Abstract
Objective
Von Willebrand factor (VWF), which is synthesized in endothelial cells and megakaryocytes, is known to worsen stroke outcome. In vitro studies suggest that platelet-derived VWF is biochemically different from the endothelial cell-derived VWF. However, little is known about relative contribution of different pools of VWF in stroke.
Approach and Results
Using bone marrow transplantation, we generated chimeric platelet derived-VWF mice (Plt-VWF), platelet derived-VWF mice that lack ADAMTS13 in platelets and plasma (Plt-VWF/Adamts13−/−), and endothelial cell derived-VWF mice (EC-VWF) to determine relative contribution of different pools of VWF in stroke. In brain ischemia/reperfusion injury model, we found that infarct size, post-ischemic intracerebral thrombo-inflammation (fibrin(ogen) deposition, neutrophil infiltration, IL-1β and TNF-α levels) within lesions were comparable between EC-VWF and WT mice. Infarct size and post-ischemic thrombo-inflammation were comparable between Plt-VWF and Plt-VWF/Adamts13−/− mice, but decreased compared to EC-VWF and/or WT mice (P<0.05) and increased compared to Vwf −/− mice (P<0.05). Susceptibility to FeCl3 injury-induced carotid artery thrombosis was comparable between WT and EC-VWF mice, whereas Plt-VWF and Plt-VWF/Adamts13−/− mice exhibited defective thrombosis. Although most of the injured vessels did not occlude, slope over time showed that thrombus growth rate was increased in both Plt-VWF and Plt-VWF/Adamts13−/− mice compared to Vwf −/− mice (P<0.05), but decreased compared to WT or EC-VWF mice.
Conclusions
Platelet-derived VWF, either in presence or absence of ADAMTS13, partially contributes to VWF-dependent injury and post-ischemic thrombo-inflammation following stroke. Endothelial cell-derived VWF is the major determinant that mediates VWF-dependent ischemic stroke by promoting post-ischemic thrombo-inflammation.
Keywords: Von Willebrand factor, thrombosis, platelets, brain ischemia/reperfusion injury
Introduction
Von Willebrand factor (VWF) is a large multimeric protein that provides the initial adhesive link between circulating platelets by binding to platelet GPIbα, and sites of vascular injury by binding to components of extracellular matrix. VWF also serves as a carrier for coagulation Factor VIII in circulation, significantly prolonging its half-life.1 VWF synthesis is limited to endothelial cells and megakaryocytes. It is stored as ultra large VWF (ULVWF) multimers or high molecular weight multimers (HMWM) in endothelial Weibel-Palade bodies and platelet α-granules.2 Upon secretion in blood and under shear conditions, ULVWF multimers are cleaved by the protease ADAMTS13 into the less active VWF multimers that support normal hemostasis and thrombosis.3, 4 A majority of VWF present in plasma is derived from endothelial cells through a constitutive secretory pathway or regulated secretion in response to secretagogues such as histamine, thrombin and TNF-α,5 whereas, ~ 15–20 % of VWF measured in plasma is released from activated platelets.6
VWF is implicated in hemostatic and thrombotic processes7, 8 including deep vein thrombosis,9 non-hemostatic processes including tumor metastasis and angiogenesis,10, 11 and in thrombo-inflammatory processes including myocardial infarction,12, 13 atherosclerosis14, 15 and acute ischemic stroke.16–18 However, very little is known about the relative contributions of platelet-derived VWF (Plt-derived VWF) versus endothelial-cell derived VWF (EC-derived VWF) in these conditions. Several in vitro studies suggest that there are biochemical and functional differences between Plt-derived VWF from megakaryocytic origin and plasma VWF from endothelial cell origin: 1) Unlike EC-derived VWF, Plt-derived VWF does not bind to factor VIII,19 2) A recent in vitro study suggested that Plt-derived VWF differs in glycosylation profile, particularly with a reduction in N-linked sialic acid expression when compared to EC-derived VWF.20 Although platelets contain ADAMTS13, this study suggested that due to different glycosylation profile, Plt- derived VWF is resistant to ADAMTS13 cleavage and therefore enriched in ULVWF multimers.20 In contrast, other in vitro studies have shown that Plt-derived ULVWF multimers are only observed when platelets are activated or lysed in presence of EDTA.21, 22 3) Despite ULVWF, Plt-derived VWF was demonstrated to be less capable of binding to GPIbα.23 Other studies suggested that Plt-derived VWF binds efficiently to activated αIIbβ3 and unfractionated heparin.23, 24 Although ~ 15–20 % of VWF measured in plasma is released from activated platelets,6 aforementioned studies suggest that Plt-derived VWF may alone be sufficient to mediate thrombosis and stroke.
