Abstract
Background & Aims
The ETS-transcription factor ETV1 is involved in the epithelial–mesenchymal transition (EMT) during pancreatic development and is induced in mouse pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC). We investigated the function of ETV1 in stromal expansion of PDAC and metastasis, as well as its effects on its downstream target Sparc, which encodes a matricellular protein found in the PDAC stroma that has been associated with invasiveness and metastasis and poor outcomes of patients.
Methods
Pancreatic ductal cells were isolated from Pdx1Cre;KrasG12D/+ mice (PanIN), Pdx1Cre;KrasG12D/+;p53fl/+ and Pdx1Cre;KrasG12D/+;p53fl/+;Rosa26YFP mice (PDAC), and Pdx1Cre;KrasG12D/+; p53fl/+;Sparc-/- mice. Cells were grown in 3-dimensional organoid culture to analyze morphology, proliferation, and invasion. Human PanIN and PDAC tissues were evaluated for ETV1 expression. Orthotopic transplants of ETV1-overexpressing PDAC and control cells were assessed in mice.
Results
Analyses of orthotopic xenografts revealed that ETV1 induced significantly larger primary tumors than controls, with significantly increased stromal expansion and significantly more ascites and metastases. Three-dimensional organoids that overexpressed ETV1 had a disrupted cyst architecture, underwent the EMT, and were more invasive. ETV1 expression was increased in human PanINs and even more so in primary and metastatic PDACs. We identified Sparc as a functional gene target of ETV1 by luciferase assays, and SPARC and ETV1 proteins co-localized in vivo. Disruption of Sparc reduced the phenotype of stromal expansion and metastasis found with ETV1 overexpression in vivo. We identified Has2 as another downstream factor of ETV1; it may mediate ETV1's significant expansion of hyaluronic acid. Conversely, disruption of Etv1 in PDAC mice (Pdx1Cre;KrasG12D/+;p53fl/+;Rosa26YFP;Cre;Etv1fl/fl) reduced levels of SPARC and hyaluronic acid in the stroma.
Conclusions
ETV1 is critical in the desmoplastic stromal expansion and metastatic progression of pancreatic cancer in mice, mediated functionally in part through SPACR2 and HAS2.
Keywords: EMT, pancreas, cancer, gene regulation
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the United States and is projected to be the second leading cause by 2020.1 Underlying the poor median survival of 6 months after diagnosis is that the vast majority of patients present with clinically evident metastatic disease, and even those who exhibit no signs of metastasis and undergo surgical resection for small tumors eventually develop metastatic disease.2,3 It has been theorized that epithelial-mesenchymal transition (EMT) is required for cancer progression, including in PDAC.4-7 The pancreatic tumor microenvironment is characterized by an intense desmoplastic stroma that contributes to therapeutic resistance through biophysical limitations in drug delivery and prominent immune cell infiltration.8,9 However, the factors that shape the tumor microenvironment and promote EMT remain incompletely understood.
A growing and complex body of evidence is emerging that the role of the stroma in pancreatic cancer can be varied. There are elements that are hallmarks of poor prognosis and an impediment to therapeutics that may benefit from depletion, such as hyaluronic acid (HA),10 and yet other elements of the stroma that may serve to restrain carcinogenesis.11,12 Secreted protein acidic and rich in cysteine (Sparc/osteonectin) has been examined in pancreatic cancer and found to be a frequent constituent of tumor associated desmoplastic stroma, but absent in most PDAC and PDAC derived cell lines.13 It has been found subsequently that peritumoral fibroblast expression of Sparc in PDAC has been associated with poorer patient outcomes in association with differentiation status.14-16 Most recently, Sparc has been identified as a critical factor in circulating tumor cells in pancreatic cancer that may mediate invasiveness and metastatic capacity as well as regulating extravasation and metastasis in melanoma.17 {Tichet:2015gu} However, the factors upstream of Sparc that may regulate this behavior remain unknown.
To date, key pancreatic transcriptional factors have been identified that govern endocrine cell lineage (Pdx1) and exocrine cell lineage fates (Ptf1a or p48 for acinar cells and likely, Sox9, Hnf1beta, Hnf6 and Prrx1 for ductal cells).18 Transcriptional factors that regulate convergence on ductal cell fate have remained elusive apart from Prrx1 and Sox9.19 In an effort to identify transcriptional factors that may serve as “drivers” of pancreatic ductal fate, the transcriptional profile of cells derived from embryonic ductal pancreata, pancreatitis injury/regeneration model (acinar-ductal metaplasia), and early neoplasia (pancreatic epithelial neoplasia or PanIN) were analyzed. Strikingly elevated levels of the Ets-transcriptional factor Etv1 were observed in these three processes.19
Approximately 30 Ets factors have been described in mammals, characterized by their conserved helix-turn-helix binding motif, called the Ets domain that binds to the GGAA/T core consensus sequence.20 Ets factors have been classified into 9 subfamilies, with Etv1 and Etv5 belonging to the PEA3 subfamily.21 These factors are known downstream targets of Kras (mutated in >90% of human PDAC) and have been implicated in cell proliferation, senescence, apoptosis, and differentiation.20 In development, the PEA3 family of Ets transcription factors has been observed in organs that undergo branching morphogenesis such as the lung, salivary gland and pancreas, with Etv5 expressed in epithelial buds and Etv1 found in the mesenchymal compartment, indicating a potential role for these factors in epithelial-mesenchymal plasticity.22,23 Given their distinct epithelial and mesenchymal expression in branching morphogenesis and potential role in EMT in carcinomas, we sought to examine the role of Etv1 in EMT and metastasis in PDAC.
Herein we define novel functional roles for Etv1 in the regulation of EMT, stromal expansion, and metastasis. To examine the process of pancreatic carcinogenesis in vitro, we utilized cell lines from mice that yield Pancreatic Intraepithelial Neoplasia (PanIN) (Pdx1-Cre;LSL-KrasG12D) (KC) and from mice that develop tumors and metastatic disease similar to human PDAC (Pdx1-Cre;LSL-KrasG12D;Trp53fl/+; Rosa26YFP and Pdx1-Cre;LSL-KrasG12D;Trp53fl/+) (KPfC-Y and KPfC, respectively).24-26 Utilizing orthotopic transplantation, the overexpression of Etv1 caused the development of significantly larger tumors with significantly increased stroma, including hyaluronic acid (HA) correlating to increased tumor volume. Etv1 was associated with significantly more liver and lung metastasis. Mechanistically, Etv1 overexpression disrupted cyst architecture in 3D organoid culture models; induced EMT-regulators, mesenchymal markers and matrix degrading enzymes; and increased invasiveness. In an effort to identify the regulators underlying these striking findings, we have identified two novel targets of Etv1: hyaluronic acid synthase 2 (Has2) and Sparc. Indeed, genetic in vivo experiments demonstrated that the loss of Sparc completely abrogated the dramatic phenotype of increased primary tumor volume, stromal expansion, and increased metastasis associated with Etv1 overexpression. Furthermore, preliminary data from conditional knockout of Etv1 in KPfCY mice demonstrates decreased Sparc and HA expression. Taken together, we demonstrate a novel regulatory axis governing EMT, stromal expansion and metastasis in pancreatic cancer mediated through Etv1 and its downstream targets Has2 and Sparc. These targets may potentially provide a new platform for translational therapeutics.
Materials and Methods
Cell lines, cell culture and in vitro assays
Cell lines were generated from PanIN-bearing LSL-KrasG12D/+;Cre mice (termed KC),19,24 PDAC-bearing LSL-KrasG12D/+;p53fl/+;Cre mice (termed KPfC),25 and PDAC-bearing LSL-KrasG12D/+;p53fl/+;Rosa26YFP;Cre (termed KPfCY).26 In addition, cell lines from PDAC-bearing mice with a global SPARC deletion that have been previously described and characterized27 were utilized (termed KPfC Sparc-/-). Mouse primary pancreatic cells were cultured and maintained as described previously.19,28 KPfC and KPfC Sparc-/-cells were transduced with dTomato to allow for lineage labeling in vivo. Sorting and Isolation of Ecad+ and Ecad- fractions from KPfCY mice is described in detail in Supplemental Experimental Procedures. Invasion assays were performed as described previously19 and as detailed in Supplemental Experimental Procedures. Quantitative RT-PCR and Western Blotting were performed as described previously.28 Details of these techniques, small interference RNA (siRNA) knockdown, luciferase assay, and site directed mutagenesis are included in Supplemental Experimental Procedures.
