Abstract
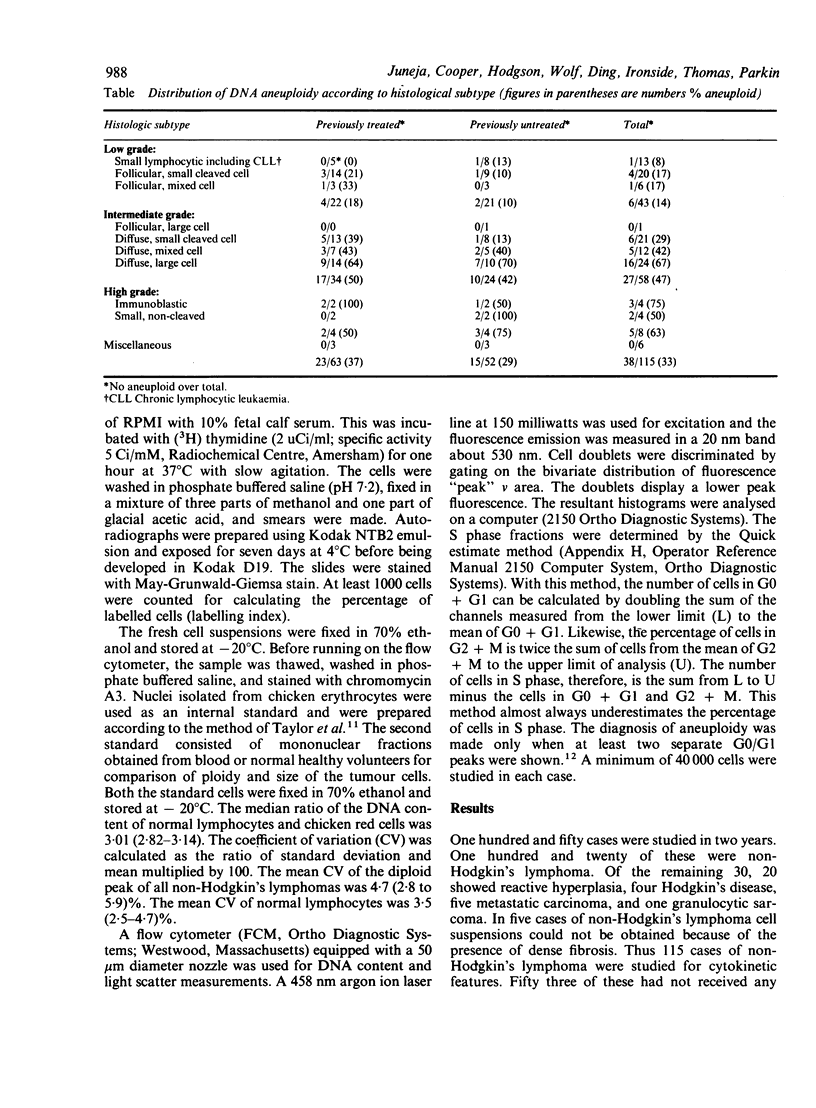
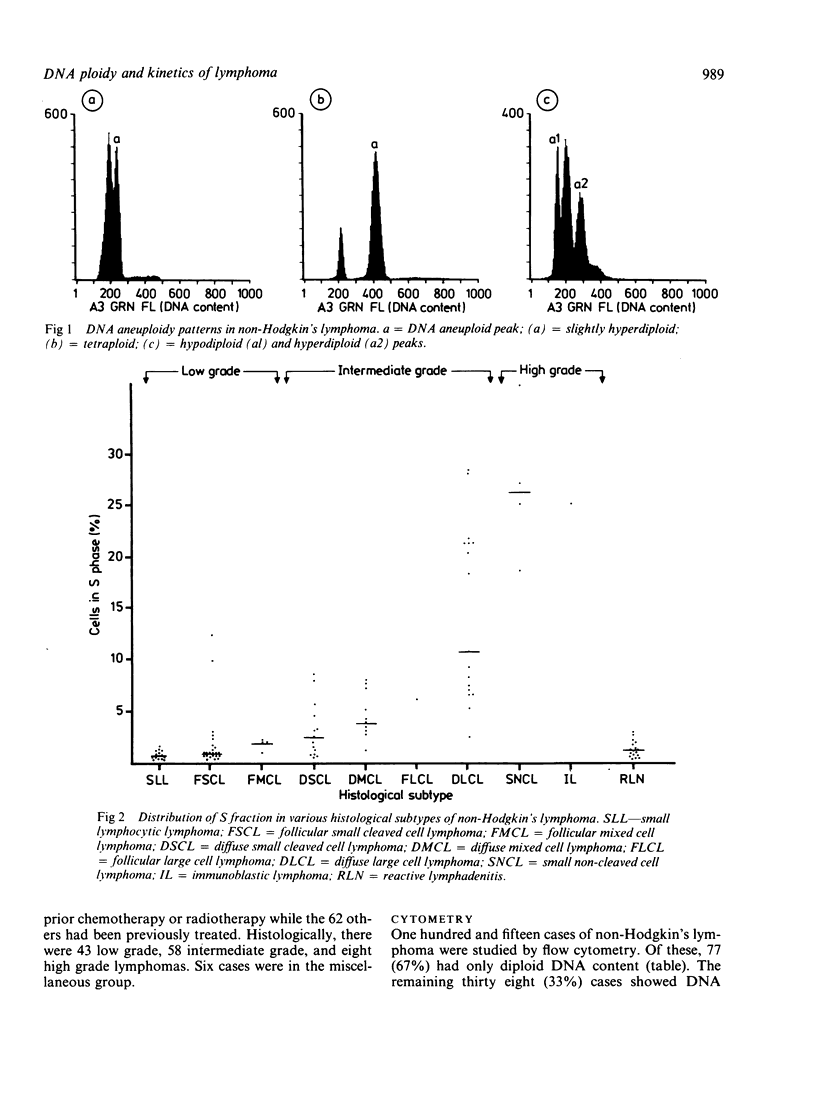
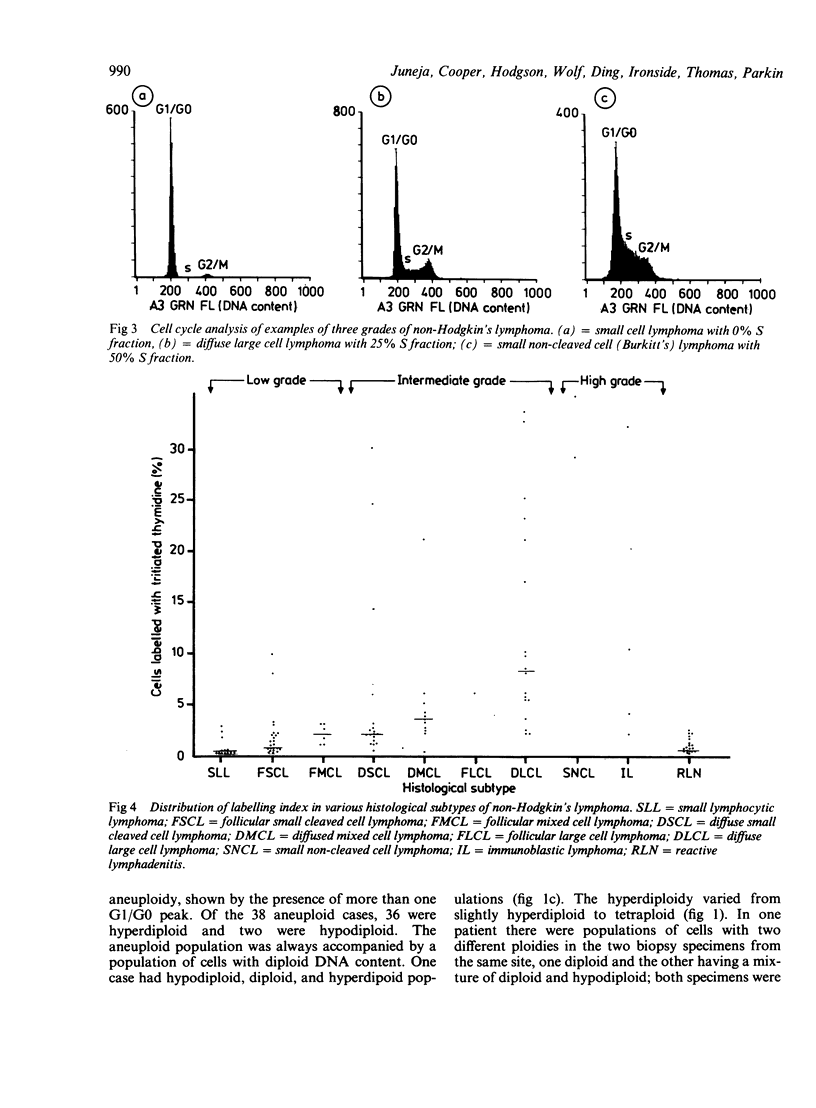
Flow cytometry studies for cellular DNA analysis were performed in 115 cases of non-Hodgkin's lymphoma, 53 of which had not received any prior chemotherapy or radiotherapy. DNA content was measured in ethanol fixed cells stained with chromomycin A3. According to the criteria of the International Working Formulation there were 43 low grade, 58 intermediate grade, and eight high grade lymphomas; six cases were in the miscellaneous group. Seventy seven (67%) had only diploid DNA content. Thirty eight (33%) showed DNA aneuploidy; 20 of these had been previously treated with chemotherapy or radiotherapy, or both. DNA aneuploidy was seen as hyperdiploidy in all cases except one, and it varied from slightly hyperdiploid to tetraploid. The incidence of aneuploidy increased significantly with increasing histological grade (p = 0.0002) and was not related to previous treatment. The low, intermediate, and high grade lymphomas had 14% (six of 43), 47% (27 of 58), and 62.5% (five of eight) cases, respectively, that showed DNA aneuploidy. The percentage of cells in S phase increased significantly with a higher histological grade (p less than 0.0001). The median S fraction in the low, intermediate, and high grade lymphomas was 1.0 (0.5 to 10)% 4 (0.4 to 35)%, and 27 (4.6-56)%, respectively. There is a significant correlation between histological grade and S fraction and the presence or absence of aneuploidy. There is heterogeneity, however, within both histological grade and a histological subtype.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlogie B., Raber M. N., Schumann J., Johnson T. S., Drewinko B., Swartzendruber D. E., Göhde W., Andreeff M., Freireich E. J. Flow cytometry in clinical cancer research. Cancer Res. 1983 Sep;43(9):3982–3997. [PubMed] [Google Scholar]
