Abstract
Adipose tissue dysfunction occurs with aging and has systemic effects, including peripheral insulin resistance, ectopic lipid deposition, and inflammation. Fundamental aging mechanisms, including cellular senescence and progenitor cell dysfunction, occur in adipose tissue with aging and may serve as potential therapeutic targets in age-related disease. In this review, we examine the role of adipose tissue in healthy individuals and explore how aging leads to adipose tissue dysfunction, redistribution, and changes in gene regulation. Adipose tissue plays a central role in longevity, and interventions restricted to adipose tissue may impact lifespan. Conversely, obesity may represent a state of accelerated aging. We discuss the potential therapeutic potential of targeting basic aging mechanisms, including cellular senescence, in adipose tissue, using type II diabetes and regenerative medicine as examples. We make the case that aging should not be neglected in the study of adipose-derived stem cells for regenerative medicine strategies, as elderly patients make up a large portion of individuals in need of such therapies.
Keywords: Aging, Adipose Tissue, Insulin Resistance, Preadipocyte, Stem Cell, Cellular Senescence
1. Adipose Tissue: Relevance in Aging
Adipose tissue is a large and dynamic endocrine, immune, and regenerative organ that can readily adapt to changes such as temperature, nutrient availability, beta-adrenergic tone, and tissue damage in young, healthy individuals. Adipose tissue is responsible for energy storage, nutrient sensing, and temperature regulation and has important functions in immune modulation, wound healing, and tissue regeneration. Aging is associated with a decline in tissue function and an increase in disease burden, and adipose tissue is no exception. With aging, adipose tissue undergoes significant changes in abundance, distribution, cellular composition, and endocrine signaling, and plays a central role in the development of insulin resistance, metabolic dysfunction, inflammation, and impaired regenerative capacity with age (Fig. 1) (Tchkonia and others 2010). Several fundamental age-related changes occur at the cellular level in adipose tissue that may contribute to age-related adipose dysfunction. Adipose tissue is often the largest organ, making up over 40% of total body mass for example in women with BMI >35 (Bonora and others 1992; Romero-Corral and others 2008). However even in lean individuals, adipose tissue has systemic influence on inflammatory signaling and insulin sensitivity through secretion of adipokines and adipose-derived hormones (Tilg and Moschen 2006). It can affect the function of other organs including muscle, bone, liver, and the brain. Adipose tissue is also being turned to as a key source of mesenchymal stem cells, which are being increasingly explored as therapeutics for multiple degenerative diseases (Zuk and others 2002). Many interventions that extend lifespan in lower organisms and mammals have significant effects in adipose tissue, often through the modulation of nutrient availability (Huffman and Barzilai 2010; Picard and Guarente 2005; Tchkonia and others 2010). Furthermore, when single-gene mutations found to extend lifespan in lower animals are restricted to adipose tissue, similar lifespan extension can be seen (Bluher and others 2003; Giannakou and others 2004). Dysfunction of adipose tissue, such as that in obesity, is associated with a shortened lifespan and increase in age-related disease prevalence, including cancer and dementia (Gilbert and Slingerland 2013; Kivipelto and others 2005). The abundance of adipose tissue, along with its varied functions, importance to whole-body physiology, and constellation of aging changes, makes it a highly relevant organ for the study of aging. Further understanding of aging adipose tissue could be valuable for the discovery and testing of therapeutic strategies to target fundamental aging processes and age-related disease.
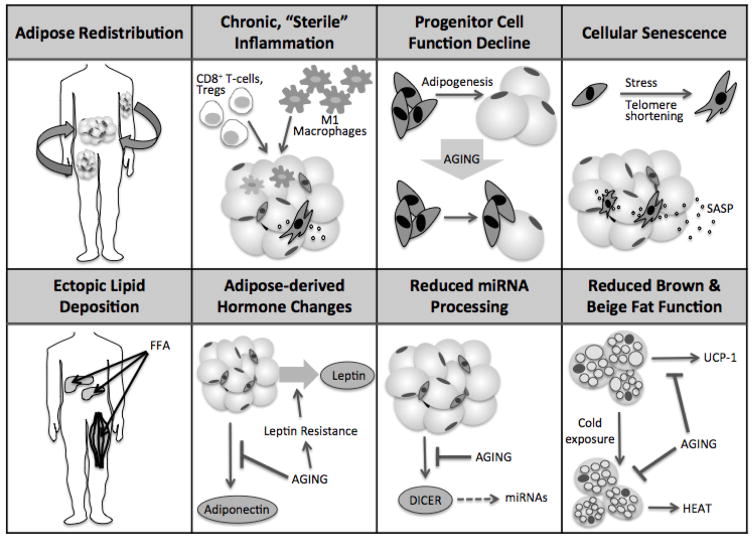
Figure 1. Adipose tissue changes with aging.
With aging, adipose tissue undergoes numerous changes, affecting distribution, inflammatory status, progenitor function, senescent cell burden, deposition of lipid in ectopic sites, adipose-derived hormone production and action, miRNA processing, and brown and beige adipose function.
2. Age-Related Changes in Adipose Tissue Composition and Function
2.1 Fat redistribution with aging
Through middle- or early old age (e.g., 40–65 years of age), body mass and body fat percentage increase in both men and women (Guo and others 1999). This age-related increase in adiposity has been suggested to underlie reduced insulin sensitivity with age (Karakelides and others 2010). From middle age, the distribution of adipose tissue shifts from primarily subcutaneous depots to visceral depots, which are more closely associated with development of metabolic syndrome and insulin resistance (Fox and others 2007; Preis and others 2010). In addition to metabolic effects, redistribution of adipose tissue is also partially responsible for aesthetic changes with aging that are related to subcutaneous fat loss, such as sunken cheeks, thinning of the skin over the hands and legs, and increased prominence of wrinkles (Coleman and Grover 2006; Donofrio 2000)
The location and function of adipose tissue have been suggested to be more important than the absolute amount of adipose tissue in terms of its effect on insulin sensitivity (Jensen 2008). It is also important to note that insulin resistance occurs even in individuals with normal BMI, not only in obese individuals (McLaughlin and others 2004). Gluteofemoral adipose tissue is associated with increased insulin sensitivity and lower diabetes and cardiovascular disease risk, while increased waist circumference, which reflects visceral adipose mass, is associated with insulin resistance and metabolic syndrome (Manolopoulos and others 2010; Snijder and others 2003). In fact, waist to hip ratio may be a better predictor of 5-year mortality than BMI alone (Folsom and others 1993). In the elderly, the ratio of limb fat to trunk fat was found to correlate positively to insulin sensitivity, while no differences in percent body fat or absolute amount of trunk fat were found in the same groups (Gavi and others 2007). Increased limb:trunk fat ratio, but not limb fat or trunk fat independently, was also correlated with higher levels of adiponectin, an adipose-derived hormone associated with improved insulin sensitivity (Gavi and others 2007). This indicates that the distribution of adipose tissue may be more important than the amount of adipose tissue in the regulation of adiponectin levels.