Lentiviral transduction and vector constructs
Coding sequences of mEtv1 and Has2 were amplified from genomic cDNA (Supplemental Table 1). mEtv1 was Flag-tagged at the N terminus (Supplemental Table 1). Following PCR amplification, coding sequences were digested using Age1 and Mlu1 and subcloned into pTRIPZ (RHS4743; Open Biosystems). dTomato was obtained from Addgene (FUdGW-Tomato, Plasmid #22771). Lentiviral transduction was completed as described previously.28 Parental cell lines were transduced in parallel with Etv1-Flag constructs or empty vector constructs (Control).
Three-dimensional (3D) pancreatic ductal cell organoids
Three-dimensional (3D) pancreatic ductal cell organoids were performed as described previously28,29 and as detailed in Supplemental Experimental Procedures.
Immunohistochemical/immunofluorescence staining and histopathology
Immunohistochemisty (IHC) and immunofluorescence (IF) staining were carried out as described previously.30 For details on the construction and grading of paired primary and metastatic pancreatic cancer human tissue microarray (TMA) please see Supplemental Experimental Procedures. For antibodies, conditions, and grading parameters see Supplemental Experimental Procedures.
Orthotopic Transplant Procedures
The Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania approved all animal studies (Protocol #8049-20). Eight week old female Ncr nude mice were purchased from Taconic (USA) and orthotopic transplantation in the pancreas was performed as described previously.31 The subcapsular space of the pancreas of nude mice was injected with 1×105 KPfCY control, KPfCY mEtv1, KPfC control, KPfC mEtv1, or KPfC Sparc -/- mEtv1 cells suspended in 25μl of DMEM/10%FBS. The mice were sacrificed 15 days after the procedure. Pancreata, livers, and lungs were harvested for examination. For details on tumor volume,32 ascites, and metastasis33,34 grading please see Supplemental Experimental Procedures.
Generation and analysis of conditional Etv1 knockout in KPfCY background
A previously generated conditional knockout of Etv1,35 was bred into the KPfCY background to generate LSL-KrasG12D/+;p53fl/+;Rosa26YFP;Cre;Etv1fl/fl mice. The Pdx1Cre was derived from Melton and colleagues36 and recombination was verified by robust expression of YFP on sacrifice.
Statistical Analysis
For all in vitro experiments, statistical analyses were performed using the Mann-Whitney-Wilcoxon test, except where noted otherwise. For in vivo experiments, statistical analyses were conducted using Welch's t-test for IF, trichrome/hyaluronic acid staining, or tumor volume. Frequency of metastases was analyzed using a Mann-Whitney Test. Chi-square test and Pearson correlation coefficients were used to examine the relationship between hyaluronic acid or trichrome staining area with tumor volume. p<0.05 was considered statistically significant. Number of mice or replicates (n) is indicated in each experiment. Values are expressed as mean ± SEM or SD.
Results
Etv1 is expressed in mouse and human PanIN, PDAC, and metastasis
To evaluate Etv1 as being potentially important in pancreatic carcinogenesis, we first examined tissue from normal mouse pancreata (C57BL/6), PanIN lesions from KC mice, invasive PDAC from KPfCY mice, and matched liver metastasis from KPfCY mice (Figure 1A, Supplemental Figure 1D). While Etv1 is limited to the ductal compartment in the normal pancreas, it becomes more pronounced in PanIN lesions, and is detected in PDAC and metastasis, especially at the invasive front (Figure 1A). We examined Etv1 expression in a TMA of human PanIN which demonstrated similar expression in 88% (15/17) of low-grade PanIN and 100% (5/5) of high-grade PanIN lesions (Supplemental Figure 1A). Next we examined Etv1 expression in a unique cohort of matched human primary PDAC and associated metastasis from a rapid autopsy cohort TMA. We found 88%(14/16) primary and 94%(15/16) matched metastatic lesions expressed Etv1, with a similar distribution in staining intensity. IHC staining in both PanIN and PDAC TMAs was predominantly nuclear but also occasionally mixed nuclear and cytoplasmic (Supplemental Figure 1A, 1B). There were poorly differentiated elements in 12/16 primary and metastatic PDAC, and thus Etv1 status and degree of differentiation is difficult to extrapolate. In a separate cohort of surgically resected human PDAC, we then examined Etv1 expression in paired tumor and tumor associated desmoplastic stroma. Interestingly, Etv1 was expressed strongly in both compartments (Supplemental Figure 1C). Finally, in silico analysis of Etv1 gene expression revealed a significant increase in human PDAC compared with normal controls (Supplemental Figure 1E).37
Figure 1. Overexpression of Etv1 in KPfCY-cells significantly expands the desmoplastic stroma.
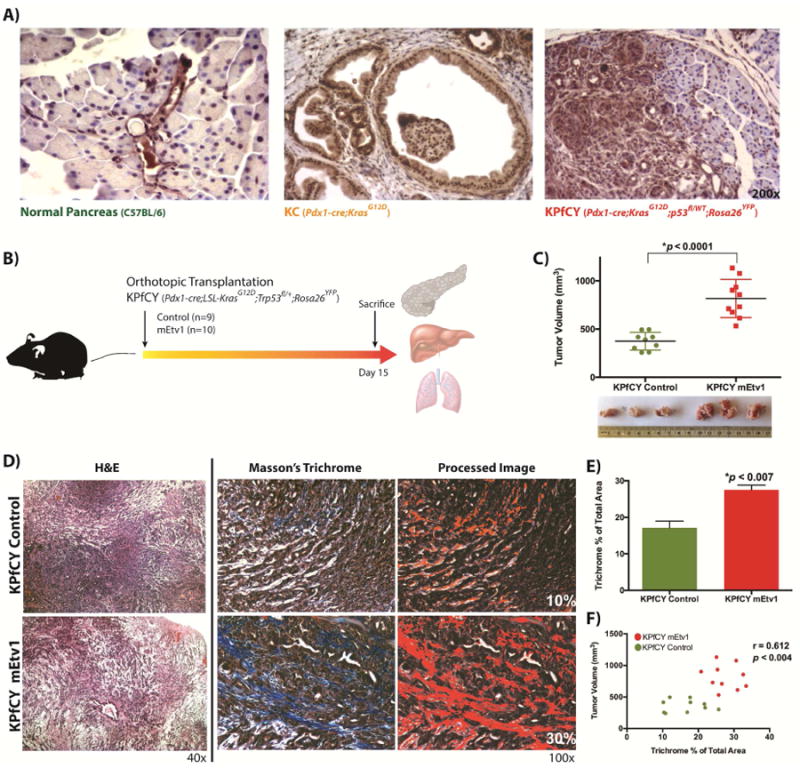
(A) Etv1 immunohistochemistry in mouse sections with the indicated genotypes. Expression of Etv1 is restricted to the ductal compartment in the normal pancreas. Pancreatic Intraepithelial Neoplasia (PanIN) lesions display elevated levels of Etv1, while Etv1 is diffusely expressed in Pancreatic Ductal Adenocarcinoma (PDAC). (B) Schematic experimental design of orthotopic xenograft transplantation experiments with KPfCY and KPfCY mEtv1 cells. (C) Primary tumor volumes of KPfCY control and KPfCY mEtv1-overexpressiong orthotopic xenograft tumors. Etv1 overexpressing KPfCY cells form significantly larger primary tumors. (D) Hematoxylin/Eosin and Masson's trichrome staining of KPfCY Control (upper row) and KPfCY mEtv1 (lower row) orthotopic xenograft tumors. Image processing to perform semi-quantitation on trichrome staining is depicted adjacent. (E) Etv1 overexpressing orthotopic xenograft tumors display a significant increase in tumor stroma as measured by trichrome staining compared to control animals. (F) Correlation of tumor volume and trichrome positive area between KPfCY control (green dots) and KPfCY mEtv1 (red dots). The percentage of the trichrome positive area measured in (E) correlates significantly to the increase in tumor volume measured in (C).