Herein, using reciprocal bone marrow transplantation (BMT), we generated the following strains of mice: 1) chimeric Plt-VWF mice that express VWF only in megakaryocytes, 2) chimeric Plt-VWF/Adamts13−/− mice that express VWF in megakaryocytes and are deficient for ADAMTS13 both in plasma and platelet, and 3) EC-VWF mice that express VWF only in endothelial cells. We found that Plt-derived VWF partially contributes to VWF-dependent cerebral injury and post-ischemic inflammation following acute ischemic stroke, but alone is not sufficient to maintain hemostasis and thrombosis. Furthermore, we show that EC-derived VWF is the major determinant that mediates VWF-dependent acute ischemic stroke by promoting post-ischemic thrombo-inflammation.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Characterization of chimeric mice expressing Plt-derived VWF and EC-derived VWF
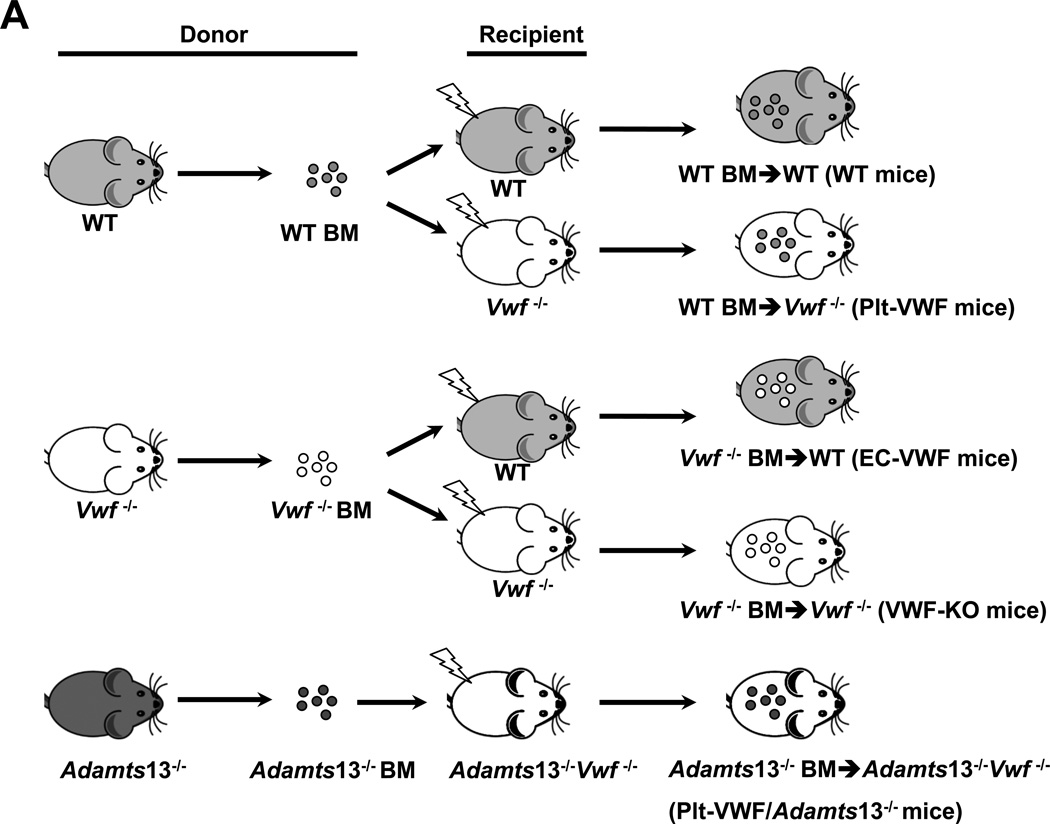
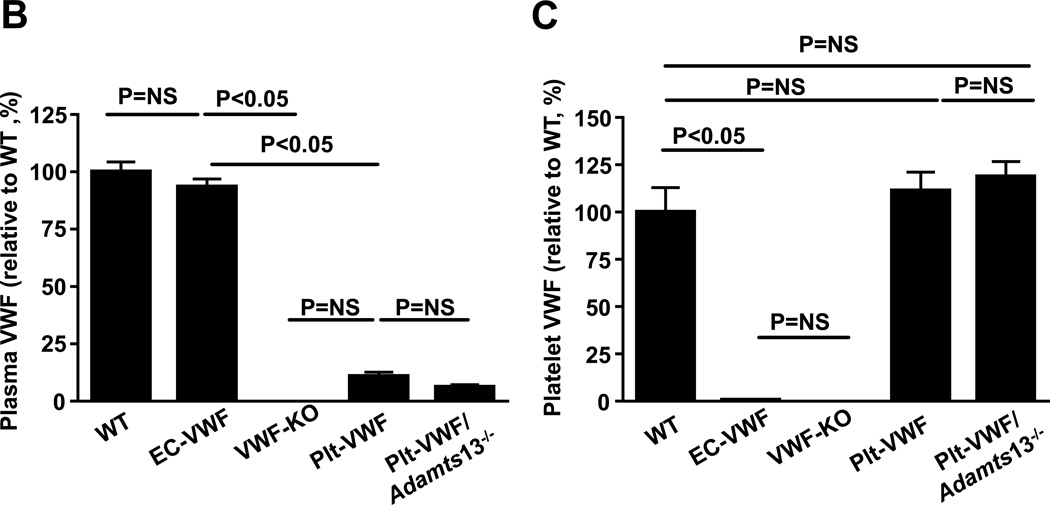
In the remainder of the manuscript: 1) irradiated WT mice reconstituted with BM from WT donors (WT-BM→ WT mice) are controls and are referred as WT mice (source of VWF: endothelial cells and platelets), 2) irradiated Vwf −/− mice reconstituted with BM from Vwf −/− donors (Vwf −/−-BM→ Vwf −/− mice) are referred as VWF-KO mice, 3) irradiated WT mice reconstituted with bone marrow from Vwf −/− donors (Vwf −/−-BM→ WT mice) are referred as EC-VWF mice (express VWF in endothelial cells, but lack VWF in platelet), 4) irradiated Vwf −/− mice reconstituted with bone marrow from WT donors (WT-BM→ Vwf −/− mice) are referred as Plt-VWF mice (have platelet-derived VWF, but lack VWF in endothelial cells), and 5) irradiated Vwf −/−Adamts13−/− mice reconstituted with bone marrow from Adamts13−/− donors (Adamts13−/−-BM→ Vwf −/−Adamts13−/− mice) are referred as Plt-VWF/Adamts13−/− mice (Deficient for ADAMTS13 in plasma and platelet and lack VWF in endothelial cells) (Figure 1A). Complete blood counts were comparable suggesting that BMT did not affect the number of BM-derived blood cells (Table SI). To confirm successful engraftment of bone marrow the relative content of VWF in plasma and platelets was quantified by ELISA. Plasma VWF levels were comparable between WT mice (103.3 ± 4.4 %, n=22) and EC-VWF mice (96.6 ± 3.6 %, n=22, Figure 1B). No detectable levels of plasma VWF were found in VWF-KO mice (n=18), whereas a minor amount of plasma VWF, although non-significant (Figure 1B) was found in Plt-VWF (11.3 ± 1.8 %, n=12) and Plt-VWF/Adamts13−/− mice (6.4 ± 1.1 %, n=20). Plt-derived VWF levels were comparable between WT, Plt-VWF mice and Plt-VWF/Adamts13−/− mice (Figure 1C). No detectable level of Plt-derived VWF was found in VWF-KO or EC-VWF mice (Figure 1C).
Figure 1. Generation and characterization of chimeric mice, which express VWF either in megakaryocytes or endothelial cells.
A, Schematic representation of bone marrow transplantation protocol. Reciprocal bone marrow (BM) from WT or Vwf −/− mice were transplanted into either WT or Vwf −/− recipient mice to generate 4 experimental groups of mice: 1) WT mice; source of VWF is platelets and endothelial cells, control, 2) Plt-VWF mice: source of VWF is platelets, 3) EC-VWF mice; source of VWF is endothelial cells, 4) VWF-KO mice; lack VWF in platelets and endothelial cells. Additionally, BM from Adamts13−/− mice was transplanted into Adamts13−/−Vwf −/− mice to generate Plt-VWF/Adamts13−/− mice that contain VWF in platelets, but are deficient for ADAMTS13 in both platelets and plasma. B, Plasma VWF levels. N=12–20 mice/group. C, Platelet VWF levels. N=8–12 mice/group. Data are presented as mean ± SEM.
