storage was limited, and its inhibition by 5G6 Fab did not appear to impact significantly the platelet response to agonist stimulation (Fig. 1,2). Finally, desialylation of GPIbα can induce GPIbα shedding39, thus potentially inducing platelet clearance via this shedding-mediated mechanism. Additional studies are needed to distinguish between these possibilities and to elucidate the molecular consequences of GPIbα shedding.
Supplementary Material
Highlights.
Antibody 5G6 Fab inhibits specifically GPIbα shedding in platelets during storage.
Specific inhibition of GPIbα shedding improves post-transfusion recovery and hemostatic function of stored platelets.
Specific inhibition of GPIbα shedding may be utilized to optimize platelet storage conditions.
Acknowledgments
WC, XL, JW, CDJ and RL designed research; WC, XL, AKS, PJ and WC performed research and analyzed results; JW provided critical reagents; WC, XL, and RL wrote the paper; JW and CDJ edited manuscript and provided critical comments. We thank the Emory Children’s Pediatric Research Center Flow Cytometry Core for technical support.
Sources of Funding
This work was supported in part by National Institutes of Health grants HL082808 and HL123984.
Nonstandard Abbreviations and Acronyms
- GP
glycoprotein
- hTg
transgenic mouse expressing human GPIbα
- SCID
severe combined immunodeficient
- IL4R-IbαTg
Transgenic mouse expressing chimeric IL4Rα/GPIbα
- VWF
von Willebrand factor
- CFSE
carboxyfluorescein succinimidyl ester
- LR-ADP
human leukoreduced apheresis-derived platelets
- PRP
platelet-rich plasma
- PS
phosphatidylserine
- WT
wild-type
- MSD
mechanosensory domain
Footnotes
Disclosures
Emory University has filed a patent application for 5G6 and related antibodies for which RL is an inventor. All other authors declared no conflicts of interest.
References
- 1.Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30:475–487. doi: 10.1016/j.cll.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Lozano M, Knutson F, Tardivel R, Cid J, Maymo RM, Lof H, Roddie H, Pelly J, Docherty A, Sherman C, Lin L, Propst M, Corash L, Prowse C. A multi-centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet a pathogen inactivation stored for 6 or 7 d prior to transfusion. Br J Haematol. 2011;153:393–401. doi: 10.1111/j.1365-2141.2011.08635.x. [DOI] [PubMed] [Google Scholar]
- 3.Cap AP. Platelet storage: A license to chill! Transfusion. 2016;56:13–16. doi: 10.1111/trf.13433. [DOI] [PubMed] [Google Scholar]
- 4.Boomgaard MN, Gouwerok CW, Homburg CH, de Groot G, MJ IJ, de Korte D. The platelet adhesion capacity to subendothelial matrix and collagen in a flow model during storage of platelet concentrates for 7 days. Thromb Haemost. 1994;72:611–616. [PubMed] [Google Scholar]
- 5.Prudova A, Serrano K, Eckhard U, Fortelny N, Devine DV, Overall CM. Tails n-terminomics of human platelets reveals pervasive metalloproteinase-dependent proteolytic processing in storage. Blood. 2014;124:e49–e60. doi: 10.1182/blood-2014-04-569640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmeier W, Burger PC, Piffath CL, Hoffmeister KM, Hartwig JH, Nieswandt B, Wagner DD. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood. 2003;102:4229–4235. doi: 10.1182/blood-2003-04-1305. [DOI] [PubMed] [Google Scholar]
- 7.Michelson AD, Adelman B, Barnard MR, Carroll E, Handin RI. Platelet storage results in a redistribution of glycoprotein ib molecules. Evidence for a large intraplatelet pool of glycoprotein ib. J Clin Invest. 1988;81:1734–1740. doi: 10.1172/JCI113513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardiner EE, Karunakaran D, Shen Y, Arthur JF, Andrews RK, Berndt MC. Controlled shedding of platelet glycoprotein (gp)vi and gpib-ix-v by adam family metalloproteinases. J Thromb Haemost. 2007;5:1530–1537. doi: 10.1111/j.1538-7836.2007.02590.x. [DOI] [PubMed] [Google Scholar]