Etv1 induces increased tumor volume via expansion of the tumor stroma
Given these initial findings, we sought to evaluate the mechanistic underpinnings of Etv1 in PDAC. We developed cell lines from KC, KPfC, and KPfCY mice that have been reported previously.19,25,26 We found a stepwise increase in mEtv1 gene expression by qPCR in comparing wild type pancreatic ductal cells (PDC) with parental cell lines from KC and KPfC animals (Supplemental Figure 2A). Lentiviral transduction was employed to overexpress control or FLAG-tagged Etv1 constructs, and overexpression was confirmed by both qPCR for Etv1 (Supplemental Figure 2A) and western blot analysis for FLAG (Supplemental Figure 2B). With regards to overexpression, in comparison to the 184-fold increase in mEtv1 observed between PDC and KPfC parental cell lines, transduced cell lines had a 6-8 fold increase over their respective controls (Supplemental Figure 2A). Subsequently, orthotopic pancreatic transplantation of KPfCY control and KPfCY over-expressing Etv1 cells was performed into 8-week-old Ncr nude mice.31 Pilot experiments were conducted with non-transduced cell lines to evaluate the optimal time point for reliable development of primary tumors and the initiation of metastasis without the mice becoming moribund (data not shown). After 15 days, primary tumors formed with 100% penetrance and mice were sacrificed and pancreata, livers, and lungs removed for histological examination (Figure 1B). Strikingly, we found that in KPfCY mEtv1 animals, primary tumors were significantly larger than their control counterparts by >2-fold (Figure 1C). Examination of these tumors histologically demonstrated prominent stromal expansion (Figure 1D). Utilizing Masson's Trichrome staining to quantify this stromal expansion, KPfCY mEtv1 tumors were noted to have significantly more trichrome staining than respective controls (Figure 1D,E). Furthermore, the trichrome area correlated significantly with increased tumor volume that was observed in Etv1 over-expressing tumors (Figure 1F). Examination of the primary tumors from Etv1 over-expressing mice demonstrated no increase in Ki67 index or change in TUNEL staining for apoptosis (Supplemental Figure 3A,B). WST-1 assay demonstrated minimal difference in proliferation between KPfC Control and KPfC mEtv1 cells, with control cells proliferating at a slightly higher rate (Supplemental Figure 3C). Taken together, these data suggest that Etv1, through expansion of the tumor stroma, promotes a significant increase in tumor volume.
Etv1 promotes the development of increased metastasis
Upon sacrifice of animals with orthotopic transplantation of Etv1-overexpressing tumors, it was apparent that there was significantly more frequent and higher-grade ascites present in these animals (Supplemental Figure 3D). To examine the effects of Etv1 on dissemination and metastasis, we examined the liver and lungs from mice that had undergone orthotopic transplantation with KPfCY control or KPfCY mEtv1 cells. Metastases were classified into three subtypes: isolated tumor cells (ITC) that were <0.2mm and <200cells, micrometastasis that were 0.2-2mm in size with >200cells, or macrometastasis that were >2mm in size based upon a published classification scheme.33,34 We found examples of ITCs (Figure 2A), tumor thrombus (2B), micrometastasis (Figure 2C), and macrometastasis (Figure 2D) in mEtv1-overexpressing animals. Very interestingly, there was a marked stromal expansion in many of the micrometastases observed in Etv1 overexpressing tumors that is reminiscent of the stromally expanded primary tumors from these animals (Figure 2C). The presence of the YFP lineage label allowed us to track cancer cells as they underwent EMT and gave rise to distant metastasis. Thus, metastatic events were confirmed further utilizing immunofluorescence staining (Figure 2E). Overall, 80% of KPfCY mEtv1 animals developed metastatic events in comparison to 22% of control animals (p<0.023) (Figure 2F). Furthermore, irrespective of metastasis size, there were significantly more metastatic liver events in total in the Etv1-overexpressing animals (Figure 2F). Similarly, we found significantly more frequent lung metastasis in the Etv1-overexpressing animals in comparison to their controls although all these events were ITCs (Supplemental Figure 3E,F).
Figure 2. Overexpression of Etv1 in KPfCY -cells significantly increases liver metastasis (A-D).
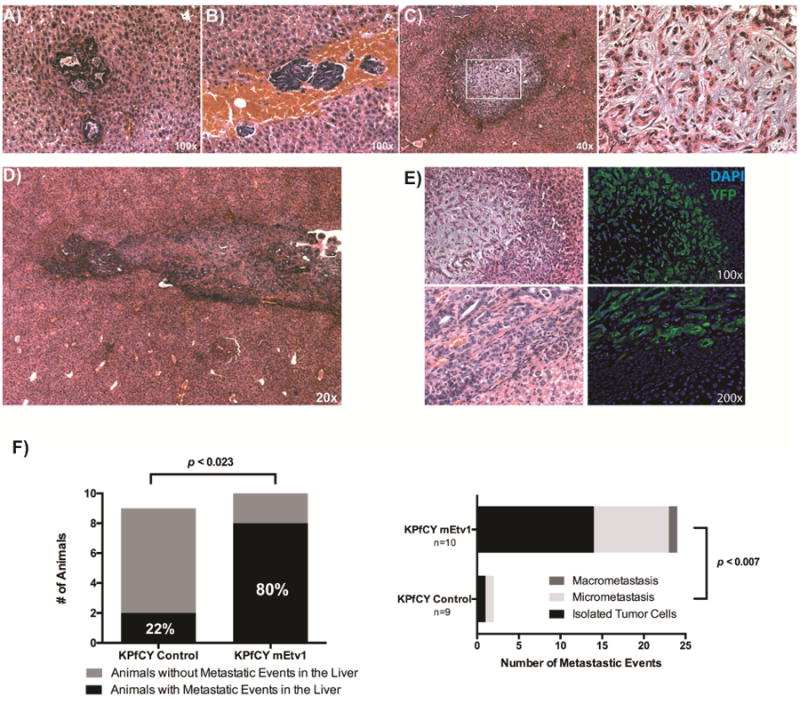
Types of liver metastases observed in Etv1 overexpressing tumors including Isolated Tumor Cells (A), tumor microthrombi (B), micrometastasis (C), & macrometastasis (D). (C) Metastases demonstrate stromal expansion reminiscent of primary Etv1 overexpressing tumors (right panel expanded from white box in lower magnification). (E) Matched H&E and immunofluorescence staining for YFP (green) of liver metastasis of Etv1 overexpressing KPfCY tumors. (F) The number of animals with liver metastatic events is significantly higher among Etv1 overexpressing tumors. In addition, Etv1 overexpression was associated with a significantly larger number of total metastatic events of all subtypes pictured in (A-D).
The matricellular protein Sparc is regulated by Etv1 in vitro and in vivo
As a significant proportion of the tumor associated stroma in our lineage labeled orthotopic model is YFP+ and frequently appears spindle shaped and E-cadherin (ECAD) negative (Figure 3C,E) we sought to examine factors known to be important in the desmoplastic reaction within PDAC. Examining known matricellular factors in pancreatic cancer9,38,39 we performed an in silico screen for potential Etv1 binding sites and found several possible targets (Supplemental Table 2). Given the relative frequency of Ets binding sites, we sought to validate stromal targets that are significantly modulated by Etv1 in vitro. Thus, we found in KPfC cell lines that Etv1 overexpression was associated with Sparc increase by 3.5 fold and, conversely, knockdown of Etv1 by siRNA was associated with Sparc decrease by 0.5 fold (Figure 3A). In silico screening demonstrated several putative Ets binding sites in the Sparc promoter (Supplemental Figure 3A). We generated a Sparc luciferase reporter gene construct that contains the portion of the Sparc promoter with these Ets binding sites (Supplemental Figure 4A). Etv1 overexpression significantly increased Sparc luciferase activity in comparison to the control pGL3 vector (Figure 3B). Furthermore, in vivo validation in KPfCY mEtv1 orthotopic transplant animals demonstrated significant colocalization of Sparc and YFP (Figure 3C,D). These data suggest a novel and exciting potential regulation of Sparc by Etv1.