EC-derived VWF is the major determinant that contributes to VWF-dependent cerebral ischemia/reperfusion injury
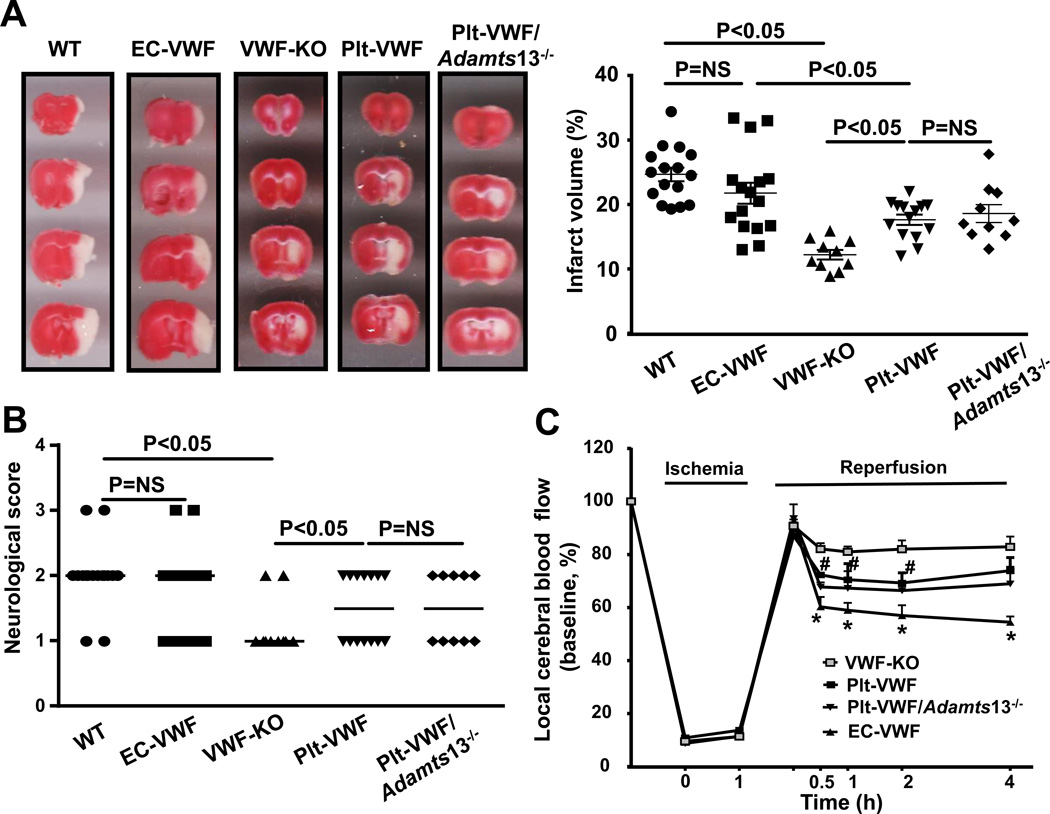
To define the relative importance of Plt-derived VWF versus EC-derived VWF pool in the pathophysiology of acute ischemic stroke, EC-VWF mice and Plt-VWF mice with controls (WT and VWF-KO) were subjected to 60 minutes of ischemia followed by 23 hours of reperfusion. We found that infarct volume and neurological outcome were comparable in WT mice (24.7 ± 1.1 %) and EC-VWF mice (21.7 ± 1.6 %, Figure 2 A&B). Similar to previous reports,16, 17 VWF-KO mice exhibited decreased infarct volume (12.2 % ± 0.7 %, P<0.05) and better neurological outcome when compared with either WT mice or EC-VWF mice (Figure 2 A and B). Plt-VWF mice exhibited significantly decreased infarct volume (17.6 ± 0.8 %, P<0.05) and better neurological outcome when compared with either WT mice or EC-VWF mice, but increased infarct volume and worse neurological outcome when compared to VWF-KO mice (Figure 2A and B). These results suggest that Plt-derived VWF by itself partially contributes to cerebral ischemia/reperfusion injury whereas EC-derived is the major determinant.
Figure 2. Stroke outcome in EC-VWF mice, but not in Plt-VWF mice, is comparable to WT mice.
A, Left panel shows representative 2, 3, 5-triphenyl-tetrazolium chloride stained serial coronal brain sections from one mouse after transient middle cerebral artery occlusion. Viable tissue is stained red whereas infarcted area is unstained (white). Right panel shows corrected mean infarct volumes of each group as mean ± SEM (n = 10–16 mice/group). Number of mice is a pool of two independent experiments. B, Neurological score. Horizontal line depicts the median. Analysis of variance on ranks was applied to test for significant differences in the neurological score. C, Doppler flow measurements of local cerebral blood flow in the territory of the right middle cerebral artery at 0.5, 1, 2, and 4 hours of reperfusion (#P < 0.05 versus VWF-KO mice, *P versus VWF-KO mice, repeated measures ANOVA; N=8–9 mice/group).
Next, we determined whether Plt-VWF, in the absence of ADAMTS13 in plasma and platelets, is able to induce stroke outcome comparable to EC-derived VWF. Infarct volume and neurological outcome were comparable in Plt-VWF mice (17.6 ± 0.8) and Plt-VWF/Adamts13−/− mice (18.6 ± 1.4 %), but significantly decreased when compared to WT mice (24.7 ± 1.1 %) or EC-VWF mice (21.7 ± 1.6 %, P<0.05, Figure 2A and B). This result suggests that Plt-derived VWF even in the absence of ADAMTS13, by itself was not sufficient to promote cerebral ischemia/reperfusion injury similar to EC-derived VWF. Laser Doppler flow measurements (Table SII) were similar among groups before, during, and after ischemia. To determine whether worse stroke outcome in WT mice was concomitant with reduced local cerebral blood flow (CBF), laser Doppler flowmetry was performed at different time points (0.5–4 hours). We found that local CBF was significantly decreased at 0.5, 1, 2, and 4 hours after reperfusion in EC-VWF mice (P<0.05 vs. VWF-KO or Plt-VWF or Plt-VWF/Adamts13−/− mice, Figure 2C). Local CBF was significantly decreased at 1 and 2 hours after reperfusion in Plt-VWF and Plt-VWF/Adamts13−/− mice when compared to VWF-KO mice but significantly increased when compared with EC-VWF mice (Figure 2C).