- Braylan R. C., Benson N. A., Nourse V. A. Cellular DNA of human neoplastic B-cells measured by flow cytometry. Cancer Res. 1984 Nov;44(11):5010–5016. [PubMed] [Google Scholar]
- Braylan R. C., Diamond L. W., Powell M. L., Harty-Golder B. Percentage of cells in the S phase of the cell cycle in human lymphoma determined by flow cytometry. Cytometry. 1980 Nov;1(3):171–174. doi: 10.1002/cyto.990010302. [DOI] [PubMed] [Google Scholar]
- Diamond L. W., Braylan R. C. Flow analysis of DNA content and cell size in non-Hodgkin's lymphoma. Cancer Res. 1980 Mar;40(3):703–712. [PubMed] [Google Scholar]
- Diamond L. W., Nathwani B. N., Rappaport H. Flow cytometry in the diagnosis and classification of malignant lymphoma and leukemia. Cancer. 1982 Sep 15;50(6):1122–1135. doi: 10.1002/1097-0142(19820915)50:6<1122::aid-cncr2820500616>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S., Slocum H. K., Rustum Y. M., Arbuck S. G., Pavelic Z. P., Petrelli N., Huben R. P., Pontes E. J., Greco W. R. Flow cytometric analysis of DNA aneuploidy in primary and metastatic human solid tumors. Cytometry. 1984 Jan;5(1):71–80. doi: 10.1002/cyto.990050111. [DOI] [PubMed] [Google Scholar]
- Hattori T., Hosokawa Y., Fukuda M., Sugihara H., Hamada S., Takamatsu T., Nakanishi K., Tsuchihashi Y., Kitamura T., Fujita S. Analysis of DNA ploidy patterns of gastric carcinomas of Japanese. Cancer. 1984 Oct 15;54(8):1591–1597. doi: 10.1002/1097-0142(19841015)54:8<1591::aid-cncr2820540821>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W. Application of DNA flow cytometry to paraffin-embedded archival material for the study of aneuploidy and its clinical significance. Cytometry. 1985 Jul;6(4):327–333. doi: 10.1002/cyto.990060409. [DOI] [PubMed] [Google Scholar]
