Abstract
Parkinson’s disease is a major age-related neurodegenerative disorder. As the classical disease-related motor symptoms are associated with the loss of dopamine-generating cells within the substantia nigra, tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of catecholamines has become an important target in the development of Parkinson’s disease drug candidates, with the focus to augment TH levels or its activity. By contrast, TH inhibitors are of relevance in the treatment of conditions associated with catecholamine over-production, as occurs in pheochromocytomas. To aid characterizing new drug candidates, a molecular docking study of catecholamines and a novel hypothetical compound [4-(propan-2-yl) phenyl]carbamic acid (PPCA) with TH is described. Docking was performed using Autodock4.2 and results were analyzed using Chimera1.5.2. All the studied ligands were found to bind within a deep narrow groove lined with polar aromatic and acidic residues within TH. Our results corroborated a ‘hexa interacting amino acids unit’ located in this deep narrow groove crucial to the interaction of PPCA and the studied catecholamines with TH, whereby the ‘His361-His336 dyad’ was found to be even more crucial to these binding interactions. PPCA displayed a binding interaction with human TH that was comparable to the original TH substrate, L-tyrosine. Hence PPCA may warrant in vitro and in vivo characterization with TH to assess its potential as a candidate therapeutic.
Keywords: Catecholamine, docking study, novel inhibitor, Parkinson’s disease, tyrosine hydroxylase
BACKGROUND
Parkinson’s disease (PD) is a common age-related progressive neurodegenerative disorder that manifests as an essentially sporadic condition, and afflicts some 1–3% of the population over 65 years of age. It is characterized by progressive loss of muscle control that leads to tremor of the limbs and head while at rest, bradykinesia, rigidity, slowness of movement, and impaired balance. The abnormal motor behavior observed in PD patients primarily is consequent to a progressive loss of dopaminergic neurons underpinned by degeneration within the substantia nigra pars compacta of the brain, resulting in diminished striatal dopamine production [1, 2]. PD development appears to be a consequence of a complex amalgamation of genetic and environmental factors, equally contributing to the onset of disease; while these can be very different across individuals, a common pathogenic cascade of molecular events ensues [3]. Pharmacological and surgical therapies are currently available that can alleviate some of the cardinal symptoms; however, such interventions present their own side effects, some potentially serious, and generally gradually lose their efficacy over time [4].
As tyrosine hydroxylase (TH) catalyses the formation of L-dihydroxyphenylalanine (L-DOPA), the rate limiting step in the biosynthesis of dopamine, PD may be simplistically viewed as a TH-deficiency syndrome of the striatum. In this regard, TH not only provides a potential marker to follow PD development and progression, with relatively normal locomotor activity often persisting until a major loss of TH (>70%) occurs due to the plasticity and compensatory mechanisms available with brain, but TH is additionally a primary target of PD treatment strategies. In a search for potential drug candidates, the interaction pattern between TH and both natural endogenous (catecholamines) and unnatural ligands can be characterized by in silico molecular docking analyses. Such docking provides a basis to support predictions of ligand conformations inside the active site of the target and, additionally, affords insight in relation to interactions between the receptor and ligand [5]. In the present study, molecular docking of human TH, a tetramer containing four identical subunits and a Zn+2-dependent enzyme that catalyzes the conversion of tyrosine to L-DOPA, was performed with a novel potential inhibitor, [4-(propan-2-yl) phenyl]carbamic acid (PPCA), and the catecholamines, L-tyrosine, L-DOPA, norepinephrine and epinephrine. This was done to ascertain the exact residues involved in the interaction between TH and these agents and the role of the metal ion. To support and validate our investigation we additionally performed docking studies of of these ligands with rat TH Fe+3 dependent tetrameric protein, which is the closest ortholog having strong sequence and structural similarities to the human target (Fig. 1). Whereas as a loss of TH-positive dopaminergic cells is a cardinal hallmark of PD, tumors originating from adrenal gland chromaffin cells, pheochromocytomas, express excess TH and thereby overproduce catecholamines to induce substantially different symptoms (elevated blood pressure and heart rate, panic attacks and diaphoresis) [6, 7]. Hence TH ligands have a number of potential pharmacological uses, depending on their mode of interaction.