Transfusion of high number of WT platelets into Plt-VWF or Adamts13−/− platelets into Plt-VWF/Adamts13−/− mice does not aggravate stroke outcome
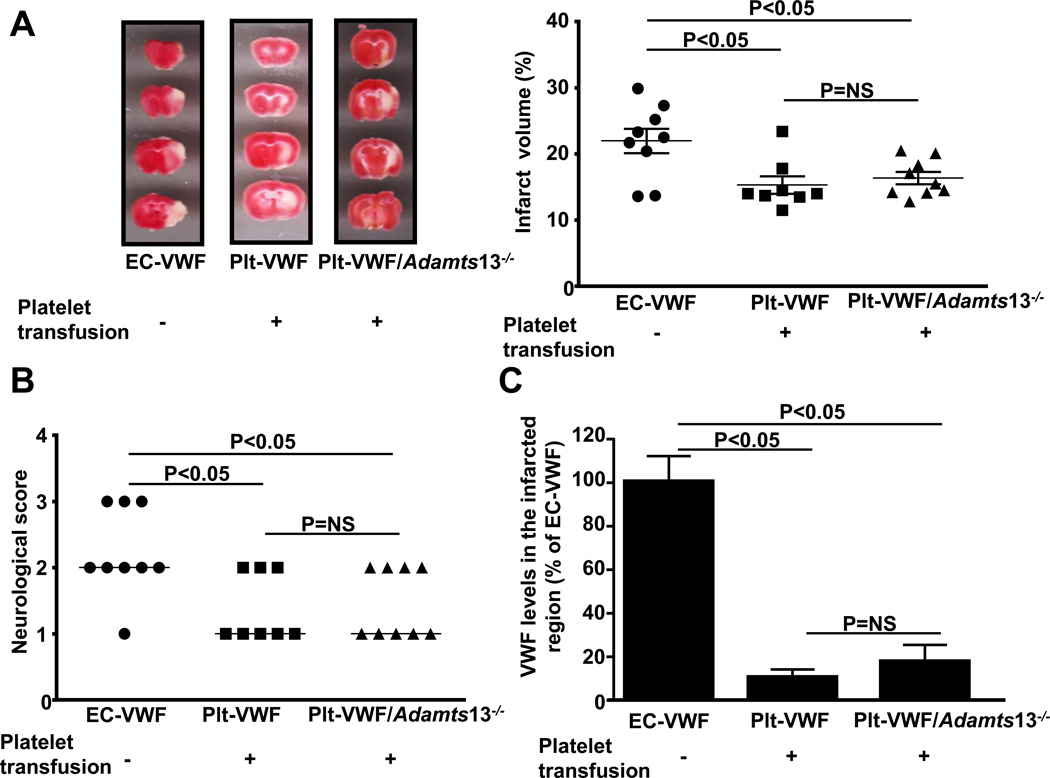
Next, we determined whether transfusion of high number of WT platelets (~3.2 ×108) into Plt-VWF mice and Adamts13−/− platelets (~3.2 ×108) into Plt-VWF/Adamts13−/− mice would result in stroke outcome similar to EC-VWF mice. Compared with EC-VWF mice, Plt-VWF mice and/or Plt-VWF/Adamts13−/− mice transfused with additional high number of platelets did not aggravate stroke outcome (Figure 3 A and B). Quantification of VWF in the brain homogenates from the infarcted and surrounding regions revealed a marked decrease in local VWF antigen levels in both Plt-VWF and Plt-VWF/Adamts13−/− mice transfused with additional VWF+ platelets when compared to EC-VWF mice (Figure 3C). Together, these results suggest that although Plt-derived VWF contributes to partial brain injury following stroke, it is the EC-derived VWF that drives VWF-dependent ischemic stroke.
Figure 3. Transfusion of high number of WT platelets into Plt-VWF mice or Adamts13−/− platelets into Plt-VWF/Adamts13−/− mice does not aggravate stroke outcome.
A, Left panel shows representative 2, 3, 5-triphenyl-tetrazolium chloride stained serial coronal brain sections from one mouse after transient middle cerebral artery occlusion. Right panel shows corrected mean infarct volumes of each group as mean ± SEM (n = 8–9 mice/group). B, Neurological score. Horizontal line depicts the median. C, Quantification of VWF in the tissue homogenates of infarcted and surrounding region by ELISA. Data are presented as mean ± SEM (n = 8–9 mice/group).
Post-ischemic thrombo-inflammation was comparable between EC-VWF and WT mice
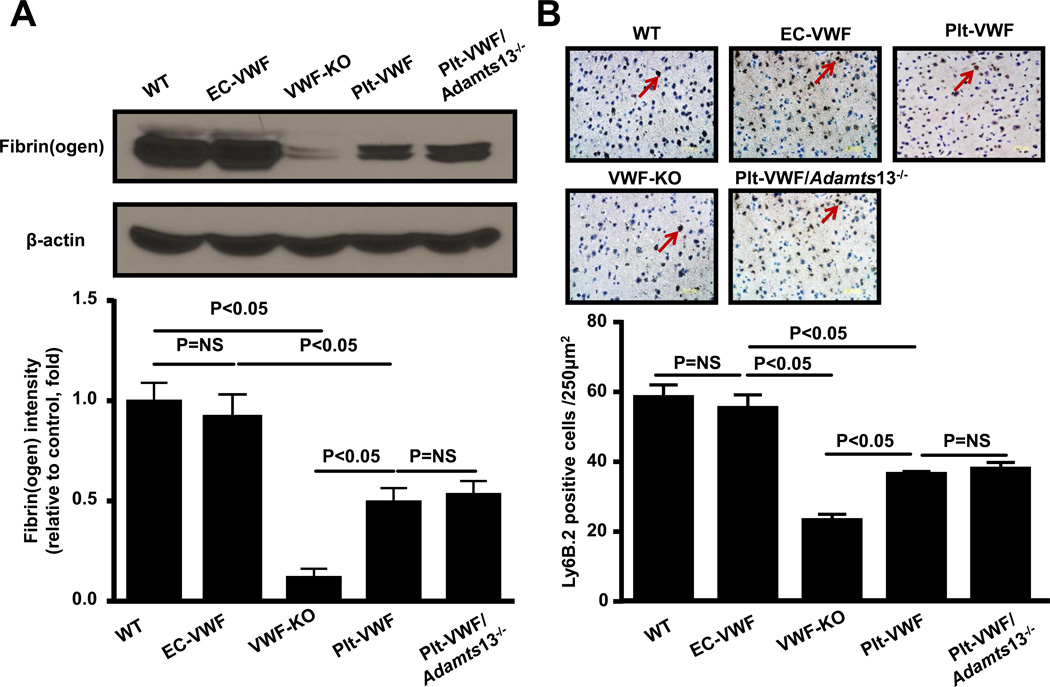
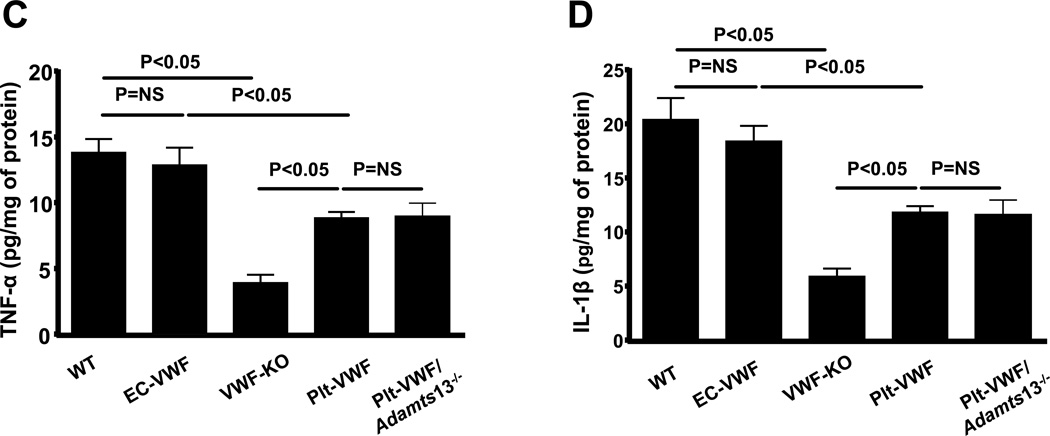
Both thrombosis and inflammatory processes contribute to acute stroke. To quantify thrombosis in cerebral microvasculature, we measured intracerebral fibrin(ogen) deposition, whereas to quantify inflammation, we determined neutrophil infiltration and inflammatory cytokines (TNF-α and IL-1β levels) within the infarct and peri-infarct region of the perfused brain after 60 minutes of tMCAO followed by 23 hours of reperfusion. We found that intracerebral fibrin(ogen) deposition, as determined by Western blot, was comparable between EC-VWF and WT mice but significantly increased when compared to either Plt-VWF mice or Plt-VWF/Adamts13−/− mice (Figure 4A). Fibrin(ogen) deposition was similar in Plt-VWF and Plt-VWF/Adamts13−/− mice but significantly increased when compared to VWF-KO mice (Figure 4A). Immunostained sections of the ischemic region revealed that neutrophil infiltration was similar in EC-VWF and WT mice but significantly increased when compared with either Plt-VWF or Plt-VWF/Adamts13−/− mice (P<0.05, Figure 4B). On the other hand, neutrophil infiltration within the ischemic region was comparable in Plt-VWF and Plt-VWF/Adamts13−/− mice and significantly increased when compared with VWF-KO mice (Figure 4B). Total leukocyte counts were similar among genotypes (Table SI). In line with these findings, ELISA experiments showed a significant increase in TNF-α and IL-1β levels in both WT and EC-VWF mice (P < 0.05 vs. VWF-KO or Plt-VWF or Plt-VWF/Adamts13−/− mice, Figure 4C and D). Together these findings suggest that although Plt-derived VWF partially contributes to post-ischemic thrombo-inflammation, it is the EC-derived VWF that is the major determinant.