- Klein F. A., Herr H. W., Whitmore W. F., Jr, Sogani P. C., Melamed M. R. An evaluation of automated flow cytometry (FCM) in detection of carcinoma in situ of the urinary bladder. Cancer. 1982 Sep 1;50(5):1003–1008. doi: 10.1002/1097-0142(19820901)50:5<1003::aid-cncr2820500531>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Shackney S. E., Levine A. M., Fisher R. I., Nichols P., Jaffe E., Schuette W. H., Simon R., Smith C. A., Occhipinti S. J., Parker J. W. The biology of tumor growth in the non-Hodgkin's lymphomas. A dual parameter flow cytometry study of 220 cases. J Clin Invest. 1984 Apr;73(4):1201–1214. doi: 10.1172/JCI111306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srigley J., Barlogie B., Butler J. J., Osborne B., Blick M., Johnston D., Kantarjian H., Reuben J., Batsakis J., Freireich E. J. Heterogeneity of non-Hodgkin's lymphoma probed by nucleic acid cytometry. Blood. 1985 May;65(5):1090–1096. [PubMed] [Google Scholar]
- Taylor I. W., Milthorpe B. K. An evaluation of DNA fluorochromes, staining techniques, and analysis for flow cytometry. I. Unperturbed cell populations. J Histochem Cytochem. 1980 Nov;28(11):1224–1232. doi: 10.1177/28.11.6159392. [DOI] [PubMed] [Google Scholar]
- Wantzin G. L., Larsen J. K., Christensen I. J., Ralfkiaer E., Thomsen K. Early diagnosis of cutaneous T-cell lymphoma by DNA flow cytometry on skin biopsies. Cancer. 1984 Oct 1;54(7):1348–1352. doi: 10.1002/1097-0142(19841001)54:7<1348::aid-cncr2820540719>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Wijkström H., Granberg-Ohman I., Tribukait B. Chromosomal and DNA patterns in transitional cell bladder carcinoma. A comparative cytogenetic and flow-cytofluorometric DNA study. Cancer. 1984 Apr 15;53(8):1718–1723. doi: 10.1002/1097-0142(19840415)53:8<1718::aid-cncr2820530817>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]