Subcutaneous and visceral adipose depots are very different in terms of their effects on metabolism (Atzmon and others 2002; Jensen 2008; Tchkonia and others 2013a). In mice, transplantation of subcutaneous fat into the visceral depot of recipient mice caused improvements in glucose homeostasis as well as decreased body weight and fat mass, whereas the opposite experiment, visceral adipose tissue transplanted to the subcutaneous depot of recipient mice, had little effect (Tran and others 2008). Adipose tissue dysfunction is likely to originate in subcutaneous fat, which is a larger depot than visceral fat in young individuals. With age, macrophages accumulate in subcutaneous fat, but no significant change is seen in visceral depots, which suggests that the sentinel site of inflammation with aging is likely the subcutaneous fat (E. Jerschow 2007; Lakowa and others 2015). Telomere length is also shorter in subcutaneous versus visceral adipose tissue, independently of BMI or diabetic status, and this difference is localized to the stromovascular fraction, not adipocytes (Lakowa and others 2015; Tchkonia and others 2006b). Accordingly, subcutaneous but not visceral adipose telomere length shortens with aging, and also correlates with obesity and diabetes (Lakowa and others 2015). This shorter basal length and age-related shortening of telomeres in subcutaneous depots may make subcutaneous adipose tissue a key contributor to increasing senescent cell burden with aging. Because of the impact that subcutaneous fat has on systemic metabolism, sentinel changes in subcutaneous fat with aging could be the harbinger for metabolic dysfunction, and represent an opportunity for intervention that could have significant impact on age-related disease.
Identification of the amount and distribution of adipose tissue that is healthy in elderly individuals has been difficult. Loss of subcutaneous fat is a common feature with advancing age (Sepe and others 2011). Although visceral fat is associated with worse metabolic phenotypes in younger individuals, the maintenance of adipose tissue seems to be beneficial in old age, sometimes independently of its location. For example, one study found that elderly individuals classified as “robust” according to their functional status had increased visceral and pericardial adipose tissue, normally considered to be sites of ectopic lipid deposition, compared to their “frail” counterparts (Idoate and others 2015). However, increased waist diameter was associated with the highest frailty within each BMI category in another study, indicating that adipose distribution favoring visceral adipose is still harmful when normalized for total adipose tissue (Hubbard and others 2010). The relationship between frailty and BMI is U-shaped, with extremely low and extremely high BMI predicting frailty and mortality (Allison and others 1997; Blaum and others 2005; Hubbard and others 2010). Interestingly, certain interventions that improve frailty in mice also maintain adipose tissue mass (Xu and others 2015a; Xu and others 2015b).
2.2. Chronic, sterile inflammation
Sterile inflammation, or the presence of inflammation in the absence of known, identifiable infection, is a common feature of aging and is certainly increased in aging adipose tissue. Adipose tissue is thought to be a major contributor to the chronic, low-grade inflammation seen in aging (Tchkonia and others 2010; Wu and others 2007). A variety of endogenous substances or stimuli, for example hypoxia, excess nutritional elements such as fatty acids, or products of cell death, which is persistent at a low level in obesity, may trigger sterile inflammation in adipose tissue (Chen and Nunez 2010; Itoh and others 2011). Adipose-derived cytokines and chemokines, termed adipokines, play a key role in immune cell recruitment to adipose tissue. Adipose tissue macrophages (ATMs) represent a significant source of pro-inflammatory substances such as IL-6 with aging (Tilg and Moschen 2006; Wu and others 2007). Numbers of ATMs increase in human subcutaneous fat until 30–35 years of age and then slightly decline in lean individuals (Ortega Martinez de Victoria and others 2009). ATMs in visceral adipose tissue do not change substantially in number during aging, but the ratio of pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages appears to increase with aging (Garg and others 2014; Lumeng and others 2011). Adipose tissue T cell populations also change with aging. Specifically, CD4+ T-lymphocytes, particularly regulatory T cells (Tregs), increase in visceral adipose tissue in aging mice, along with an increase in CD8+ T-cells (Lumeng and others 2011). Senescent cells also accumulate significantly with age in adipose tissue. Through their senescence-associated secretory phenotype (SASP), senescent cells are themselves the source of many pro-inflammatory cytokines and chemokines (Lumeng and others 2011; Xu and others 2015a; Xu and others 2015b).
In addition to the chronic changes in adipose inflammatory signaling and macrophage populations with aging, adipose tissue immune system components can also undergo acute changes in response to nutrient status, stress, or other short-term changes. For example, M2/M1 macrophage ratios have been shown to increase in response to a 24-hour fast in mice (Asterholm and others 2012). Interestingly, these acute changes are dampened in mice on a high fat diet, suggesting an impairment of adipose tissue to respond to acute changes when under metabolic stress (Asterholm and others 2012).
2.3. Progenitor cell function decline
Adipose tissue is composed of many different cell types, generally divided into two fractions: the adipocyte fraction (AF), which contains primarily mature adipocytes, and the stromovascular fraction (SVF), which comprises progenitor cells, lymphocytes, endothelial cells, pericytes, and fibroblasts. Adipose progenitor cells isolated from old individuals have reduced function and adipogenic potential compared to progenitors isolated from their young counterparts (Caso and others 2013; Karagiannides and others 2001; Tchkonia and others 2010). This is also seen in progenitors isolated from obese subjects when compared to lean age-matched controls (Tchkonia and others 2010).
Preadipocytes acquire insulin sensitivity during differentiation to adipocytes through increased expression of the critical transcription factors peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer binding protein alpha (C/EBPα) (Hamm and others 1999). Preadipocytes that are less able to differentiate appear with aging and may contribute to the limited ability of adipose tissue to be insulin responsive. This may be a significant contributor to the insulin resistance of old age, in addition to contributions from other organs (e.g. pancreas, skeletal muscle, and liver) (Tchkonia and others 2010).
Additionally, the replication and differentiation of preadipocytes is crucial for hyperplasia and hypertrophy, the two ways that adipose tissue responds to an increased demand for energy storage. Decline in adipogenic potential has been postulated to drive insulin resistance by limiting the ability of adipose tissue to expand in the face of excess nutrients (Danforth 2000). In addition, inability to recruit progenitor cells to the adipogenic lineage to execute hyperplasia can lead instead to hypertrophy of existing adipocytes. The resulting increase in adipocyte size has been associated with insulin resistance, especially in obesity (Gustafson and others 2015). Limited plasticity of adipose tissue with aging, leading to adipocyte hypertrophy in the face of nutrient excess, may be one mechanism by which older individuals are predisposed to insulin resistance (Kim and others 2014).