Figure 3. The matricellular protein Sparc is regulated by Etv1 in vitro and in vivo (A-E).
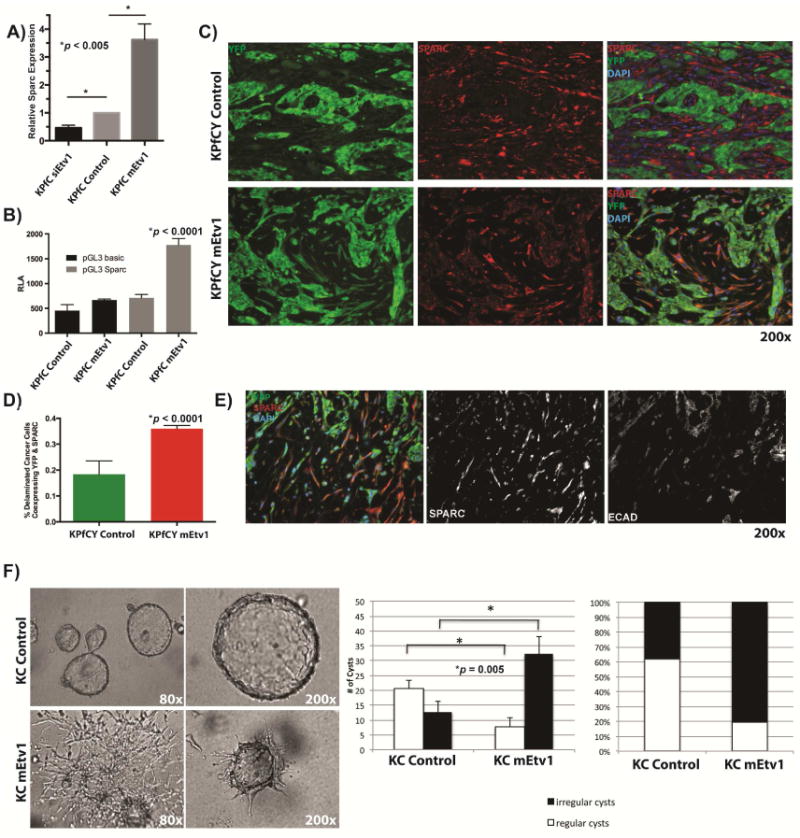
(A) Quantitative PCR of Sparc after knockdown oroverexpression of Etv1 in KPfC cells. Knockdown of Etv1 through siRNA by is associated with a significant decrease of Sparc. Conversely, Sparc is upregulated by 3.5-fold in Etv1 overexpressing cells compared to control. (B) Luciferase reporter assay of the Sparc promoter. Sparc promoter activity is significantly increased in Etv1 overexpressing KPfC cells compared to control. (C) Immunofluorescence staining for YFP (green) and Sparc (red) in KPfCY tumors. Sparc is mainly expressed in the YFP-negative stroma of KPfCY control tumors (upper row). However, KPfCY mEtv1 tumors display a large population of delaminated, YFP+ cells. These cells are found to express both YFP and Sparc (lower row). (D) The fraction of delaminated, YFP-positive tumor cells that coexpress Sparc is significantly higher in KPfCY mEtv1 tumors than in KPfCY control tumors. (E) Delaminated tumor cells that co-express YFP and Sparc (left panel) are negative for E-cadherin (right panel) and display a spindle shaped morphology. (F) 3D organoid culture of KC control and KC mEtv1 cells. KC control cells form regular three dimensional cysts with a hollow lumen surrounded by a single layer of epithelial cells reminiscent of pancreatic ductal structures (left panel, upper row). KC mEtv1 cells form significantly more disrupted/irregular cysts and display a high proportion of spindle shaped cells that fail to form cystic structures (left panel lower row).
Etv1 induces EMT and invasion in vitro
Careful examination of the primary pancreatic tumors from KPfCY mEtv1 orthotopic transplants demonstrated that the population of cells with cololocalization of YFP and SPARC were essentially uniformly ECAD negative (Figure 3C,E). These cells were also notably spindle shaped in their morphology (Figure 3E). In a separate analysis of KPfCY genetically engineered mice (GEMM), we isolated YFP+ tumor cells from primary pancreatic tumors and sorted them into Ecad+ and Ecad- fractions. We found a significant increase in 2/3 of these tumors for Etv1 and 3/3 of these tumors for SPARC in the ECad- fraction in comparison to the Ecad+ fraction (Supplemental Figure 4B,C). Given these observations, namely that Etv1 overexpression was associated with an expansion of a population of cells that apparently had undergone EMT with loss of ECAD expression and the association of Etv1 with increased development of distant metastasis, we sought to examine the role of Etv1 in EMT in vitro. To examine the potential role of Etv1 in pancreatic ductal morphogenesis, we utilized a 3D organoid culture system.28 Using KC cells, we noted that these ductal organoids suspended in collagen tend to form regular cysts with hollow lumens (Figure 3F). However, in Etv1 overexpressing cells, we saw significantly more cysts that are highly irregular/disrupted and demonstrate a spindle-shape morphology (Figure 3F). Concurrent with this change in morphology we see significant upregulation in classical regulators of EMT as well as matrix metalloproteases including Slug, Snail, Twist, Vimentin, Zeb1, Zeb2, mmp3, and mmp9 (Supplemental Figure 4D). Conversely, knockdown of Etv1 was associated with significant reductions in Zeb1 in vitro (Supplemental Figure 4F). In experiments utilizing TGF-β to induce EMT in vitro in KC cells, we see a stepwise, significant increase in Etv1 with induction of EMT in parallel with Snail, Zeb1, Zeb2, N-cad, and a corresponding decrease in Etv1 and these markers with withdrawal of TGF-β (Supplemental Figure 5A). Canonical invasion assays were also performed to examine the functional role of Etv1 in dissemination. We found that in comparison to control KC cells, KC mEtv1 cells demonstrated a significant increase in invasive capacity (Supplemental Figure 4E). Conversely, in comparison to control KPfC cells, KPfC cells with knockdown of Etv1 demonstrated significantly less invasive capacity (Supplemental Figure 4F).
The loss of Sparc abrogates the increased expansion of stroma and development of metastasis mediated by Etv1
To examine the loss of the downstream target Sparc on Etv1's effect on tumor stroma and metastasis in vivo, we utilized KPfC, KPfC mEtv1, and KPfC Sparc-/- mEtv1 mouse pancreatic cancer cell lines in an independent orthotopic pancreatic transplantation model (Figure 4A). The KPfC and KPfC Sparc-/- parental lines used in these experiments were derived from littermates. All cell lines were transduced lentivirally with dTomato in addition to mEtv1-FLAG as indicated to allow for lineage labeling in vivo (Supplemental Figure 2A,B; Supplemental Figure 5C). KPfC Sparc-/- mice have been characterized as developing PDAC tumors with similar latency and metastasis frequency to the original descriptions of the KPfC model with the notable absence of Sparc in tumor associated stroma and some differences in collagen maturation.25,27 In vitro, we observed that while Etv1 overexpression was associated with increases in levels of EMT regulators like Snail, Zeb1, and Zeb2, the loss of Sparc abrogated the capacity of Etv1 to induce these factors (Supplemental Figure 5B). Orthotopic transplantation was performed with NCr nude mice for 15 days. Once again, in an independent cell line, in comparison with control KPfC cells, Etv1 overexpressing KPfC cells were associated with a significant expansion of primary tumor volume by 5.4 fold (Figure 4B,C). As was noted previously, these tumors were associated with significantly expanded tumor stroma, as measured by Masson's Trichrome staining (Figure 4D). Strikingly, the loss of Sparc in KPfC Sparc-/- mEtv1 cells completely abrogated this increase in tumor volume (Figure 4B,C) and the expansion of the tumor stroma (Figure 4D). Similarly, there was a significant correlation of primary tumor volume to trichrome staining among the KPfC, KPfC mEtv1, and KPfC Sparc-/- mEtv1 tumors (Figure 4E). Primary tumors were validated by immunofluoresence staining for dTomato and Sparc. While there was continued co-localization of dTomato and Sparc in the KPfC mEtv1 tumors, this was very low in the KPfC Control and absent in KPfC Sparc-/- mEtv1 tumors (Supplemental Figure 5C). Interestingly, there was apparent host-derived Sparc detected in the KPfC Sparc-/- mEtv1 tumors in the dTomato negative tumor associated stroma (Supplemental Figure 5C).