Figure 4. EC-VWF mice, but not Plt-VWF mice, exhibit thrombo-inflammation similar to WT mice following cerebral ischemia/reperfusion injury.
A, Top panel shows representative immunoblots showing fibrin(ogen) accumulation in the tissue homogenates of infarcted and surrounding region. Bottom panel shows densitometric quantification (top bands) normalized to corresponding β-actin (loading control). Data are presented as mean ± SEM (N = 4–5 mice/group). B, Top panel shows representative coronal brain sections from each group stained for neutrophils (Ly6 B.2 positive cells stained as brown are indicated by arrows) and counterstained with hematoxylin (blue). The scale bar = 50 µm. Bottom panel shows quantification. Mean for individual mouse was calculated from 4 coronal sections/mouse (separated by 100 µm). Data are presented as mean ± SEM (n = 5 mice/group). C and D, Quantification of TNF-α and IL-1β levels by ELISA in brain homogenates prepared from the infarcted and surrounding areas following tMCAO. Data are presented as mean ± SEM (n= 6 mice/group).
EC-derived VWF is a major determinant for occlusive thrombus formation
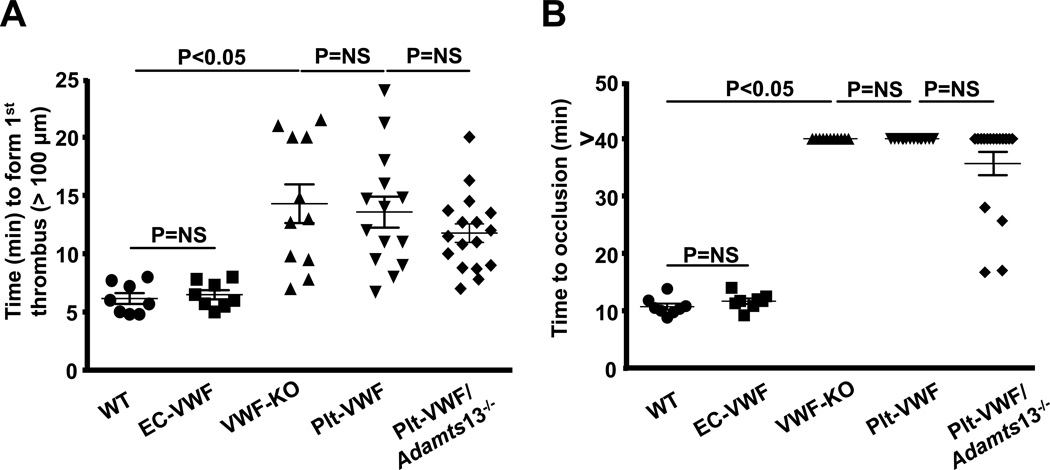
To test the hypothesis that EC-derived VWF is the major determinant that promotes cerebral thrombosis, and, thereby contributes to brain injury following acute stroke, we determined relative contribution of Plt-VWF versus EC-VWF to susceptibility to FeCl3 injury-induced carotid artery thrombosis. All mice used were similar in age and weight to those used for stroke experiments. Using in vivo imaging, we found that the mean time to form first thrombus (≥ 100 µm) and the mean time to occlusion were similar in EC-VWF mice when compared with WT mice (Figure 5). The mean time to form the first thrombus were similar in Plt-VWF and Plt-VWF/Adamts13−/− mice when compared with VWF-KO mice but significantly prolonged when compared to EC-VWF or WT mice (Figure 5A). Similar to VWF-KO mice, none of the injured vessels occluded in Plt-VWF mice (Figure 5B). Interestingly, 23% of Plt-VWF/Adamts13−/− mice (4 out of 17) had occlusive thrombus. Although the mean time to occlusion was shortened, it was not significant when compared to Plt-VWF or VWF-KO mice (Figure 5B). In tail-transection bleeding assay, EC-VWF mice exhibited normal hemostasis, whereas Plt-VWF and Plt-VWF/Adamts13−/− mice exhibited defective hemostasis (Figure SI). Together these results suggest that EC-derived VWF, but not Plt-derived VWF, is the major determinant for occlusive thrombus formation.
Figure 5. EC-derived VWF, but not Plt-derived VWF, alone is sufficient for formation of occlusive thrombus in FeCl3 injury-induced carotid thrombosis model.
A, Time to first thrombus formation. B, Mean time to complete occlusion of FeCl3 injured carotid artery. Each dot represents single mouse. Occlusion time was compared by Fisher's exact test. Data are presented as mean ± SEM (N = 8 to 17 mice/group).