Targeting age-related changes in adipose tissue, such as cellular senescence, has some promise in improving adipose progenitor function (Xu and others 2015a) and can prevent or reverse age-related fat loss in mice (Xu and others 2015a). Individuals with Hutchinson-Gilford progeria syndrome suffer from lipodystrophy, which may be explained in part by progenitor exhaustion in the adipose tissue (Mansilla and others 2011; Mazereeuw-Hautier and others 2007). Similarly, mice with progeria have dysfunctional progenitor cells and lipodystrophic features, which may be attributable to progenitor dysfunction, cellular senescence, or immune clearance of cells with persistent DNA damage (Baker and others 2008; Karakasilioti and others 2013).
In addition to having implications for regenerative medicine, as will be discussed, adipose progenitor cell function plays a crucial role in lipid handling, adipose tissue expansion, and insulin sensitivity. Further study is needed to understand the role that age-related adipose tissue progenitor cell decline plays in age-related metabolic disease. Progenitor cell function decline with age in other organs, such as skeletal muscle, may also have contributory or independent roles in age-related insulin resistance. The respective contributions of reduced adipogenesis, versus dysregulation of lipid metabolism and/or glucose metabolism, to the development of age-related insulin resistance is unknown. In addition, it is not known what percentage of progenitor cells become dysfunctional with age, what threshold of progenitor dysfunction is necessary to cause physiologic changes such as lipodystrophy or insulin resistance, or whether progenitor dysfunction alone is sufficient to cause such changes (Kirkland and Dobson 1997; Kirkland and others 2002).
2.4. Cellular Senescence
Adipose tissue is a site of considerable senescent cell accumulation, in the settings of both obesity and aging (Tchkonia and others 2010). Cellular senescence is an essentially irreversible cell fate, in which cells stop dividing in response to an insult such as telomere shortening, oncogene activation, or metabolic stress (Tchkonia and others 2013b). Senescent cells adopt an enlarged phenotype, exhibit positivity for senescence-associated beta galactosidase, and can secrete a multitude of chemokines, cytokines, growth factors, and matrix metalloproteinases, comprising the SASP (Coppe and others 2008). SASP factors are expressed in vivo, as demonstrated in experiments where PAI-1 and IL-6 expression were increased in p16-positive cells isolated from mouse inguinal fat (Baker and others 2011). In addition, the SASP has been identified in vivo in mouse models of wound healing, liver fibrosis, and embryonic development (Demaria and others 2014; Krizhanovsky and others 2008; Storer and others 2013). The composition of the SASP may vary based on the senescent cell type, tissue, or senescence inducer, an area that merits further research.
In co-culture experiments, senescent cells have been shown to affect the function of adipose-derived progenitors as well as impair insulin sensitivity of adipose tissue (Xu and others 2015a; Xu and others 2015b). Therapeutic targeting of the SASP may be beneficial in alleviating age-related insulin resistance (Xu and others 2015a; Xu and others 2015b). We recently reported that removing senescent cells from older mice allows for improved adipogenesis (Xu and others 2015a). Removing senescent cells might therefore facilitate adipose tissue expansion in the face of nutrient excess, concurrent with improved insulin sensitivity, promoting a “metabolically healthy obesity” phenotype. Indeed, adipose tissue expansion occurs in humans after treatment with thiazolidinediones, which activate PPARγ, or JAK1/2 inhibitiors, which we recently reported to be SASP inhibitors (Xu and others 2015b), both of which improve insulin sensitivity (Fonseca 2003; Verstovsek and others 2010), On the other hand, senescent cell removal may allow for proper nutrient handling, mitochondrial function, and lipolysis that would limit expansion. However, the effects of senescent cell removal on adipose tissue dynamics have yet to be tested.
Strategies for selective elimination of senescent cells are beginning to emerge (Roos and others 2016; Zhu and others 2015a; Zhu and others 2015b). These advances follow the discovery that genetic clearance of senescent cells in mice is effective in preventing or reversing age-related dysfunction including loss of subcutaneous adipose tissue (Baker and others 2011; Xu and others 2015a). Because strategies to eliminate senescent cells generally only remove a portion of senescent cells, the effects of removing all senescent cells from a tissue is unknown. However, reduction of 30–70% of senescent cells does not appear to have detrimental effects on health of experimental animals (Baker and others 2011; Demaria and others 2014). In addition, several beneficial roles of senescent cells have been identified, for example in wound healing, which may have implications for adipose tissue remodeling (Demaria and others 2014). If they exist, detrimental effects of senescent cell clearance could be avoided through the use of strategic, intermittent treatment, which is possible with senolytic therapies (Roos and others 2016). This is in contrast to SASP inhibitors such as JAK1/2 inhibitors, which would need to be continually administered to exert their effects. The high burden of senescent cells found in adipose tissue, and its role at the nexus of aging, obesity, and insulin resistance, makes adipose tissue senescence a promising target for alleviating age-related metabolic dysfunction (Palmer and others 2015; Tchkonia and others 2013b).
2.5. Ectopic lipid deposition
Aged preadipocytes are less able to differentiate and properly store lipid, leading to a spillover of toxic free fatty acids that can cause ectopic lipid deposition in sites such as the liver, muscle, and pancreas (Cartwright and others 2007; Guo and others 2007; Marcus and others 2010; Tchkonia and others 2006a). This infiltration of lipid into non-adipose tissues can cause lipotoxicity and accelerate age-related disease. For example, ectopic lipid deposition may contribute to the increased prevalence of non-alcoholic fatty liver disease with aging, in addition to other risk factors such as increased fasting glucose and serum triglycerides (Koehler and others 2012). In the pancreas, lipotoxicity can cause apoptosis of beta cells, having a significant impact on an individual’s ability to produce insulin (Unger and Zhou 2001). Intermuscular adipose tissue, which increases with aging, can contribute to declining muscle quality, a feature of sarcopenia and frailty (Delmonico and others 2009). Some have proposed that the increase in visceral adipose tissue with aging is also a type of ‘ectopic’ lipid deposition, resulting from overflow of toxic free fatty acids due to the inability of subcutaneous depots to properly store lipid due to age-related dysfunction (Jensen 2008). Another site of fat infiltration with age is the bone marrow, where adipocytes are thought to have a negative impact on hematopoiesis, which could have implications for bone marrow transplantation in patients of advanced age (Naveiras and others 2009).