Figure 4. Loss of Sparc abrogates the increased expansion of stroma and development of metastasis mediated by Etv1.
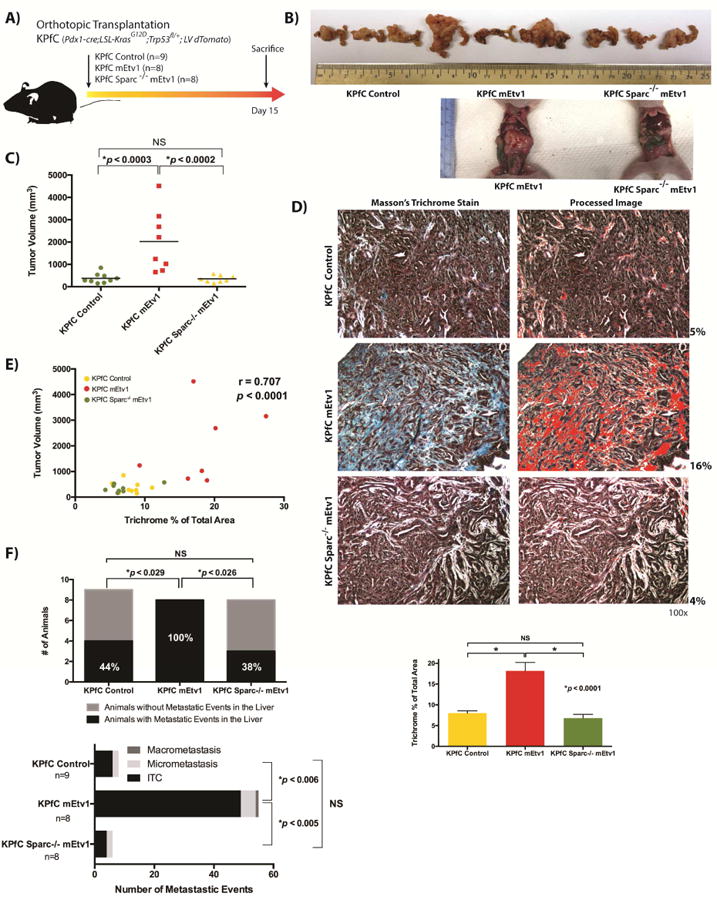
(A) Schematic representation of independent orthotopic pancreatic xenograft transplantation experiments of KPfC Control, KPfC mEtv1, and KPfC Sparc-/- mEtv1 cells. All cells were lentivirally transduced with dTomato to allow for lineage labeling. (B) Representative pancreata of KPfC control, KPfC mEtv1 and KPfC Sparc-/- mEtv1 xenografts. Examples of large tumors in KPfC mEtv1 animals in comparison to KPfC Sparc-/- mEtv1 in situ in the abdomen of sacrificed mice are noted below. (C) Overexpression of Etv1 is associated with significant increase in tumor volume in comparison to KPfC control. However, the loss of Sparc completely abrogates the increase in tumor volume observed with mEtv1 overexpression. (D) Masson's trichrome staining of KPfC Control, KPfC mEtv1, and KPfC Sparc-/- mEtv1 tumors. Representative images are shown and semi-quantitative image processing was used as demonstrated adjacent. The increase in trichrome positive tumor stroma observed with Etv1 overexpression is abrogated by the loss of Sparc in KPfC Sparc-/- mEtv1 xenografts. (E) Correlation of tumor volume and trichrome positive area between KPfC Control (yellow dots), KPfC mEtv1 (red dots), and KPfC Sparc-/- mEtv1 (green dots) tumors. The percentage of the trichrome positive area measured in (D) correlates significantly to the increase in tumor volume measured in (C). (F) The overexpression of Etv1 is associated with the development of significantly more liver metastasis in comparison to KPfC Control. However, the loss of Sparc completely abrogates this Etv1 associated increase in metastasis development.
In addition to the abrogation of stromal expansion and increased tumor volume, the loss of Sparc also abrogated significant Etv1 associated increases in the frequency and grade of ascites present in the animals at sacrifice (Supplemental Figure 5D). Careful examination of livers from these animals demonstrated that while 44% (4/9) of KPfC Control animals had metastatic events, significantly more (100% (8/8), p<0.029) KPfC mEtv1 animals had metastatic events (Figure 4F). In comparison, the loss of Sparc returned the frequency of liver metastasis to levels similar to the control group (4/9 vs 3/9, p<NS). Dividing metastatic events into isolated tumor cells, micrometastasis, or macrometastasis, KPfC mEtv1 had significantly more total events in comparison to KPfC control or KPfC Sparc-/- mEtv1 (Figure 4F). Taken together, this data demonstrate that Etv1 mediates its effects on stromal expansion and metastasis in part via Sparc.
Etv1 mediated stromal expansion is associated with hyaluronic acid synthase 2 (Has2)
We next performed immunofluorescence analysis of the primary tumors from KPfCY and KPfCY mEtv1 orthotopic transplants for αSMA, classically associated with activated fibroblasts, and GFAP. We found no significant difference in αSMA or GFAP in association with mEtv1 over-expression (Supplemental Figure 6A,B). Given the observation of differences in Masson's Trichrome staining and a possible association with collagen crosslinking, we next examined the primary tumors for lysyl-oxidase (LOX) and Collagen I by immunofluorescence but similarly found no significant difference (Supplemental Figure 6C,D). We also evaluated KPfCY Control and KPfCY mEtv1 cell lines for Collagen I, III, and FAP by qPCR and found no significant differences (Supplemental Figure 6E).
Further examination of our in silico screen of potential matricellular downstream targets of Etv1 demonstrated a potential interaction with Hyaluronan Synthase 2 (Has2) (Supplemental Table 2). Has2 has been identified in vitro as significantly overexpressed in fibroblast co-culture experiments with PDAC cells39 and has been associated with significantly increased hyaluronic acid secretion.40,41 Hyaluronic acid has been identified as a critical factor in the pancreatic tumor microenvironment that increases interstitial fluid pressure, collapsing potential vascular access to tumors.10 PDAC xenografts utilizing over-expression of Has2 demonstrated increased tumor growth, loss of membranous E-cadherin and increased accumulation of cytoplasmic β-catenin.41 Indeed, Has2 has been associated with poorer survival in human PDAC.42 We found Etv1 overexpression was associated with Has2 increase by 4 fold (Figure 5A). In silico screening demonstrated 3 putative Ets binding sites in the Has2 promoter (Figure 5B). We generated a Has2 luciferase reporter gene construct that contains the portion of the Has2 promoter with these Ets binding sites (Figure 5B). Etv1 overexpression significantly increased Has2 luciferase activity and knockdown of Etv1 significantly decreased Has2 luciferase activity in comparison to the control pGL3 vector (Figure 5C). Subsequently, we performed site directed mutagenesis of a putative Etv1-binding site located 1119bp upstream of the transcriptional start site, which significantly abrogated Etv1 associated luciferase activity (Figure 5D). The proximity of this binding site to the promoter suggests direct activation of the Has2 promoter through Etv1.
Figure 5. Etv1 mediated stromal expansion is associated with Has2.