Both EC-derived VWF and Plt-derived VWF modulate thrombus growth
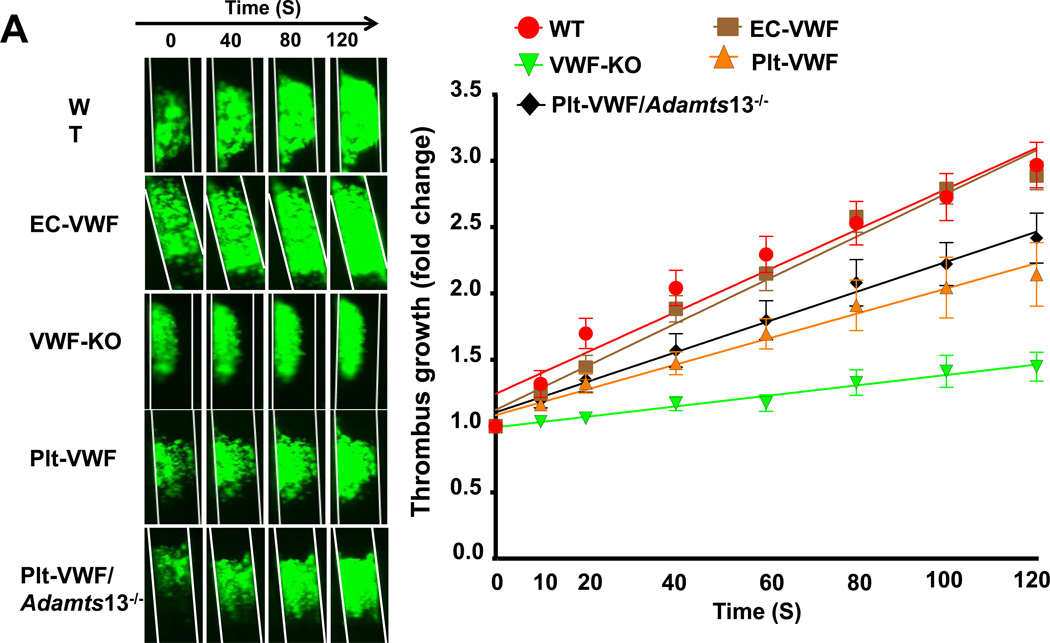
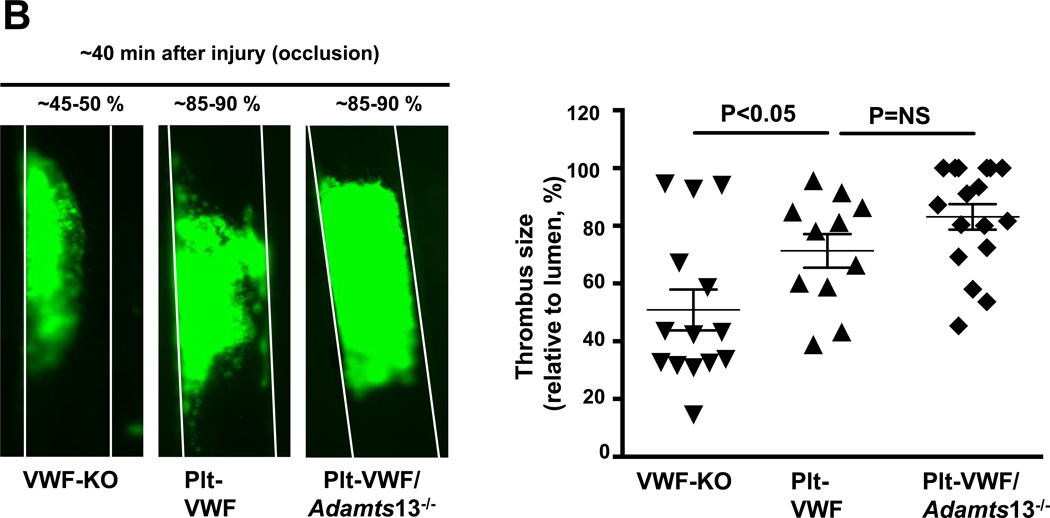
Next, we determined whether Plt-derived VWF alone is able to support thrombus growth to a certain extent in the absence of EC-derived VWF. Using intravital microscopy, we measured thrombus growth kinetics and percentage occlusion (relative to vessel lumen) in injured vessels. Slope over time showed that the rate of thrombus growth was comparable in EC-VWF and WT mice and significantly increased when compared to VWF-KO mice (Figure 6A). Interestingly, rate of thrombus growth was also significantly increased in Plt-VWF mice when compared to VWF-KO mice (Figure 6A) but decreased when compared to EC-VWF or WT mice, suggesting that Plt-derived VWF partially contributes to thrombus growth. To our surprise, rate of thrombus growth was comparable between Plt-VWF and Plt-VWF/Adamts13−/− mice (Figure 6A). Consistent with these results Plt-VWF mice or Plt-VWF/Adamts13−/− mice developed larger thrombi (~85–90% occlusion) when compared with VWF-KO mice (~45–50% occlusion) within 40 minutes after FeCl3 injury (Figure 6B). Next, we determined whether Plt-derived VWF is able to support thrombus growth in another experimental model of thrombosis (laser injury-induced mesenteric artery thrombosis). We transfused 6 × 108 (~50% platelets) from either WT or VWF-KO mice into VWF-KO mice. Following laser injury, we quantified thrombus growth kinetics and % occlusion. Slope over time showed that rate of thrombus growth was increased in VWF-KO mice transfused with WT platelets when compared with VWF-KO mice transfused with VWF-KO platelets (Figure SIIA). Consistent with these results, VWF-KO mice transfused with WT platelets developed larger thrombi (~60–70% occlusion) when compared with VWF-KO mice transfused with VWF-KO platelets (~30–40% occlusion; Figure SIIB). Together, these results suggest that Plt-derived VWF by itself partially contributes to transient thrombus growth whereas EC-derived VWF is the major determinant.
Figure 6. Although Plt-derived VWF partially contributes to thrombus growth, EC-derived VWF present in plasma is the driving factor that contributes to occlusive thrombus formation in injured vessel.
A, Left panel shows representative microphotographs of thrombus growth in FeCl3-injured carotid arteries as visualized by upright intravital microscopy. Right panel shows thrombus growth kinetics. The fold increase in diameter was calculated by dividing the diameter of the thrombus at time (n) by the diameter of the same thrombus at time (0) (defined as the time point at which the thrombus diameter first reached 100 µm). Slopes over time showed that the rate of thrombus growth were comparable in EC-VWF mice and WT mice. Rate of thrombus growth was significantly increased in Plt-VWF or Plt-VWF/Adamts13−/− mice when compared to VWF-KO mice, but decreased when compared to EC-VWF mice or WT mice. Data are presented as mean ± SEM (N=8–17 mice/group). B, Left panel shows representative microphotographs depicting percentage occlusion ~40 minutes after FeCl3-induced injury. Right panel shows quantification. Platelets were labeled with calcein green. White lines delineate the arteries. Data are presented as mean ± SEM (N=11–17 mice/group).
Discussion
The results presented here shed mechanistic insights on the relative importance of different pools of VWF in the pathophysiology of acute ischemic stroke. The key findings are: 1) EC-derived VWF is the major determinant that mediates VWF-dependent cerebral ischemia/reperfusion injury by promoting thrombo-inflammation. 2) Plt-derived VWF, in the presence or absence of ADAMTS13, promotes transient non-occlusive thrombus growth and contributes partially to brain injury and post-ischemic thrombo-inflammation following cerebral ischemia/reperfusion injury.
Herein, we found that chimeric EC-VWF mice express normal VWF levels in the plasma and no detectable VWF in platelets. On the other hand, chimeric Plt-VWF mice or chimeric Plt-VWF/Adamts13−/− mice express normal VWF levels in platelets and only trace amount of VWF in the plasma. Most likely, the source of trace amount of VWF in the plasma of chimeric Plt-VWF mice or chimeric Plt-VWF/Adamts13−/− mice may be due to an artifact of platelet activation during the blood draw. Together, these results imply that origin of plasma VWF is endothelium, and not quiescent circulating platelets.