Fig. 1.
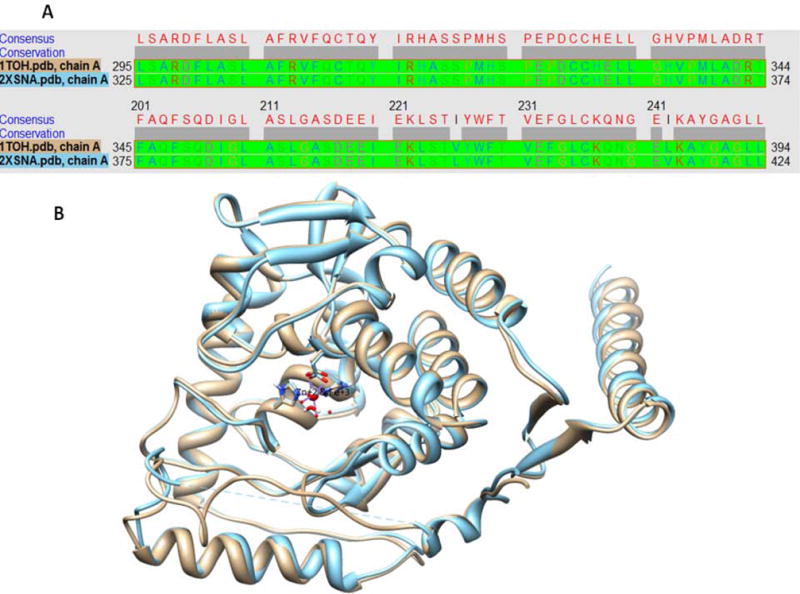
(A) Sequence alignment of chain A of rat TH (1TOH) with human TH (2XSNA) (B) Structural superimposition of rat TH with human TH showing high structural similarity.
METHODS
Structural and sequence comparison and molecular docking study was performed on a DELL Inspiron Core i3 work station under open source Ubuntu 11.10, and the molecular structures of PPCA, L-tyrosine, L-DOPA, epinephrine and norepinephrine were drawn using CHEMOFFICE2010. The energy minimization and geometry optimization were performed by MOPAC2009 using a RM1 semi-empirical method. Autodock4.2 was used for flexible docking [8]. Results were analyzed using Chimera1.5.2 [9]. The receptor structure (TH) was retrieved from the Protein Data Bank (PDB) having PDB ID “2XSN”. Prior to docking, the PDB structure, 2XSN, was subjected to structural alignment with rat TH (PDB ID: 1TOH) to confirm the active chain. 2XSN chain A, which was confirmed to be the active chain, was used for docking studies. Detailed docking and grid parameters are presented in Table 1.
Table 1.