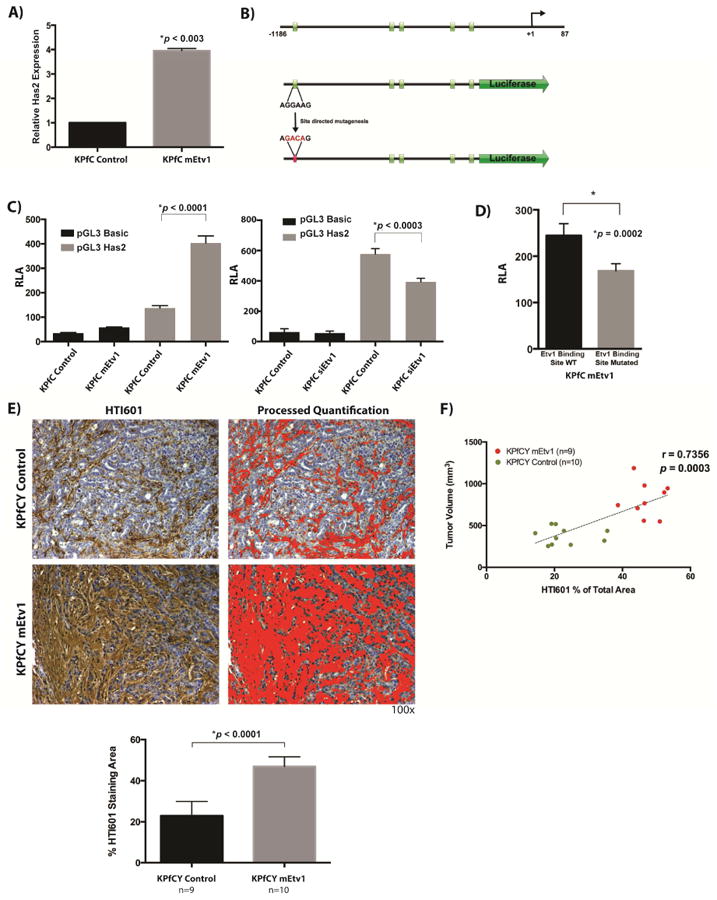
(A) Quantitative PCR analysis of Has2 expression. Etv1 overexpression induces the expression of Has2 by 4 fold in KPfC cells. (B) Schematic diagram of the Has2 promoter upstream of the 5′ untranslated region. Green boxes indicate predicted Etv1 binding sites. The promoter fragment was subcloned into the pGL3 Vector for Luciferase reporter analysis. PCR based site directed mutagenesis was used to mutate a predicted Etv1 binding site located 1119bp upstream of the transcriptional start site. Mutated bases are indicated in red. (C) Luciferase reporter Assay of the Has2 promoter. Has2 promoter activity is significantly increased in Etv1 overexpressing KPfC cells compared to control while knockdown of Etv1 via siRNA significantly decreases Has2 promoter activity. (D) Luciferase reporter assay of the Has2 promoter with wildtype and mutated Etv1 binding site, respectively in KPfC mEtv1 cells. Mutation of the putative Etv1 binding site is associated with loss of Has2 promoter activity. (E) Staining for hyaluronic acid with the molecular probe HTI601 and quantification. KPfCY mEtv1 tumors display significantly more hyaluronic acid positive areas than KPfCY control. (F) Correlation of tumor volume and hyaluronic acid positive area between KPfCY Control (green dots) and KPfCY mEtv1 (red dots). The percentage of the hyaluronic positive area measured in (E) correlates significantly to the increase in tumor volume associated with mEtv1 overexpression.
Furthermore, in vivo validation in KPfCY mEtv1 orthotopic transplant animals was completed utilizing staining for hyaluronic acid with the molecular probe HTI601. KPfCY mEtv1 orthotopic transplants demonstrated significantly more hyaluronic acid staining in comparison to the control animals (Figure 5E). In addition, the hyaluronic acid staining and tumor volume significantly correlated, more so in fact than trichrome staining (Figure 5F, Figure 1F). Taken together, these data suggest a novel and exciting potential regulation of Has2 and hyaluronic acid deposition in the pancreatic tumor stroma by Etv1.
The conditional loss of Etv1 in KPfCY mice is associated with decreased Sparc and HA
We generated a conditional knockout of Etv1 in the KPfCY background to better understand the role of Etv1 in tumorigenesis and the tumor stroma. In a preliminary evaluation, we compared, three mice aged 6-8 months as this was when tumors were clinically apparent, to three KPfCY mice aged 2-3 months with clinically apparent tumors. Two additional KPfcY Etv1fl/fl mice sacrificed at 3 months demonstrated no evidence of primary tumors. Liver metastasis were not seen in the three KPfCY Etv1fl/fl mice sacrificed at 6-8 months examined (data not shown). Interestingly, in a qualitative analysis by IF, SPARC expression appeared dramatically decreased in KPfCYEtv1fl/fl mice in comparison with KPfCY controls (Figure 6A). There was still expression of SPARC, but this was seen only in the YFP- fraction. Also, while there were several examples of delaminated YFP+/Ecad- cells in the KPfCY tumors that also expressed SPARC, these cells were not appreciated in the KPfCY Etv1fl/fl mice. With regards to HA, staining confirmed reduced, although not absent, HA in KPfCYEtv1fl/fl tumor associated stroma in comparison to KPfCY tumors (Figure 6B). Thus, this preliminary data from a novel GEMM is validation of our orthotopic transplantation model's findings of Etv1's relationship with increased Sparc expression in delaminated YFP+ cells and HA expression.
Figure 6.
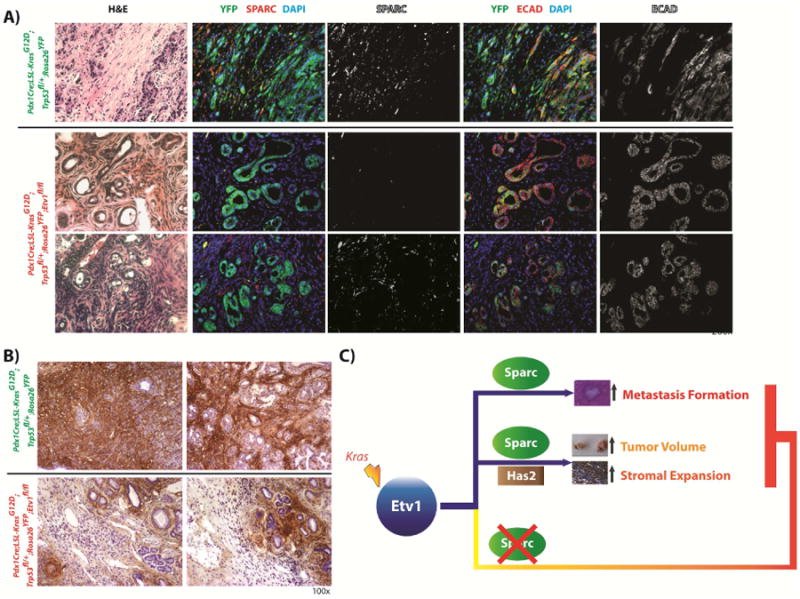
(A) Immunofluorescence staining for YFP (green) and Sparc (red) in Pdx1Cre;LSL-KrasG12D;TRP53fl/+;Rosa26YFP- and Pdx1Cre;LSL-KrasG12D;Trp53fl/+;Rosa26YFP;Etv1fl/fl-mice (KPfCY Etv1fl/fl). Loss of Etv1 is associated with decreased expression of Sparc (row 2), however Sparc expression is not completely abrogated and retained in some areas of the YFP-negative stromal compartment (row 3). The fraction of delaminated, YFP-positive E-cadherin-negative tumor cells that coexpress Sparc is lower in KPfCY Etv1fl/fl in comparison to KPfCY mice. (B) Staining for hyaluronic acid with the molecular probe HTI601. Hyaluronic acid is reduced in tumors of KPfCY Etv1fl/fl in comparison to KPfCY mice. (C) Model of Etv1 induced effects on tumor volume, stromal expansion, and metastasis formation in pancreatic cancer.
Discussion
We demonstrate that the Ets-transcription factor Etv1 induces stromal expansion in PDAC leading to the development of significantly larger primary tumors. Correlating with this increased tumor volume we also observe both increased frequency and severity of ascites as well as increased frequency of liver and lung metastasis in a murine model of pancreatic cancer. Utilizing 3D pancreatic organoids, we demonstrate that Etv1 in concert with an oncogenic KrasG12D mutation disrupt cyst architecture, induce known EMT associated genes, and increase invasive capacity. We identify the matricellular protein Sparc as a novel downstream factor of Etv1, the loss of which completely abrogates the phenotype of stromal expansion and metastasis seen with Etv1 overexpression in vivo. Additionally, we identify Has2 as a second novel downstream factor that mediates the expansion of hyaluronic acid in the tumor microenvironmentm(Figure 6C). Preliminary experiments demonstrate that the conditional loss of Etv1 in the KPfCY mouse model is associated with decreased HA and SPARC.