2.6. Adipose-derived hormone secretion and sensitivity change with age
Prevalence of insulin resistance and type II diabetes risk increases with age, mainly due to decreased peripheral insulin sensitivity and age-related pancreatic beta cell dysfunction (Chen and others 1985; Defronzo 1979). In addition to insulin resistance, aging predisposes to resistance to other metabolic hormones, such as leptin. Leptin levels increase with aging in rodents and humans, suggesting that leptin sensitivity declines with age (Gabriely and others 2002). Old rats given recombinant leptin do not have a decrease in visceral fat, as occurred in young rats, and do not suppress leptin gene expression in adipose tissue as well as young rats (Ma and others 2002). In contrast to other adipose-derived hormones, adiponectin, which is produced by mature adipocytes, is positively correlated with metabolic health. Adiponectin improves preadipocyte differentiation and enhances insulin sensitivity (Fu and others 2005). Adiponectin levels decline with aging, but are positively correlated with longevity. For example, one study found that centenarians had higher levels of circulating adiponectin than BMI-matched young controls (Atzmon and others 2008). Adiponectin signaling has therefore been explored as a therapeutic target in obesity and diabetes (Yamauchi and Kadowaki 2013). More research is needed to determine whether modulation of adipose-derived hormones could have an impact on age-related metabolic dysfunction or other age-related diseases.
2.7. Adipose tissue miRNA processing declines with age
Increased stochasticity in gene expression with aging occurs in several tissues and may contribute to the loss of resilience, or ability to properly respond to external stressors, with age (Raj and van Oudenaarden 2008). Differential expression of one particular class of gene regulatory elements, microRNAs (miRNAs), is a generalized phenomenon in aging and has been identified in multiple tissues including adipose, brain, liver, and skeletal muscle (Pincus and others 2011). Expression of Dicer, which processes miRNAs, declines with aging in the adipose tissue of mice, the intestine (primary fat storage organ) of C. elegans, and human preadipocytes (Mori and others 2012). In mice, an adipose-specific knockout of dicer, with reductions of dicer expression in subcutaneous, perigonadal, and brown adipose tissues, caused lipodystrophy. These mice exhibited decreased white adipose tissue mass and “whitening” of the brown adipose tissue, as well as inflammation and insulin resistance (Mori and others 2014). Remarkably, dicer was also found to be downregulated in patients with HIV, who exhibit a similar partial lipodystrophy syndrome. Caloric restriction, which extends lifespan, prevented age-related downregulation of dicer, and knockdown of dicer led to premature senescence in murine preadipocytes (Mori and others 2012). Downregulation of miRNAs may impair the ability of adipose tissue to respond to metabolic stress or fluctuation and may play a role in age-related metabolic dysfunction. Therefore adipose miRNA processing may represent a useful target for drugs that modulate fundamental aging processes and age-related metabolic disease (Mori and others 2012).
2.8. Brown and beige adipose changes with age
Although activation of brown adipose tissue is known to occur most significantly in response to cold exposure, it may also offer a defense against age-related weight gain by increasing energy expenditure. A significant amount of functional brown fat is present in adult humans in the interscapular fat depot (Cypess and others 2009; van Marken Lichtenbelt and others 2009; Virtanen and others 2009), which shares characteristics of beige fat, rather than classical brown fat (Wu and others 2012). UCP1 expression declines with aging in rodents in subcutaneous white fat, suggesting that brown-like adipose tissue function (“beige” or “brite” adipocytes) is reduced with aging (Rogers and others 2012; Tan and others 2015). Additionally, brown fat itself may become dysfunctional with age (Saito and others 2009; Yamashita and others 1999; Yoneshiro and others 2011). The absolute amount of brown adipose tissue is decreased in elderly humans, and its activation as measured by FDG-PET during cold exposure is dampened in old versus young individuals (Saito and others 2009). Whether loss of brown-like features of adipose tissue might predict the development of insulin resistance with aging is not clear. In mice, age-related decline in BAT function is prevented by dietary restriction, which is known to delay age-related dysfunction in other tissues (Valle and others 2008). Brown fat may play a role in preventing age-related dysfunction and disease, but this remains to be established (Mattson 2010).
3. Adipose Plays an Integral Role in Longevity
3.1. Interventions that improve longevity impact adipose tissue
The majority of interventions that are known to affect lifespan, including single gene mutations (e.g., in the GH/IGF-1 axis) and dietary or pharmacologic interventions (e.g., caloric restriction, metformin, rapamycin, 17α-estradiol, acarbose) affect fat tissue directly or indirectly, often through pathways related to nutrient signaling or processing. For example, growth-hormone-deficient and -resistant mice, which exhibit lifespan extension, have less ectopic lipid deposition, reduced cellular senescence in adipose tissue, and improved adipose progenitor function (Stout and others 2014). Caloric restriction, which extends lifespan in species ranging from flies to primates, acts partially through a decrease in total adipose tissue mass, in addition to intracellular mechanisms within adipose tissue such as decreasing inflammation, increasing autophagy and DNA repair mechanisms, reducing cellular senescence, and preventing age-related changes in gene expression (Fontana and Klein 2007; Linford and others 2007). 17α-estradiol, which has lifespan extension effects in male mice, reduces inflammation in visceral adipose and decreases visceral adiposity (Stout and others 2016). Metformin, which causes modest yet significant lifespan extension in rodents and may have lifespan-extending effects in humans, reduces body mass in humans mainly through a decrease in adipose tissue (Martin-Montalvo and others 2013; Stumvoll and others 1995). The effects of metformin on lifespan in humans have recently been demonstrated by a surprising increase in survival of diabetic patients treated with metformin beyond that of age-matched, non-diabetic controls (Bannister and others 2014). However, these data should be interpreted in light of a 4–9% rate of undiagnosed type II diabetes in the general population over 65, the onset of which can occur years before symptoms emerge or a diagnosis is made (Harris and others 1992; Menke and others 2015). In sum, these examples indicate that adipose tissue is a useful target for the development of therapies to extend healthspan and alleviate or delay age-related disease.
3.2. Adipose-specific interventions affect longevity
In lower animals, lifespan-extending mutations that are restricted to adipose tissue can confer the same lifespan extension as whole-organism mutations (Katic and others 2007). Other than the brain, adipose tissue is arguably the only tissue in which organ-specific interventions extend lifespan. For example, reduction of insulin/insulin-like growth factor signaling specifically in the fat body of female Drosophila extends lifespan (Giannakou and others 2004). Fat-specific insulin receptor knockout (FIRKO) mice are leaner and exhibit a significant lifespan extension, as also occurs in whole-body insulin receptor knockout animals (GIRKO). Because FIRKO mice have similar food intake to WT mice, this suggests that adiposity itself has some effect on longevity (Bluher and others 2003). Bariatric surgery, which causes caloric restriction and reduction in adipose tissue mass, reduces mortality in severely obese individuals (Sjostrom and others 2007). Interestingly, it does not seem that surgical removal of adipose tissue in humans has the same benefits for metabolism as reduction of adipose tissue by other methods such as caloric restriction or exercise (Klein and others 2004). However, these experiments have been focused on removal of subcutaneous fat by liposuction. The effects of removing adipose from different depots, such as visceral adipose tissue, may have different effects. For example in rats, selective surgical removal of visceral adipose tissue increased median and maximum lifespan (Muzumdar and others 2008). Removal of omental adipose also showed beneficial effects on insulin sensitivity in healthy dogs (Lottati and others 2009). Experiments to remove omental adipose tissue in humans were conducted in obese, diabetic individuals undergoing gastric bypass surgery and did not find additional beneficial effect on metabolic health (Fabbrini and others 2010; Herrera and others 2010). However, it may be possible that removal of visceral adipose tissue would be beneficial in different contexts, for example in aging or before the development of diabetes (Tchkonia and others 2013a). Therefore more research is needed to determine whether visceral adipose tissue is a useful target for interventions to alleviate age-related metabolic diseases in humans (Huffman and Barzilai 2009). Similarly, little is known about the effects that interventions to reduce adipose tissue mass or create negative energy balance, such as exercise, bariatric surgery, or calorie restriction, may have on markers of aging, for example cellular senescence. Research is underway to address these questions.