The Following Grid and Docking Parameters Were Used while Performing Docking
| Grid Parameters | Docking Parameters | ||
|---|---|---|---|
| Spacing | 0.631 | Translation step | 2 |
| Grid Center | 61.286X | Quatemion step | 50 |
| 4.319Y | Torsion step | 50 | |
| 20.957Z | Torsional degrees of freedom | 6 | |
| Rate of Gene Mutation | 0.02 | ||
| Rate of Crossover | 0.8 | ||
| Cluster Translation Step | 2 | ||
RESULTS AND DISCUSSION
TH is a homotetramer comprising four chains, A to D [10]. Initially we ran open docking for all the chains of human TH and did not obtain valid docking results. Later, we performed sequence and structural alignment of human TH (PDB: 2XSN) with rat TH (PDB: 1TOH) and found an identity of 89.25% along with structural similarity of all chains having a root mean square deviation (RMSD) < 0.54 angstrom, as shown in Fig. (1). To obtain an optimal docking conformation we undertook 100 runs of docking for the five characterized ligands (PPCA, L-tyrosine, L-DOPA, norepinephrine and epinephrine) individually with human TH. The chemical structures of these ligands are shown in Fig. (2). A grid was set on the complete receptor to find the free binding site. Conserved water molecules were duly added to the binding pocket in order to mimic the in vivo environment prior to docking. Details concerning interacting sites, in terms of system enthalpy and distance from reference point, are presented in Table 2. The remaining parameters were set to default Autodock values, and a genetic algorithm was used as the main searching protocol. All the studied ligands were found to bind within a deep narrow groove lined with polar aromatic and acidic residues within the TH receptor in the presence of metal ions. Six amino acid residues located in this groove, namely Leu 324, Phe 330, His 361, His 366, Tyr 401 and Glu 406, were found to be crucial to the interaction of both PPCA and the studied catecholamines with human as well as rat TH. This finding is in accordance with Goodwill et al., 1997 [11]. Norepinephrine provided the best binding interaction with human TH, with an estimated free energy (ΔG) of −8.52kcal/mol and an inhibition constant (Ki) of 0.569 μM. Furthermore, this analysis demonstrated that the presence of Zn+2 within human TH and Fe+3 within rat TH is crucial to the binding of the studied ligands within the enzyme active site. Fig. (3, constructed using LIGPLOT) clearly depicts the importance of metal ions within the active site for ligand binding in rat TH. In Fig. (3B, D), the Fe+3 metal ion is present where L-DOPA and PPCA are interacting with rat TH in the active site. At this very location, the ‘His331-His336 dyad’ additionally appears prominent. However, in Fig. (3A, D), albeit some interaction arises, this is determined to be weak as it occurs at a surface groove. Figs. (4, 5) depict a detailed interaction of the catecholamine and PPCA ligands with the human TH within the deep narrow groove.
Fig. 2.
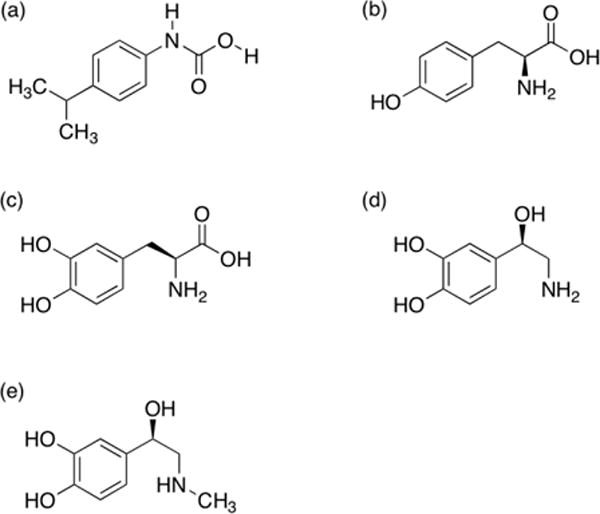
Chemical structures of ligands: (a) the novel potential inhibitor [4-(propan-2-yl) phenyl]carbamic acid, (b) L-tyrosine, (c) L-DOPA, (d) norepinephrine and (e) epinephrine. Each was docked with receptor tyrosine hydroxylase “2XSN”.
Table 2.
Bioinformative Details of the Studied Interactions
| Ligand/Properties | [4-(Propan-2-yl) Phenyl]Carbamic Acid | L-Tyrosine | L-DOPA | Norepinephrine | Epinephrine |
|---|---|---|---|---|---|
| Number of conformations in best cluster | 3 | 13 | 14 | 92 | 22 |
| RMSD from reference structure (angstrom) | 63.31 | 63.48 | 63.59 | 64.51 | 54.45 |
| Estimated Free Energy of Binding (kcal/mol) | −6.95 | −5.96 | −5.6 | −8.52 | −4.56 |
| Estimated Inhibition Constant, Ki (μM) | 8 | 42.74 | 79.17 | 0.569 | 457.64 |
| Final Intermolecular Energy (kcal/mol) | −7.85 | −7.75 | −7.68 | −10.31 | −6.35 |
| vdW + Hbond + desolv Energy (kcal/mol) | −3.28 | −2.17 | −2.55 | −5.98 | −4.79 |
| Electrostatic Energy (kcal/mol) | −4.79 | −5.58 | −5.13 | −4.33 | −1.56 |
| Final Total Internal Energy (kcal/mol) | −0.05 | −1.28 | −1.83 | 0.1 | −0.59 |
| Torsional Free Energy (kcal/mol) | 0.89 | 1.79 | 2.09 | 1.79 | 1.79 |
| Unbound System’s Energy (kcal/mol) | −0.05 | −1.28 | −1.83 | 0.1 | −0.59 |
| Temperature (K) | 298.15 | 298.15 | 298.15 | 298.15 | 298.15 |
Fig. 3.