- 9.Canault M, Duerschmied D, Brill A, Stefanini L, Schatzberg D, Cifuni SM, Bergmeier W, Wagner DD. P38 mitogen-activated protein kinase activation during platelet storage: Consequences for platelet recovery and hemostatic function in vivo. Blood. 2010;115:1835–1842. doi: 10.1182/blood-2009-03-211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang X, Russell SR, Estelle S, Jones LH, Cho S, Kahn ML, Berndt MC, Bunting ST, Ware J, Li R. Specific inhibition of ectodomain shedding of glycoprotein ibalpha by targeting its juxtamembrane shedding cleavage site. J Thromb Haemost. 2013;11:2155–2162. doi: 10.1111/jth.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Y, Zhang X, Liang X, Zang J, Mo X, Li R. Structural basis for the specific inhibition of glycoprotein ibalpha shedding by an inhibitory antibody. Sci Rep. 2016;6:24789. doi: 10.1038/srep24789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang X, Syed AK, Russell SR, Ware J, Li R. Dimerization of glycoprotein ibalpha is not sufficient to induce platelet clearance. J Thromb Haemost. 2016;14:6. doi: 10.1111/jth.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piper JT, Gelderman MP, Vostal JG. In vivo recovery of human platelets in severe combined immunodeficient mice as a measure of platelet damage. Transfusion. 2007;47:1540–1549. doi: 10.1111/j.1537-2995.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 14.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: The bernard-soulier syndrome. Proc. Natl. Acad. Sci. USA. 2000;97:2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwertz H, Koster S, Kahr WH, Michetti N, Kraemer BF, Weitz DA, Blaylock RC, Kraiss LW, Greinacher A, Zimmerman GA, Weyrich AS. Anucleate platelets generate progeny. Blood. 2010;115:3801–3809. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieffer N, Guichard J, Farcet JP, Vainchenker W, Breton-Gorius J. Biosynthesis of major platelet proteins in human blood platelets. Eur. J. Biochem. 1987;164:189–195. doi: 10.1111/j.1432-1033.1987.tb11010.x. [DOI] [PubMed] [Google Scholar]
- 17.DiMinno G, Silver MJ, Murphy S. Stored human platelets retain full aggregation potential in response to pairs of aggregating agents. Blood. 1982;59:563–568. [PubMed] [Google Scholar]
- 18.Veeraputhiran M, Ware J, Dent J, Bornhorst J, Post G, Cottler-Fox M, Pesek G, Theus J, Nakagawa M. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51:1030–1036. doi: 10.1111/j.1537-2995.2010.02897.x. [DOI] [PubMed] [Google Scholar]
- 19.Winkler AM, Sheppard CA, Culler EE, Myers RL, Duncan A, Castillejo MI, Hillyer CD, Josephson CD. Effects of storage duration and volume on the quality of leukoreduced apheresis-derived platelets: Implications for pediatric transfusion medicine. Transfusion. 2010;50:2193–2198. doi: 10.1111/j.1537-2995.2010.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankert RB, Hess SD, Egilmez NK. Scid mouse models to study human cancer pathogenesis and approaches to therapy: Potential, limitations, and future directions. Front Biosci. 2002;7:c44–c62. doi: 10.2741/A758. [DOI] [PubMed] [Google Scholar]
- 21.Siljander PR, Munnix IC, Smethurst PA, Deckmyn H, Lindhout T, Ouwehand WH, Farndale RW, Heemskerk JW. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood. 2004;103:1333–1341. doi: 10.1182/blood-2003-03-0889. [DOI] [PubMed] [Google Scholar]
- 22.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine bernard-soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 23.Bergmeier W, Piffath CL, Goerge T, Cifuni SM, Ruggeri ZM, Ware J, Wagner DD. The role of platelet adhesion receptor gpibalpha far exceeds that of its main ligand, von willebrand factor, in arterial thrombosis. Proc Natl Acad Sci U S A. 2006;103:16900–16905. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Wang Z, Liao Y, Zhang W, Shi Q, Yan R, Ruan C, Dai K. The glycoprotein ibalpha-von willebrand factor interaction induces platelet apoptosis. J Thromb Haemost. 2010;8:341–350. doi: 10.1111/j.1538-7836.2009.03653.x. [DOI] [PubMed] [Google Scholar]