Recently, Verhenne et al., showed that chimeric Plt-VWF mice and chimeric EC-VWF mice had infarct size similar to WT mice in brain ischemia/reperfusion model, suggesting that VWF by itself, independent of its origin and overall local levels, is sufficient to mediate VWF-dependent stroke exacerbation.25 Of note, this study did not quantify the overall local levels of VWF in the infarcted and surrounding region following stroke. Similar to the Verhenne et al. study, we found that chimeric Plt-VWF mice exhibited significantly bigger infarcts compared to VWF-KO mice. However, in contrast to their study, we found that chimeric Plt-VWF mice infarcts were significantly smaller when compared either to chimeric EC-VWF mice or WT mice following cerebral ischemia/reperfusion injury. The discrepancy between these studies remains unclear but could be related to different experimental set-up used to induce cerebral ischemia/reperfusion injury. Of note, our studies were in progress when Verhenne et al. published their findings.25 We speculated that the transfusion of additional WT platelets (~30%) in Plt-VWF mice might worsen stroke outcome similar to EC-VWF mice because of overall increased local levels of VWF released by activated platelets following injury. Surprisingly, we found that transfusion of additional high number of VWF+ platelets did not rescue the phenotype in either Plt-VWF or Plt-VWF/Adamts13−/− mice. Based on our findings, we suggest that although Plt-derived VWF could partially contribute to stroke exacerbation, it is the EC-derived VWF that drives VWF-dependent ischemic stroke. Additional studies, will be required to determine whether increasing plasma VWF levels in the Vwf −/− mice similar to WT, independent of the source of VWF, will worsen stroke outcome comparable to WT mice.
It is well established that cerebral ischemia/ reperfusion injury elicits a strong post thrombo-inflammatory response that promotes brain tissue damage in the ischemic penumbra.26 VWF is known to promote intracerebral thrombosis and post-inflammatory response following acute stroke.17, 27 Herein, we found that chimeric EC-VWF mice exhibited increased intracerebral fibrin(ogen) deposition when compared to chimeric Plt-VWF, which was concomitant with lower local CBF as estimated by non-invasive laser Doppler flowmetry. This result suggests that EC- derived VWF present in the plasma most likely promotes cerebral thrombosis, and thereby decreases local CBF at a number of isolated points in the cortical area. Additionally, compared to Plt-derived VWF, we found that EC-derived VWF significantly increases post-ischemic inflammatory response characterized by an increase in neutrophil infiltration and inflammatory cytokines, such as TNF-α and IL-1β within ischemic region.
The relative in vivo role of different pools of VWF in arterial thrombosis has not been studied in detail yet. We found that EC-derived VWF alone, but not Plt-derived VWF, was sufficient to form occlusive thrombus formation. Our findings are in accordance with previous studies done in chimeric mice25 and pig models,28 which demonstrate that EC-derived VWF present in the plasma is essential for development of arterial thrombosis. It is important to note that these studies did not determine whether Plt-derived VWF by itself contributes to thrombus growth. We found that although Plt-derived VWF is not able to support occlusive thrombus formation, it partially contributes to transient thrombus growth both in FeCl3-induced carotid thrombosis and laser injury-induced mesenteric thrombosis models. Our findings are in agreement with previous in vitro studies, which suggested that Plt-derived VWF might contribute to platelet aggregation under flow conditions.29, 30
Platelets contain ADAMTS13, and few studies have suggested that Plt-derived ULVWF multimers are only observed if platelets are activated and lysed in presence of EDTA.21, 22 In contrast, another in vitro study by Mcgrath et al., suggested that platelet-derived VWF has different glycosylation profile and is therefore resistant to ADAMTS13 cleavage.20 ULVWF multimers are considered hyperactive because they bind avidly to the extracellular matrix31 and form high strength bonds with platelet glycoprotein GPIbα.32 We speculated that presence of ULVWF multimers in platelets in absence of ADAMTS13 might be sufficient to support hemostasis and thrombosis. To answer this question, we transplanted irradiated Vwf −/−Adamts13−/− mice with BM from Adamts13−/− donors, so that ULVWF released after platelet activation is not cleaved by ADAMTS13. We found that majority (75%) of chimeric Plt-VWF/Adamts13−/− mice exhibited defective thrombosis. In ~25% chimeric Plt-VWF/Adamts13−/− mice injured vessels had occlusive thrombus, however the time to mean occlusion was significantly prolonged compared to WT or EC-VWF mice. We speculate that this could be due to a significant heterogeneity in platelet VWF levels which has been observed in clinical studies with type 1 VWD patients.33 Finally, cerebral ischemia/reperfusion injury in chimeric Plt-VWF/Adamts13−/− mice was comparable to Plt-VWF mice, but significantly less when compared with EC-VWF mice. Results from these murine studies clearly suggest that under physiological conditions it is the EC-derived VWF that drives brain injury following acute stroke most likely through platelet GPIbα.18
In conclusion, our results provide insights on the role of different pools of VWF in pathophysiology of acute stroke. This study further supports the notion that therapeutically targeting VWF may have the potential to reduce brain injury and improve outcomes in patients at high risk for stroke.
Supplementary Material
Highlights.
VWF is synthesized in endothelial cells and megakaryocytes.
Platelet-derived VWF partially contributes to VWF-dependent cerebral brain injury and post-ischemic inflammation following acute ischemic stroke, but alone is not sufficient to maintain hemostasis and thrombosis.
Endothelial cell-derived VWF is the major determinant that mediates VWF-dependent ischemic stroke, hemostasis, and thrombosis in mice.
Acknowledgments
Sources of funding
A.K.C lab is supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health grants (R01 HL118246 and R01 HL118742) and by innovative grant 16IRG27490003 from American Heart Association.
Non-standard Abbreviations and Acronyms
- VWF
Von willebrand factor
- ULVWF
Ultra large von willebrand factor
- EC-VWF
Endothelial cell-derived VWF
- Plt-VWF
Platelet-derived VWF
- BMT
Bone marrow transplantation
- ADAMTS13
A Disintegrin And Metalloprotease with Thrombospondin type I repeats-13
Footnotes
Disclosures
None.