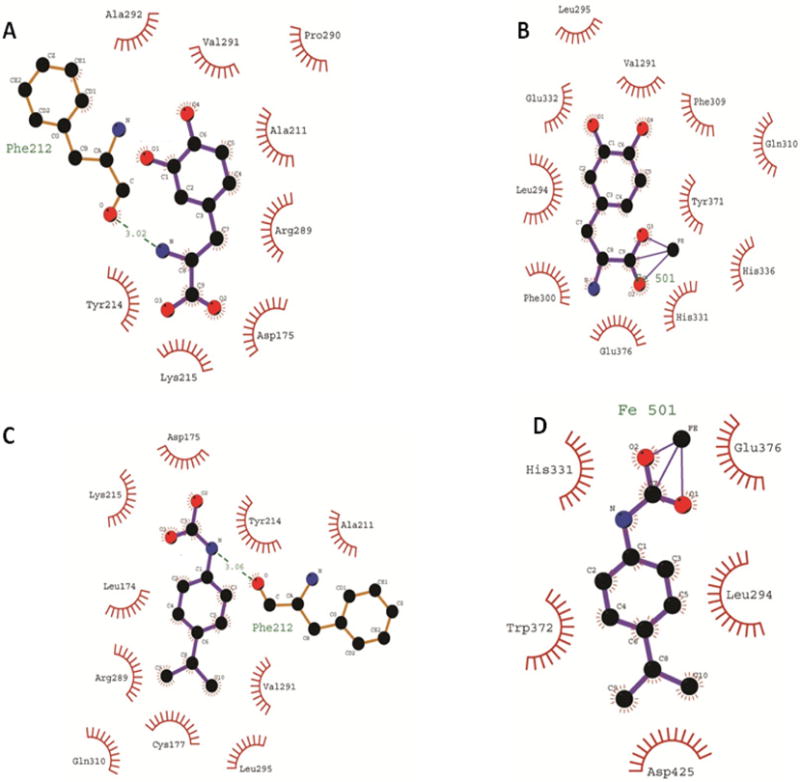
The interaction of rat TH with the studied ligands in the presence and absence of Fe+3 ions. (A) L-DOPA; Fe+3 absent. (B) L-DOPA; Fe+3 present. (C) PPCA; Fe+3 absent (D) PPCA; Fe+3 present.
Fig. 4.
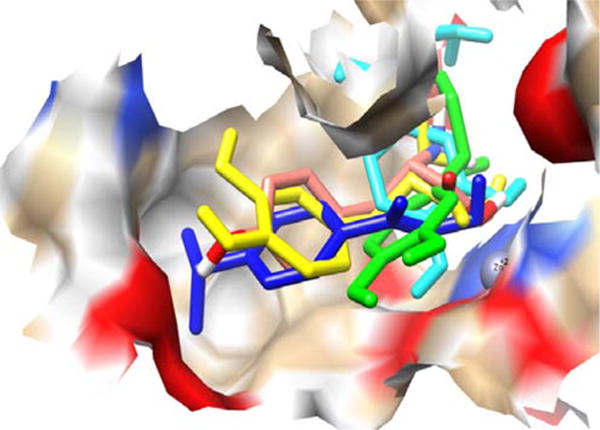
A frontal view of the complex of receptor TH having Zn+2 as prosthetic group along with the five ligands after docking is completed. Ligands are shown in different colors: PPCA in blue, L-tyrosine in pink, L-DOPA in yellow, norepinephrine in cyan, and epinephrine in green. The ligands are shown in “stick” format whereas the receptor is shown in “surface” format.