- 25.Bergmeier W, Rackebrandt K, Schroder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von willebrand factor receptor gpib-ix with novel monoclonal antibodies. Blood. 2000;95:886–893. [PubMed] [Google Scholar]
- 26.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 27.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 28.Inoue O, Suzuki-Inoue K, Ozaki Y. Redundant mechanism of platelet adhesion to laminin and collagen under flow: Involvement of von willebrand factor and glycoprotein ib-ix-v. J Biol Chem. 2008;283:16279–16282. doi: 10.1074/jbc.C700241200. [DOI] [PubMed] [Google Scholar]
- 29.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur JF, Gardiner EE, Matzaris M, Taylor SG, Wijeyewickrema L, Ozaki Y, Kahn ML, Andrews RK, Berndt MC. Glycoprotein vi is associated with gpib-ix-v on the membrane of resting and activated platelets. Thromb Haemost. 2005;93:716–723. doi: 10.1160/TH04-09-0584. [DOI] [PubMed] [Google Scholar]
- 31.Heemskerk JW, Sakariassen KS, Zwaginga JJ, Brass LF, Jackson SP, Farndale RW. Biorheology Subcommittee of the SSCotI. Collagen surfaces to measure thrombus formation under flow: Possibilities for standardization. J Thromb Haemost. 2011;9:856–858. doi: 10.1111/j.1538-7836.2011.04230.x. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen AL, Rumjantseva V, Nayeb-Hashemi S, Clausen H, Hartwig JH, Wandall HH, Hoffmeister KM. Role of sialic acid for platelet life span: Exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114:1645–1654. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grozovsky R, Begonja AJ, Liu K, Visner G, Hartwig JH, Falet H, Hoffmeister KM. The ashwell-morell receptor regulates hepatic thrombopoietin production via jak2-stat3 signaling. Nat Med. 2015;21:47–54. doi: 10.1038/nm.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, van der Wal DE, Zhu G, Xu M, Yougbare I, Ma L, Vadasz B, Carrim N, Grozovsky R, Ruan M, Zhu L, Zeng Q, Tao L, Zhai ZM, Peng J, Hou M, Leytin V, Freedman J, Hoffmeister KM, Ni H. Desialylation is a mechanism of fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan R, Chen M, Ma N, Zhao L, Cao L, Zhang Y, Zhang J, Yu Z, Wang Z, Xia L, Ruan C, Dai K. Glycoprotein ibalpha clustering induces macrophage-mediated platelet clearance in the liver. Thromb. Haemost. 2015;113:107–117. doi: 10.1160/TH14-03-0217. [DOI] [PubMed] [Google Scholar]
- 36.Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Yin H, Stojanovic-Terpo A, Xu W, Corken A, Zakharov A, Qian F, Pavlovic S, Krbanjevic A, Lyubimov AV, Wang ZJ, Ware J, Du X. Role for platelet glycoprotein ib-ix and effects of its inhibition in endotoxemia-induced thrombosis, thrombocytopenia, and mortality. Arterioscler Thromb Vasc Biol. 2013;33:2529–2537. doi: 10.1161/ATVBAHA.113.302339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Deng W, Zhou L, Xu Y, Yang W, Liang X, Wang Y, Kulman JD, Zhang XF, Li R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein ib-ix complex. Blood. 2015;125:562–569. doi: 10.1182/blood-2014-07-589507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jansen AJ, Josefsson EC, Rumjantseva V, Liu QP, Falet H, Bergmeier W, Cifuni SM, Sackstein R, von Andrian UH, Wagner DD, Hartwig JH, Hoffmeister KM. Desialylation accelerates platelet clearance after refrigeration and initiates gpibα metalloproteinase-mediated cleavage in mice. Blood. 2012;119:1263–1273. doi: 10.1182/blood-2011-05-355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.