References
- 1.Brinkhous KM, Sandberg H, Garris JB, Mattsson C, Palm M, Griggs T, Read MS. Purified human factor viii procoagulant protein: Comparative hemostatic response after infusions into hemophilic and von willebrand disease dogs. Proc Natl Acad Sci USA. 1985;82:8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von willebrand protein in weibel-palade bodies of human endothelial cells. The Journal of cell biology. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. Adamts-13 rapidly cleaves newly secreted ultralarge von willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 4.Zheng XL. Adamts13 and von willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211–225. doi: 10.1146/annurev-med-061813-013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai HM, Nagel RL, Hatcher VB, Seaton AC, Sussman II. The high molecular weight form of endothelial cell von willebrand factor is released by the regulated pathway. British journal of haematology. 1991;79:239–245. doi: 10.1111/j.1365-2141.1991.tb04528.x. [DOI] [PubMed] [Google Scholar]
- 6.Sporn LA, Chavin SI, Marder VJ, Wagner DD. Biosynthesis of von willebrand protein by human megakaryocytes. J Clin Invest. 1985;76:1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von willebrand disease: Defects in hemostasis and thrombosis. Proc Natl Acad Sci USA. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. Von willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer AT, Suckau J, Frank K, Desch A, Goertz L, Wagner AH, Hecker M, Goerge T, Umansky L, Beckhove P, Utikal J, Gorzelanny C, Diaz-Valdes N, Umansky V, Schneider SW. Von willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood. 2015;125:3153–3163. doi: 10.1182/blood-2014-08-595686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD, Cutler DF, Laffan MA, Randi AM. Endothelial von willebrand factor regulates angiogenesis. Blood. 2011;117:1071–1080. doi: 10.1182/blood-2010-01-264507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi C, Motto DG, Jensen M, Lentz SR, Chauhan AK. Adamts13 deficiency exacerbates vwf-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5224–5230. doi: 10.1182/blood-2012-06-440255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, Dietrich B, Rottensteiner H, Scheiflinger F, Wagner DD. Protective anti-inflammatory effect of adamts13 on myocardial ischemia/reperfusion injury in mice. Blood. 2012;120:5217–5223. doi: 10.1182/blood-2012-06-439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Methia N, Andre P, Denis CV, Economopoulos M, Wagner DD. Localized reduction of atherosclerosis in von willebrand factor-deficient mice. Blood. 2001;98:1424–1428. doi: 10.1182/blood.v98.5.1424. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi C, Ahmad A, Wilson KM, Chauhan AK. Adamts13 modulates atherosclerotic plaque progression in mice via a vwf-dependent mechanism. J Thromb Haemost. 2014;12:255–260. doi: 10.1111/jth.12456. [DOI] [PubMed] [Google Scholar]
- 16.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. Von willebrand factor-cleaving protease adamts13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinschnitz C, De Meyer SF, Schwarz T, Austinat M, Vanhoorelbeke K, Nieswandt B, Deckmyn H, Stoll G. Deficiency of von willebrand factor protects mice from ischemic stroke. Blood. 2009;113:3600–3603. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 18.De Meyer SF, Schwarz T, Deckmyn H, Denis CV, Nieswandt B, Stoll G, Vanhoorelbeke K, Kleinschnitz C. Binding of von willebrand factor to collagen and glycoprotein ibalpha, but not to glycoprotein iib/iiia, contributes to ischemic stroke in mice--brief report. Arterioscler Thromb Vasc Biol. 2010;30:1949–1951. doi: 10.1161/ATVBAHA.110.208918. [DOI] [PubMed] [Google Scholar]
- 19.Yarovoi HV, Kufrin D, Eslin DE, Thornton MA, Haberichter SL, Shi Q, Zhu H, Camire R, Fakharzadeh SS, Kowalska MA, Wilcox DA, Sachais BS, Montgomery RR, Poncz M. Factor viii ectopically expressed in platelets: Efficacy in hemophilia a treatment. Blood. 2003;102:4006–4013. doi: 10.1182/blood-2003-05-1519. [DOI] [PubMed] [Google Scholar]
- 20.McGrath RT, van den Biggelaar M, Byrne B, O'Sullivan JM, Rawley O, O'Kennedy R, Voorberg J, Preston RJ, O'Donnell JS. Altered glycosylation of platelet-derived von willebrand factor confers resistance to adamts13 proteolysis. Blood. 2013;122:4107–4110. doi: 10.1182/blood-2013-04-496851. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez MF, Ginsberg MH, Ruggeri ZM, Batlle FJ, Zimmerman TS. Multimeric structure of platelet factor viii/von willebrand factor: The presence of larger multimers and their reassociation with thrombin-stimulated platelets. Blood. 1982;60:1132–1138. [PubMed] [Google Scholar]
- 22.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, Ruan C, Moake JL, Dong JF. Platelet-derived vwf-cleaving metalloprotease adamts-13. J Thromb Haemost. 2005;3:2536–2544. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 23.Williams SB, McKeown LP, Krutzsch H, Hansmann K, Gralnick HR. Purification and characterization of human platelet von willebrand factor. British journal of haematology. 1994;88:582–591. doi: 10.1111/j.1365-2141.1994.tb05077.x. [DOI] [PubMed] [Google Scholar]
- 24.Mannucci PM. Platelet von willebrand factor in inherited and acquired bleeding disorders. Proc Natl Acad Sci USA. 1995;92:2428–2432. doi: 10.1073/pnas.92.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhenne S, Denorme F, Libbrecht S, Vandenbulcke A, Pareyn I, Deckmyn H, Lambrecht A, Nieswandt B, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. Platelet-derived vwf is not essential for normal thrombosis and hemostasis but fosters ischemic stroke injury in mice. Blood. 2015;126:1715–1722. doi: 10.1182/blood-2015-03-632901. [DOI] [PubMed] [Google Scholar]
- 26.Stoll G, Kleinschnitz C, Nieswandt B. Combating innate inflammation: A new paradigm for acute treatment of stroke? Annals of the New York Academy of Sciences. 2010;1207:149–154. doi: 10.1111/j.1749-6632.2010.05730.x. [DOI] [PubMed] [Google Scholar]
- 27.Khan MM, Motto DG, Lentz SR, Chauhan AK. Adamts13 reduces vwf-mediated acute inflammation following focal cerebral ischemia in mice. J Thromb Haemost. 2012;10:1665–1671. doi: 10.1111/j.1538-7836.2012.04822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols TC, Samama CM, Bellinger DA, et al. Function of von willebrand factor after crossed bone marrow transplantation between normal and von willebrand disease pigs: Effect on arterial thrombosis in chimeras. Proc Natl Acad Sci USA. 1995;92:2455–2459. doi: 10.1073/pnas.92.7.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni S, Dopheide SM, Yap CL, Ravanat C, Freund M, Mangin P, Heel KA, Street A, Harper IS, Lanza F, Jackson SP. A revised model of platelet aggregation. J Clin Invest. 2000;105:783–791. doi: 10.1172/JCI7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanaji S, Fahs SA, Shi Q, Haberichter SL, Montgomery RR. Contribution of platelet vs. Endothelial vwf to platelet adhesion and hemostasis. J Thromb Haemost. 2012;10:1646–1652. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sporn LA, Marder VJ, Wagner DD. Von willebrand factor released from weibel-palade bodies binds more avidly to extracellular matrix than that secreted constitutively. Blood. 1987;69:1531–1534. [PubMed] [Google Scholar]
- 32.Arya M, Anvari B, Romo GM, Cruz MA, Dong JF, McIntire LV, Moake JL, Lopez JA. Ultralarge multimers of von willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein ib-ix complex: Studies using optical tweezers. Blood. 2002;99:3971–3977. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- 33.Mannucci PM, Lombardi R, Bader R, Vianello L, Federici AB, Solinas S, Mazzucconi MG, Mariani G. Heterogeneity of type I von willebrand disease: Evidence for a subgroup with an abnormal von willebrand factor. Blood. 1985;66:796–802. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.