4. Obesity: Accelerated Aging?
4.1. Markers of aging increase in obesity
Several changes seen in aging adipose tissue also occur in the setting of obesity. For example, plasma and adipose tissue from obese mice exhibit increased oxidative stress compared to lean mice, and systemic markers of oxidative stress are elevated in obese humans (Furukawa and others 2004). Adipose tissue from obese individuals also contains an increased burden of senescent cells compared to lean age-matched controls (Minamino and others 2009; Tchkonia and others 2010). In addition, telomere shortening is found in white blood cells and in subcutaneous adipose tissue from obese patients (Valdes and others 2005). As in aging, chronic, low-grade sterile inflammation of adipose tissue develops in obesity, with elevated production of adipokines and decreased adiponectin levels (Fernandez-Real and others 2003). Brown adipose UCP1 expression also decreases in obesity and is lower in obese humans with diabetes than obese humans without diabetes (Timmons and Pedersen 2009). More research is needed to determine the relationship of these markers of aging to the development of obesity, namely to understand whether they have a pathogenic role in obesity, or whether they are markers of established disease. Another interesting question is whether or not markers of aging, for example senescent cell numbers, are lower in individuals with “healthy” obesity versus obese individuals with metabolic syndrome. Efforts to understand aging mechanisms in adipose tissue may inform strategies to improve outcomes in obesity, and vice versa.
4.2. Obesity predisposes to age-related diseases including cancer
Age is the leading risk factor for most chronic diseases, including dementia, cardiovascular disease, type II diabetes, and cancer, even in lean individuals. Age-related diseases are more prevalent in obese individuals at younger ages than lean individuals, suggesting that obesity predisposes to age-related disease and is in some ways a state of premature aging. Might adipose tissue dysfunction, seen in both aging and obesity, be the common denominator? An increased understanding of adipose tissue dysfunction with aging could help in understanding the predisposition to age-related diseases in obesity, and in identifying strategies to prevent these comorbidities.
As in aging, there is a significantly increased risk of cancer deaths in obesity, and this is not due to an increase in hormonally regulated cancers alone (De Pergola and Silvestris 2013; Samanic and others 2004; Wolk and others 2001). Rather, it is due to a general increase in age-related cancers. Individuals with a BMI over 40 have over 50 percent higher cancer death rates than their lean counterparts, and 14–20 percent of cancer deaths in individuals over 50 years old are estimated to be due to obesity (Calle and others 2003). Obesity-induced inflammation in adipose tissue may be one driver of cancer risk, with certain adipokines (e.g., CCL2, VEGF, IL-6, and IL-8) serving as chemoattractants that enhance migration of tumor cells and support metastasis (Gilbert and Slingerland 2013). In addition, senescent cells, which increase in aging and obesity, are known to potentiate tumorigenesis of epithelial cells, likely mediated by SASP components (Laberge and others 2015; Parrinello and others 2005; Tchkonia and others 2010). These examples highlight how fundamental aging mechanisms that occur with greater frequency in obesity could help to drive tumorigenesis.
Interestingly, calorie restriction, which is known to decrease tumor incidence in experimental animals, was unable to affect tumor incidence when hunger signals were dampened by blocking neuroendocrine pathways (Minor and others 2011). This hints that the relationship of nutrient intake and cancer incidence is complicated and may be affected more by the post-absorptive state than has been previously appreciated. Accelerated aging in obesity may not be solely due to the effects of nutrient excess and body composition, but may also be affected by mechanisms in place during the post-absorptive state.
5. Treating Age-Related Adipose Tissue Dysfunction
5.1. Targeting age-related adipose tissue dysfunction in type II diabetes
Experimental strategies to target adipose tissue-specific mechanisms such as macrophage infiltration and adipokine secretion have shown some efficacy in improving metabolic function in mouse models of diabetes (Kanda and others 2006; Lumeng and others 2007; Okada-Iwabu and others 2013). Senescent cell burden is increased in the adipose tissue of diabetic patients, and senescent cells have been identified as a potentially useful therapeutic target in type II diabetes, especially in the setting of novel senolytic agents (Palmer and others 2015; Tchkonia and others 2013b; Xu and others 2015a; Zhu and others 2015a; Zhu and others 2015b). On the other hand, some of the most effective therapeutics for diabetes, especially metformin, have significant effects on fundamental aging mechanisms, and show lifespan-extending effects in rodents (Martin-Montalvo and others 2013). This impact on longevity may also be present in humans as discussed previously (Bannister and others 2014). Treatment of age-related adipose tissue dysfunction may impact diabetes in several ways, for example prevention of lipotoxicity by improving adipogenic capacity and lipid storage, or improvement of peripheral insulin sensitivity by reducing pro-inflammatory adipokine release.
5.2. Impact of aging on adipose-based regenerative strategies
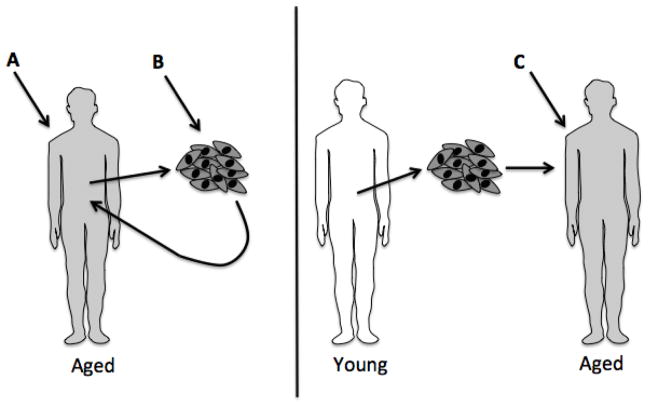
Adipose-derived progenitors have been increasingly utilized in regenerative medicine strategies, and could overtake bone marrow-derived stem cells (BMSCs) as an abundant source of progenitor cells (Strioga and others 2012; Zuk and others 2002). Adipose-derived mesenchymal stem cells (ADSCs) have been suggested to be superior to BMSCs in some orthopedic applications (Wyles and others 2015). However, most studies of this type have been conducted in subjects less than 60 years of age. It is possible that effects of aging may complicate this picture, and that strategies to mitigate effects of aging on these cells will need to be employed to optimize their use in older individuals. Progenitors isolated from different adipose depots within the same individual have different properties, and these properties can be affected by aging (Schipper and others 2008; Tchkonia and others 2013b). Aging is a significant hurdle for the advancement of regenerative medicine strategies, because many patients needing this type of intervention will be members of the growing elderly population. It is possible that age-modifying treatments will need to be given to patients before isolating progenitor cells, or to cells in vitro before being returned to the patient (Fig. 2). In the context of allogeneic applications, transplantation of young or revitalized progenitor cells into old recipients may also have limited efficacy due to the effects of an aging microenvironment (Conboy and others 2005).