Ets-transcription factors of the PEA3-family, namely Etv1 and Etv5, display a distinct expression pattern during the development of organs that undergo branching morphogenesis, such as the lung, salivary gland and pancreas. Branching morphogenesis is the result of the well-orchestrated interaction of epithelial and mesenchymal tissue compartments. In this process the expression of Etv1 is restricted to the mesenchymal compartment surrounding the growing epithelial buds that display high levels of Etv5, suggesting that Etv1 may play an important role for mesenchymal differentiation and stromal tissue identity.43 In human gastrointestinal stromal tumors (GIST) Etv1 has been demonstrated to serve as a lineage survival factor for the cKIT positive interstitial cells of Cajal that give rise to this tumor, indicating the important role of Etv1 for the mesenchymal differentiation and stromal tissue identity in this tumor.44 In this context, we are able to demonstrate that Etv1 induces EMT and invasion, in PanIN- and PDAC-cells in vitro and promotes metastasis and stromal expansion in vivo. Interestingly, two recent studies have suggested that EMT is seemingly dispensable in metastatic dissemination.45,46 It is conceivable that EMT and non-EMT mechanisms may be both involved in primary tumor formation and metastasis. However, it is interesting that Etv1 associated stromal expansion is associated so clearly with more aggressive primary and metastatic tumor behavior in vivo. Besides their well-described role as downstream effectors of Ras-signaling, Ets factors can be induced by multiple other signaling pathways that are active in PDAC and contribute to the desmoplastic tumor stroma, such as TGF-β signaling.
Besides early metastasis, PDAC is characterized by a pronounced desmoplastic stromal reaction composed of fibroblasts, immune cells, and endothelial cells embedded within a dense and complex extracellular matrix (ECM).10,39,47 We found that expression of Etv1 induces a remarkable increase of ECM in pancreatic orthotopic tumors through Sparc and Has2, suggesting that Etv1 might be an important regulator within the transcriptional networks that control the ECM in the tumor stroma of pancreatic cancer. In vitro we found that Sparc was required for Etv1associated induction of EMT factors Zeb1, Zeb2, and Snail. Very interestingly, we found that the loss of Sparc abrogated the increased hyaluronic acid deposition associated with Etv1 over-expression, suggesting an interplay between Sparc, Has2, and Etv1 in governing the ECM and tumor stroma. However, in KPfC cells in vitro, the loss of Sparc is not associated with changes in Has2, and over-expression of Has2 is not associated with changes in Sparc (Supplemental Figure 7). It is possible, if not likely, that modulation of the stroma, through both cell autonomous and non-cell autonomous mechanisms, provides a microenvironment permissive for tumor cell migration, invasion and dissemination into the circulation and ultimately, metastatic colonization. Sparc and Has2, in concert with Etv1, may be playing interrelated roles in the modification of the tumor stroma to facilitate tumor invasion, dissemination and metastasis. Further examination of the regulatory axis involving these factors is currently underway.
Sparc has been characterized as a frequent constituent of tumor associated desmoplastic stroma but was found absent in most PDAC and PDAC derived cell lines.13 Expression of Sparc in peritumoral fibroblasts has been found to be associated with poorer patient outcome.14 However, the mechanisms that regulate Sparc in PDAC still remain elusive. In our model, Etv1 drives the development of significantly larger primary tumors, more frequent metastasis and expansion of the tumor stroma, all of which are completely abrogated by the loss of Sparc. In line with our findings, Sparc has been recently identified as a critical factor in circulating tumor cells in pancreatic cancer that may mediate invasiveness and metastatic capacity.17 These and our data indicate that Sparc is not exclusively expressed in stromal cells and its expression in tumor cells contribute to the stromal expansion observed in these tumors. Moreover, expression of Sparc via Etv1 confers invasive and metastatic capacity to at least a subset of pancreatic tumor cells.
Hyaluronic acid (HA) is a major component of the ECM in many tumors and is synthesized by hyaluronic acid synthases 1-3 (Has1-3). In PDAC HA is abundant and its deposition begins with early precursor lesions.10 High amounts of HA and expression of Has2 in PDAC have been associated with shorter survival after resection, but the molecular mechanisms that lead to increased expression of HA in PDAC have not been elucidated.42 It has been demonstrated that abundance of HA and other ECM components results in increased interstitial fluid pressure within the tumor that leads to compression of intratumoral blood vessels and represents a barrier to perfusion, diffusion, and convection of chemotherapeutic agents as well as small molecule therapeutics.10 We have identified Etv1 as a transcriptional activator of Has2 expression, resulting in remarkably increased HA deposition within the PDAC stroma that in turn leads to larger tumors and is accompanied by a more metastatic phenotype. Recent data from human primary tumors and respective metastases revealed similar levels of stromal expansion and demonstrated that HA has been associated with shorter median survival.48 HA has been successfully ablated in mouse PDAC utilizing an enzymatic approach, which is associated with an improved response to conventional chemotherapy and is now undergoing testing in human clinical trials.10,49 Indeed, Etv1's association with hyaluronic acid deposition may lend it to potentially complementary therapeutic targeting in this context.
Our data now provide mechanistic insight and define novel functional roles for Etv1 in the regulation of EMT, stromal expansion, and metastasis through both Sparc and Has2. By regulating both stromal expansion as well as invasion and metastatic progression in PDAC, Etv1 might very well serve as a convergence point of different oncogenic signaling pathways making it a possible target for future therapeutic approaches.50 Moreover, identification of additional Etv1 targets in PDAC may enhance our understanding of the complex interactions of tumor cells and stroma in pancreatic cancer.
Supplementary Material
Supplemental Figure 1: (A) Immunohistochemistry for Etv1 in human low- and high- grade PanIN from a Tissue Microarray (TMA). Etv1 is expressed in 88% (15/17) of the low-grade PanIN and 100% (5/5) of the high-grade PanIN with similar staining grades in both cohorts. Staining is predominantly nuclear with occasional cytoplasmic staining also seen. (B) Immunohistochemistry for Etv1 in matched human primary PDAC and paired metastasis from a rapid autopsy cohort TMA. Etv1 is expressed in 88%(14/16) of the examined primary and in 94% (15/16) matched metastatic lesions with a similar distribution of staining intensity. Staining is predominantly nuclear with occasional cytoplasmic staining also seen. (C) Immunohistochemistry for Etv1 in the tumor areas and matched tumor associated stromal areas of human PDAC. Etv1 is expressed in both compartments in all examined tumors (7/7). (D) Immunohistochemistry for Etv1 in matched mouse primary PDAC (KPfC) and paired liver metastasis. Etv1 is expressed in 100% (5/5) of the examined primary and in 80% (4/5) matched liver metastatic lesions. (E) Etv1 expression in normal human pancreas vs. PDAC. Data from www.oncomine.org. Data from 39 samples analyzed using the Human Genome U133 Plus 2.0 Array (37). A 5.12 fold increase in Etv1 expression was found in human PDAC in comparison to matched normal controls (p<6.96×10-16).
Supplemental Figure 2: (A) Quantitative PCR for Etv1 in wild type, control and lentivirally transduced mEtv1 over-expressing cell lines: (Left) Stepwise increase in mEtv1 gene expression by qPCR in comparing wild type pancreatic ductal cells (PDC) with parental cell lines from KC (PanIN) and KPfC (PDAC) animals. There is a 15- fold increase in Etv1 between PDC and PanIN, and a 184-fold increase in Etv1 between PDC and PDAC cell lines. (Right) A consistent Etv1- overexpression of 6-9 fold after lentiviral transduction was seen across cell lines as compared to respective controls. *p<0.001 (B) Western blot for FLAG-M1 in KPfC, KPfC mEtv1, KPfC Sparc-/- Control, KPfC Sparc-/- mEtv1, KPfCY Control, and KPfCY mEtv1 cell lines used for orthotopic xenograft experiments. Respective parental cell lines were lentivirally transduced with Etv1-FLAG or empty vector (Control). β-actin served as loading control. (C) Treatment of KPfC PDAC-cells with the MEK-inhibitor U0126 resulted a significantly decreased expression of Etv1 compared to DMSO control. *p<0.001.