Fig. 5.
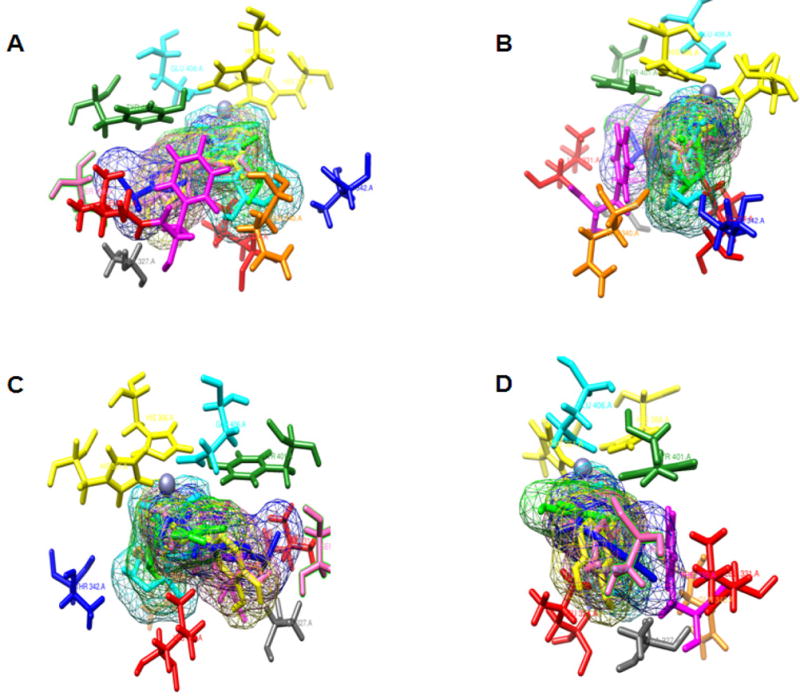
TH-ligand interactions. The six interacting residues of the receptor are shown in “stick” format and the ligands are represented in “net” format. Interacting amino acid residues are colored as follows: Leu 324, red; Phe 330, purple; His 361 and His 366, yellow; Tyr 401, green; Glu 406, cyan. Zn+2 are shown as a violet ball. Ligands are shown in different colors: PPCA, L-tyrosine in pink, L-DOPA in yellow, norepinephrine in cyan, and epinephrine in green. (A) Front view, (B) rear view, (C) right side view, (D) left side view.
Extensive studies have determined that TH can be inhibited by α-methyl-para-tyrosine (metyrosine) [12, 13], leading to a depletion of dopamine and noradrenaline within the brain consequent to reducing the availability of the initial vital precursor (L-DOPA) [14]. Clinical studies have shown that daily, long-term treatment with metyrosine may relieve symptoms in glaucoma or tardive dyskinesia in which excess catecholamines have been implicated [14], and patients with mood disorders have likewise been studied, particularly with mania [15, 16]. Metyrosine is, however, rarely used for such treatment as it can cause depression [14], but remains valuable in treating pheochromocytoma and also resistant hypertension [12–14]. Oudenone [17] and aquayamycin are further classical inhibitors of TH [18], but are structurally unrelated to catecholamines and demonstrate that structurally diverse compounds can interact with TH. In the present study, PPCA (ΔG, −6.95 kcal/mol; Ki, 8 μM) displayed a binding interaction with human TH comparable to the original TH substrate, L-Tyrosine (ΔG, −5.96 kcal/mol; Ki, 42.74 μM).
In synopsis, PPCA could serve as a potential inhibitor to transiently block TH activity to aid in characterization of its physiological role, in lieu of experimentally knocking down this gene. It, Additionally, it may inform as to the binding domains within TH and how these are be regulated. Whether or not it holds promise for the treatment of pheochromocytoma or other conditions requires in vitro and in vivo studies.