Figure 2. Possible points of intervention for adipose-derived stem cell therapies in aged individuals.
Adipose-derived stem cells are becoming increasingly utilized in regenerative medicine. Many patients who would benefit from such therapies are elderly, and the effects of aging on adipose tissue and progenitor function may reduce efficacy of these therapies. This may be due to both inherent dysfunction of isolated progenitor cells and the aged microenvironment of the recipient. Strategies to mitigate effects of aging on these cells may need to be used to optimize their use in older individuals. Points at which such strategies could be employed include (A) before isolating progenitor cells, (B) in vitro before returning cells to the patient, and (C) in allogeneic applications, administration of age-modifying therapy to the recipient before transplantation of young or revitalized progenitor cells.
For example, TGFβ family members interfere with stem cell function and have been implicated in age-related progenitor dysfunction (Carlson and others 2009; Conboy and others 2015). A member of the TGFβ superfamily, activin A, increases in plasma with aging, and is secreted by senescent adipose progenitor cells. Blocking activin A in vitro using neutralizing antibodies or receptor blockers improves adipogenic potential of adipose-derived progenitor cells (Xu and others 2015a). JAK 1/2 inhibitors prevent activin A secretion by senescent cells, and reduce circulating activin A in vivo, as does senescent cell elimination in old INK-ATTAC mice (Xu and others 2015a). JAK 1/2 inhibitors restored fat mass and insulin sensitivity in old mice, and also restored adipogenic capacity of adipose-derived progenitors to express transcription factors necessary for differentiation (Xu and others 2015a). Thus, TGFβ family members secreted by senescent cells contribute to adipose-derived progenitor dysfunction, and this is amenable to pharmacologic intervention. Administration of therapies that target age-related dysfunction, for example senolytics, to patients receiving transplanted cells may mitigate host effects that could dampen the regenerative potential of transplanted cells (Fig. 2) (Kirkland and Tchkonia 2014; Zhu and others 2015a; Zhu and others 2015b).
Cell-free regenerative strategies represent a unique opportunity to circumvent these aging “seed vs. soil” barriers by distilling the therapeutic potential of progenitor cells into deliverables not containing cells. For example, secretome components or exosomes could be administered, avoiding the use of cells isolated from aging patients (Katsuda and others 2013; Ranganath and others 2012). More investigation of these regenerative strategies is necessary to determine their efficacy in age-related disease.
Significant work is needed in preclinical and human studies to characterize ADSCs isolated from aged and obese individuals. Aging effects on progenitor quality require more study, and potential therapeutics to mitigate these effects to enhance regenerative potential should be carefully tested in preclinical models. Understanding the effects that aging has on regenerative capacity of progenitor cells as well as the potential of a recipient to garner benefit from these therapies will be essential for application of regenerative medicine approaches to the elderly population.
6. Looking Forward: Adipose Tissue as a Therapeutic Target in Aging
Many classical aging mechanisms, for example cellular senescence, chronic inflammation, and mitochondrial dysfunction, occur in adipose tissue. Therefore, emerging interventions that target fundamental aging mechanisms should have an effect on adipose tissue. This opens the possibility of using adipose tissue as an indicator of the efficacy of such therapies. In addition, adipose tissue itself may prove to be a worthwhile target for novel technologies that target fundamental aging mechanisms. Therapies that extend lifespan and target fundamental aging mechanisms tend to have major effects on adipose tissue. Conversely, it may be possible to use our knowledge of adipose tissue aging in order to design therapies that specifically target aging mechanisms that operate in fat. Because adipose tissue function is so intricately linked to insulin sensitivity and inflammation, targeting adipose tissue aging could be a new frontier for therapies to combat age-related type II diabetes, metabolic syndrome, and their complications. Frailty and age-related fat loss could be other applications of therapies that specifically target fundamental aging processes in adipose tissue. Better understanding of adipose tissue aging and its effects on progenitor cell function could also have broad implications for regenerative medicine. Thus, adipose tissue aging is valuable for the study of basic aging mechanisms, and is a potent therapeutic target for the development of new therapies to combat effects of aging and age-related disease.
Highlights.
Adipose tissue is a highly relevant organ for the study of aging.
Age-related changes occur in adipose tissue.
Adipose tissue function impacts lifespan and healthspan.
Obesity and aging have shared mechanisms and effects on adipose tissue.
Improved knowledge of adipose tissue aging could impact diabetes treatments and regenerative medicine.