Acknowledgments
Declared none.
ABBREVIATIONS
- L-DOPA
L-dihydroxyphenylalanine
- PD
Parkinson’s disease
- PPCA
[4-(propan-2-yl) phenyl]carbamic acid
- TH
Tyrosine hydroxylase
Footnotes
CONFLICT OF INTEREST
Declared none.
References
- 1.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 2.Booij J, Bergmans P, Winogrodzka A, Speelman JD, Wolters EC. Imaging of dopamine transporters with [123I]FP-CIT SPECT does not suggest a significant effect of age on the symptomatic threshold of disease in Parkinson’s disease. Synapse. 2001;39:101–108. doi: 10.1002/1098-2396(200102)39:2<101::AID-SYN1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Miller RM, Federoff HJ. Altered gene expression profiles reveal similarities and differences between Parkinson disease and model systems. Neuroscientist. 2005;11(6):539–549. doi: 10.1177/1073858405278330. [DOI] [PubMed] [Google Scholar]
- 4.Schapira AHV. Treatment options in the modern management of Parkinson disease. Arch Neurol. 2007;64(8):1083–1088. doi: 10.1001/archneur.64.8.1083. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer RT. Molecular modelling probes: docking and scoring. Biochem Soc Trans. 2003;31(Pt 5):980–984. doi: 10.1042/bst0310980. [DOI] [PubMed] [Google Scholar]
- 6.Walther MM, Keiser HR, Linehan WM. Pheochromocytoma: evaluation diagnosis and treatment. World J Urol. 1999;17(1):35–39. doi: 10.1007/s003450050102. [DOI] [PubMed] [Google Scholar]
- 7.Harari A, Inabnet WB., 3rd Malignant pheochromocytoma: a review. Am J Surg. 2011;201(5):700–708. doi: 10.1016/j.amjsurg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 10.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508(1):1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC. Crystal structure of tyrosine hydroxylase at 2.3 Å and its implications for inherited neurodegenerative diseases. Nat Struct Biol. 1997;4:578–585. doi: 10.1038/nsb0797-578. [DOI] [PubMed] [Google Scholar]
- 12.Michalakis K, Ilias I. Medical management of adrenal disease: a narrative review. Endocr Regul. 2009;43(3):127–135. [PubMed] [Google Scholar]
- 13.van der Horst-Schrivers ANA, Kerstens MN, Wolffenbuttel BHR. Preoperative pharmacological management of phaeochromocytoma. Nether J Med. 2006;64:290–295. [PubMed] [Google Scholar]
- 14.Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: review. Mol Psychiatry. 2003;8(12):951–973. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- 15.Brodie HK, Murphy DL, Goodwin FK, Bunney WE. Catecholamines and mania: the effect of alpha-methyl-para-tyrosine on manic behavior and catecholamine metabolism. Clin Pharmacol Ther. 1971;12:218–224. doi: 10.1002/cpt1971122part1218. [DOI] [PubMed] [Google Scholar]
- 16.Bunney WE, Brodie HKH, Murphy DL, Goodwin FK. Studies of alpha-methyl-para-tyrosine, L-dopa and L-tryptophan in depression and mania. Am J Psychiatry. 1971;127:872–881. doi: 10.1176/ajp.127.7.872. [DOI] [PubMed] [Google Scholar]
- 17.Ono M, Okamoto M, Kawabe N, Umezawa H, Takeuchi T. Oudenone, a novel tyrosine hydroxylase inhibitor from microbial origin. J Am Chem Soc. 1971;93(5):1285–1286. doi: 10.1021/ja00734a054. [DOI] [PubMed] [Google Scholar]
- 18.Ayukawa S, Takeuchi T, Sezaki M, Hara T, Umezawa H. Inhibition of tyrosine hydroxylase by aquayamycin. J Antibiot. 1968;21(5):350–353. doi: 10.7164/antibiotics.21.350. [DOI] [PubMed] [Google Scholar]