Acknowledgments
This work was supported by NIH grants AG13925 (JLK), AG041122 (JLK), AG31736 (Project 4: JLK), AG044396 (JLK), DK50456 (JLK), and AG46061 (AKP), CTSA Grant Number TL1 TR000137 from the National Center for Advancing Translational Science (NCATS), a Glenn/AFAR Scholarship for Research in the Biology of Aging (AKP), the Connor Group, and the Glenn, Ted Nash Long Life, and Noaber Foundations (JLK). AKP thanks the Mayo Clinic Medical Scientist Training Program for providing an excellent training environment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison DB, Faith MS, Heo M, Kotler DP. Hypothesis concerning the U-shaped relation between body mass index and mortality. American journal of epidemiology. 1997;146:339–349. doi: 10.1093/oxfordjournals.aje.a009275. [DOI] [PubMed] [Google Scholar]
- Asterholm IW, McDonald J, Blanchard PG, Sinha M, Xiao Q, Mistry J, Rutkowski JM, Deshaies Y, Brekken RA, Scherer PE. Lack of “immunological fitness” during fasting in metabolically challenged animals. Journal of lipid research. 2012;53:1254–1267. doi: 10.1194/jlr.M021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Pollin TI, Crandall J, Tanner K, Schechter CB, Scherer PE, Rincon M, Siegel G, Katz M, Lipton RB, Shuldiner AR, Barzilai N. Adiponectin levels and genotype: a potential regulator of life span in humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63:447–453. doi: 10.1093/gerona/63.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Yang XM, Muzumdar R, Ma XH, Gabriely I, Barzilai N. Differential gene expression between visceral and subcutaneous fat depots. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2002;34:622–628. doi: 10.1055/s-2002-38250. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederlander NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, Eberhardt NL, Terzic A, van Deursen JM. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nature cell biology. 2008;10:825–836. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes, obesity & metabolism. 2014 doi: 10.1111/dom.12354. [DOI] [PubMed] [Google Scholar]
- Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. Journal of the American Geriatrics Society. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science (New York, NY) 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bonora E, Del Prato S, Bonadonna RC, Gulli G, Solini A, Shank ML, Ghiatas AA, Lancaster JL, Kilcoyne RF, Alyassin AM, et al. Total body fat content and fat topography are associated differently with in vivo glucose metabolism in nonobese and obese nondiabetic women. Diabetes. 1992;41:1151–1159. [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S adults. The New England journal of medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Experimental gerontology. 2007;42:463–471. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso G, McNurlan MA, Mileva I, Zemlyak A, Mynarcik DC, Gelato MC. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism: clinical and experimental. 2013;62:337–340. doi: 10.1016/j.metabol.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews Immunology. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Bergman RN, Pacini G, Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. The Journal of clinical endocrinology and metabolism. 1985;60:13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthetic surgery journal/the American Society for Aesthetic Plastic surgery. 2006;26:S4–9. doi: 10.1016/j.asj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Rebo J. Systemic Problems: A perspective on stem cell aging and rejuvenation. Aging. 2015;7:754–765. doi: 10.18632/aging.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS biology. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nature genetics. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. Journal of obesity. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH, Health A. Body Longitudinal study of muscle strength, quality, and adipose tissue infiltration. The American journal of clinical nutrition. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 2000;26:1107–1112. [PubMed] [Google Scholar]
- Jerschow E, SA, Barzilai N, Rosenstreich D. Macrophages accumulation in visceral and subcutaneous adipose tissue correlates with age. J Allergy Clin Immunol. 2007;119:S179. [Google Scholar]
- Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Lopez-Bermejo A, Casamitjana R, Ricart W. Novel interactions of adiponectin with the endocrine system and inflammatory parameters. The Journal of clinical endocrinology and metabolism. 2003;88:2714–2718. doi: 10.1210/jc.2002-021583. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Kaye SA, Sellers TA, Hong CP, Cerhan JR, Potter JD, Prineas RJ. Body fat distribution and 5-year risk of death in older women. Jama. 1993;269:483–487. [PubMed] [Google Scholar]
- Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. The American journal of medicine. 2003;115(Suppl 8A):42S–48S. doi: 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. Jama. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. Journal of lipid research. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51:1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- Garg SK, Delaney C, Shi H, Yung R. Changes in adipose tissue macrophages and T cells during aging. Critical reviews in immunology. 2014;34:1–14. doi: 10.1615/critrevimmunol.2013006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavi S, Feiner JJ, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Limb fat to trunk fat ratio in elderly persons is a strong determinant of insulin resistance and adiponectin levels. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62:997–1001. doi: 10.1093/gerona/62.9.997. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science (New York, NY) 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annual review of medicine. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. The American journal of clinical nutrition. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J, Wang T, Wong S, Cartwright A, Hegardt FG, Corkey BE, Kirkland JL. Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. American journal of physiology Endocrinology and metabolism. 2007;292:E1041–1051. doi: 10.1152/ajpendo.00557.2006. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends in endocrinology and metabolism: TEM. 2015;26:193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Hamm JK, el Jack AK, Pilch PF, Farmer SR. Role of PPAR gamma in regulating adipocyte differentiation and insulin-responsive glucose uptake. Annals of the New York Academy of Sciences. 1999;892:134–145. doi: 10.1111/j.1749-6632.1999.tb07792.x. [DOI] [PubMed] [Google Scholar]
- Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes care. 1992;15:815–819. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- Herrera MF, Pantoja JP, Velazquez-Fernandez D, Cabiedes J, Aguilar-Salinas C, Garcia-Garcia E, Rivas A, Villeda C, Hernandez-Ramirez DF, Davila A, Zarain A. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes care. 2010;33:1413–1418. doi: 10.2337/dc09-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochimica et biophysica acta. 2009;1790:1117–1123. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman DM, Barzilai N. Contribution of adipose tissue to health span and longevity. Interdisciplinary topics in gerontology. 2010;37:1–19. doi: 10.1159/000319991. [DOI] [PubMed] [Google Scholar]
- Idoate F, Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, Marcellan T, Ruiz de Gordoa A, Rodriguez-Manas L, Bastarrika G, Marques MC, Martinez-Velilla N, Vicente-Campos D, Izquierdo M. Adipose tissue compartments, muscle mass, muscle fat infiltration, and coronary calcium in institutionalized frail nonagenarians. European radiology. 2015;25:2163–2175. doi: 10.1007/s00330-014-3555-5. [DOI] [PubMed] [Google Scholar]
- Itoh M, Suganami T, Hachiya R, Ogawa Y. Adipose tissue remodeling as homeostatic inflammation. International journal of inflammation. 2011;2011:720926. doi: 10.4061/2011/720926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD. Role of body fat distribution and the metabolic complications of obesity. The Journal of clinical endocrinology and metabolism. 2008;93:S57–63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, Kirkland JL. Altered expression of C/EBP family members results in decreased adipogenesis with aging. American journal of physiology Regulatory, integrative and comparative physiology. 2001;280:R1772–1780. doi: 10.1152/ajpregu.2001.280.6.R1772. [DOI] [PubMed] [Google Scholar]
- Karakasilioti I, Kamileri I, Chatzinikolaou G, Kosteas T, Vergadi E, Robinson AR, Tsamardinos I, Rozgaja TA, Siakouli S, Tsatsanis C, Niedernhofer LJ, Garinis GA. DNA damage triggers a chronic autoinflammatory response, leading to fat depletion in NER progeria. Cell metabolism. 2013;18:403–415. doi: 10.1016/j.cmet.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59:89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging cell. 2007;6:827–839. doi: 10.1111/j.1474-9726.2007.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, Steinhauser ML. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell metabolism. 2014;20:1049–1058. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat tissue function. Journal of the American Geriatrics Society. 1997;45:959–967. doi: 10.1111/j.1532-5415.1997.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T. Clinical Strategies and animal models for developing senolytic agents. Experimental gerontology. 2014 doi: 10.1016/j.exger.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Experimental gerontology. 2002;37:757–767. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of neurology. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. The New England journal of medicine. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- Koehler EM, Schouten JN, Hansen BE, van Rooij FJ, Hofman A, Stricker BH, Janssen HL. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. Journal of hepatology. 2012;57:1305–1311. doi: 10.1016/j.jhep.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nature cell biology. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowa N, Trieu N, Flehmig G, Lohmann T, Schon MR, Dietrich A, Zeplin PH, Langer S, Stumvoll M, Bluher M, Kloting N. Telomere length differences between subcutaneous and visceral adipose tissue in humans. Biochemical and biophysical research communications. 2015;457:426–432. doi: 10.1016/j.bbrc.2014.12.122. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Beyer RP, Gollahon K, Krajcik RA, Malloy VL, Demas V, Burmer GC, Rabinovitch PS. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging cell. 2007;6:673–688. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Lottati M, Kolka CM, Stefanovski D, Kirkman EL, Bergman RN. Greater omentectomy improves insulin sensitivity in nonobese dogs. Obesity. 2009;17:674–680. doi: 10.1038/oby.2008.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. Journal of immunology. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XH, Muzumdar R, Yang XM, Gabriely I, Berger R, Barzilai N. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. The journals of gerontology Series A, Biological sciences and medical sciences. 2002;57:B225–231. doi: 10.1093/gerona/57.6.b225. [DOI] [PubMed] [Google Scholar]
- Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. International journal of obesity. 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- Mansilla E, Diaz Aquino V, Zambon D, Marin GH, Martire K, Roque G, Ichim T, Riordan NH, Patel A, Sturla F, Larsen G, Spretz R, Nunez L, Soratti C, Ibar R, van Leeuwen M, Tau JM, Drago H, Maceira A. Could metabolic syndrome, lipodystrophy, and aging be mesenchymal stem cell exhaustion syndromes? Stem cells international. 2011;2011:943216. doi: 10.4061/2011/943216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. The journal of nutrition, health & aging. 2010;14:362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nature communications. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Perspective: Does brown fat protect against diseases of aging? Ageing research reviews. 2010;9:69–76. doi: 10.1016/j.arr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereeuw-Hautier J, Wilson LC, Mohammed S, Smallwood D, Shackleton S, Atherton DJ, Harper JI. Hutchinson-Gilford progeria syndrome: clinical findings in three patients carrying the G608G mutation in LMNA and review of the literature. The British journal of dermatology. 2007;156:1308–1314. doi: 10.1111/j.1365-2133.2007.07897.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism: clinical and experimental. 2004;53:495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. Jama. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature medicine. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- Minor RK, Lopez M, Younts CM, Jones B, Pearson KJ, Anson RM, Dieguez C, de Cabo R. The arcuate nucleus and neuropeptide Y contribute to the antitumorigenic effect of calorie restriction. Aging cell. 2011;10:483–492. doi: 10.1111/j.1474-9726.2011.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MA, Raghavan P, Thomou T, Boucher J, Robida-Stubbs S, Macotela Y, Russell SJ, Kirkland JL, Blackwell TK, Kahn CR. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell metabolism. 2012;16:336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MA, Thomou T, Boucher J, Lee KY, Lallukka S, Kim JK, Torriani M, Yki-Jarvinen H, Grinspoon SK, Cypess AM, Kahn CR. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. The Journal of clinical investigation. 2014;124:3339–3351. doi: 10.1172/JCI73468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, Barzilai N. Visceral adipose tissue modulates mammalian longevity. Aging cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW, Jr, Krakoff J. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes. 2015;64:2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. Journal of cell science. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Guarente L. Molecular links between aging and adipose tissue. International journal of obesity. 2005;29(Suppl 1):S36–39. doi: 10.1038/sj.ijo.0802912. [DOI] [PubMed] [Google Scholar]
- Pincus Z, Smith-Vikos T, Slack FJ. MicroRNA predictors of longevity in Caenorhabditis elegans. PLoS genetics. 2011;7:e1002306. doi: 10.1371/journal.pgen.1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D’Agostino RB, Sr, O’Donnell CJ, Fox CS. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity. 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell stem cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Landa A, Park S, Smith RG. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging cell. 2012;11:1074–1083. doi: 10.1111/acel.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. International journal of obesity. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging cell. 2016 doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF., Jr Obesity and cancer risk among white and black United States veterans. Cancer causes & control: CCC. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Annals of plastic surgery. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66–75. doi: 10.1159/000279755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM, Swedish Obese Subjects S. Effects of bariatric surgery on mortality in Swedish obese subjects. The New England journal of medicine. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obesity research. 2003;11:104–111. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL. 17alpha-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization. The journals of gerontology Series A, Biological sciences and medical sciences. 2016 doi: 10.1093/gerona/glv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging. 2014;6:575–586. doi: 10.18632/aging.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem cells and development. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. The New England journal of medicine. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Tan CY, Virtue S, Bidault G, Dale M, Hagen R, Griffin JL, Vidal-Puig A. Brown Adipose Tissue Thermogenic Capacity Is Regulated by Elovl6. Cell reports. 2015;13:2039–2047. doi: 10.1016/j.celrep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Corkey BE, Kirkland JL. Current Views of the Fat Cell as an Endocrine Cell: lipotoxicity. Overweight and the Metabolic Syndrome: From Bench to Bedside (Endocrine Updates) 2006a [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006b;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell metabolism. 2013a;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of clinical investigation. 2013b;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature reviews Immunology. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Pedersen BK. The importance of brown adipose tissue. The New England journal of medicine. 2009;361:415–416. doi: 10.1056/NEJMc091009. author reply 418–421. [DOI] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell metabolism. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50(Suppl 1):S118–121. doi: 10.2337/diabetes.50.2007.s118. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Valle A, Guevara R, Garcia-Palmer FJ, Roca P, Oliver J. Caloric restriction retards the age-related decline in mitochondrial function of brown adipose tissue. Rejuvenation research. 2008;11:597–604. doi: 10.1089/rej.2007.0626. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. The New England journal of medicine. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wolk A, Gridley G, Svensson M, Nyren O, McLaughlin JK, Fraumeni JF, Adam HO. A prospective study of obesity and cancer risk (Sweden) Cancer causes & control: CCC. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. Journal of immunology. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyles CC, Houdek MT, Crespo-Diaz RJ, Norambuena GA, Stalboerger PG, Terzic A, Behfar A, Sierra RJ. Adipose-derived Mesenchymal Stem Cells Are Phenotypically Superior for Regeneration in the Setting of Osteonecrosis of the Femoral Head. Clinical orthopaedics and related research. 2015;473:3080–3090. doi: 10.1007/s11999-015-4385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015a;4 doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proceedings of the National Academy of Sciences of the United States of America. 2015b;112:E6301–6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Sato Y, Mori N. Difference in induction of uncoupling protein genes in adipose tissues between young and old rats during cold exposure. FEBS letters. 1999;458:157–161. doi: 10.1016/s0014-5793(99)01143-6. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell metabolism. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity. 2011;19:1755–1760. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a Novel Senolytic Agent, Navitoclax, Targeting the Bcl-2 Family of Anti-Apoptotic Factors. Aging cell. 2015a doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging cell. 2015b;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]