Supplemental Figure 3: (A) Ki67 positive cells per high power field in KPfCY Control and KPfCY mEtv1 tumors. There is no significant difference seen. (B) TUNEL positive cells per high power field of KPfCY Control and KPfCY mEtv1 tumors. There is no significant difference seen. (C) Growth curves of KPfC Control and KPfC mEtv1 cells in vitro. WST-1 assay demonstrates an increase in proliferation between KPfC Control and KPfC mEtv1 cells, with control cells proliferating at a slightly higher rate. (D) Grading of Ascites in orthotopic transplantation experiments with KPfCY Control and KPfCY mEtv1 tumors. Mice with mEtv1 overexpressing tumors displayed significantly more frequent and higher-grade ascites. (E) Quantification of metastatic events in the lungs of animals undergoing orthotopic transplantation with KPfCY control and KPfCY Etv1. There were significantly more frequent lung metastasis in the Etv1- overexpressing animals in comparison to their controls. All metastatic events seen in the lungs were isolated tumor cells. (F) Matched H&E and Immunofluorescence staining for YFP of a representative lung metastasis from a KPfCY mEtv1 xenograft. YFP positive cells from a KPfCY Etv1 tumor have formed a lung metastasis.
Supplemental Figure 4: (A) Schematic diagram of the Sparc promoter. Green boxes indicate predicted Etv1 binding sites. The promoter fragment upstream of the 5′ untranslated region was subcloned into the pGL3 Vector for Luciferase reporter analysis. (B) KPfCY mice (n=3) were sacrificed and primary tumors were first sorted by FACS for YFP+ fraction. The YFP+ fraction was then sorted into ECAD+ and ECAD- fractions and RNA isolated. Quantitative PCR demonstrates significantly increased Etv1 and Sparc in the YFP+/Ecad- fraction of 2/3 and 3/3 of these mice, respectively. (C) Histology of tumors used for the isolation of YFP+, ECad+/ECad- cell fractions in (B). (D) Quantitative PCR for EMT-related genes in KC control and KC mEtv1 cells. KC mEtv1 cells display a significant upregulation in classical regulators of EMT as well as matrix metalloproteases. (E) Invasion assay of KC control and KC mEtv1 cells using BD BioCoat™Matrigel Invasion Chambers (8μm Pore size). The invasive capacity of KC mEtv1 cells is significantly increased compared to KC control. (F) Knockdown of Etv1 by siRNA leads to a significant downregulation of Zeb1 in KPfC-cells. The invasive capacity of KPfC-cells is significantly reduced by knockdown of Etv1.
Supplemental Figure 5: (A) TGF-β-induced EMT in KC cells. Expression of Etv1 is significantly increased with induction of EMT in parallel with Snail, Zeb1, Zeb2, N-cad, followed by a decrease in Etv1 and these markers with withdrawal of TGF-β. (B) Loss of Sparc abrogates the increased expression of the EMT-markers Snail, Zeb1 and Zeb2 mediated by Etv1 in KPfC-cells. (C) Immunofluorescence staining for dTom (green) and Sparc (red) and E-cadherin (white, lower panel) in KPfC control, KPfC mEtv1 and KPfC Sparc-/- mEtv1 pancreatic orthotopic xenograft primary tumors. Co-localization of dTom and Sparc is present in the KPfC mEtv1 tumors (arrows indicating cells colocalizing), very low in the KPfC control (arrows indicating lack of colocalization) and absent KPfC Sparc-/- Etv1 tumors (arrows indicating lack of colocalization). KPfC mEtv1 xenografts that show co-localization of dTom and Sparc are negative for E-cadherin. Host-derived Sparc is detected in the KPfC Sparc-/- mEtv1 tumors in the dTom negative tumor associated stroma. (D) Quantification of frequency and grade of ascites in KPfC Control, KPfC mEtv1, KPfC Sparc-/- mEtv1 Orthotopic Transplantation Experiment. The increased frequency and grade of ascites observed with KPfC mEtv1 overexpressing xenografts is abrogated by the loss of Sparc.
Supplemental Figure 6: (A) Immunofluorescence staining for YFP (green) and αSMA (red) in KPfCY control and KPfCY mEtv1 tumors. Automated semi-quantitation is shown adjacent. Etv1 overexpressing tumors do not display increased α-SMA expression. (B) Number of GFAP-positive cells per high power field in KPfCY control and KPfCY mEtv1 tumors. No significant difference between KPfCY control and KPfCY mEtv1 tumors was observed. (C) Immunofluorescence staining for YFP (green) and lysyl-oxidase (LOX) (red) in KPfCY control and KPfCY mEtv1 tumors. Etv1 overexpressing tumors do not show increased expression of lysyl-oxidase. (D) Immuno-fluorescence staining for YFP (green) and Collagen I (red) in KPfCY control and KPfCY mEtv1 tumors. Etv1 overexpressing tumors do not show increased expression of Collagen I. (E) Quantitative PCR for Collagen I, III, and FAP in KPfCY Control and KPfCY mEtv1 cell lines shows no significant difference.
Supplemental Figure 7: (A) Left Panel: Expression of Has2 in KPfC - and KPfC Sparc-/- -cells (left panel). There was no significant difference in Has2 between these two cell lines. Right Panel: Expression of Sparc in KPfC and KPfC-cells overexpressing Has2. KPfC mHas2 cells had a 4.7 fold increase in Has2 in comparison to respective parental control (data not shown). There was no significant difference in Has2 between these two cell lines. (B+C) Staining for hyaluronic acid with the molecular probe HTI601 in KPfC control, KPfC mEtv1 and KPfC Sparc-/- mEtv1 tumors and quantification. KPfC mEtv1 xenografts display significantly larger hyaluronic acid positive areas than KPfC control, similar to findings with KPfCY control and KPfCY mEtv1. The increase in hyaluronic acid with Etv1 overexpression is completely abrogated by the loss of Sparc. (D) Correlation of tumor volume and hyaluronic acid positive area in KPfC Control (green dots), KPfC mEtv1 (red dots), and KPfC Sparc-/- mEtv1 (yellow dots).
Supplemental Table 1: Primer Sequences
Supplemental Table 2: Predicted Ets-binding sites in ECM components of PDAC
Acknowledgments
Grant Support: This work was supported by the NIH R01 DK060694 (to ST, MR, AKR), NIH/NIDDK T32-DK007066 (KD), National Pancreas Foundation (MR), Deutsche Krebshilfe (Dr. Mildred Scheel Stiftung, 110037 (SH), Max Eder Program, 111273 (MR) and 110972 to (AN)), the AGA-Actavis Research Award in Pancreatic Disorders (MR), Honjo International Scholarship Foundation (ST), NIH/NIDDK P30-DK050306 Center for Molecular Studies in Digestive and Liver Diseases (Molecular Pathology and Imaging, Molecular Biology/Gene Expression, Cell Culture, and Transgenic and Chimeric Mouse Cores), and American Cancer Society Grant RP-10-033-01-CCE (AKR).
Footnotes
Disclosures: The authors have no disclosures.
Transcript Profiling: None
Writing Assistance: None
Author Contributions: SH, KKD, MR, BB, AKR – were all involved in the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and statistical analysis.
ST, JC, AN, NA, KW, AM, CAID, and PH – were involved in acquisition of data and analysis and interpretation of data
Author names in bold designate shared co-first authorship
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith BD, Smith GL, Hurria A, et al. Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. Journal of Clinical Oncology. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 3.Allison DC, Piantadosi S, Hruban RH, et al. DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J Surg Oncol. 1998;67:151–159. doi: 10.1002/(sici)1096-9098(199803)67:3<151::aid-jso2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RYJ, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 6.Krantz SB, Shields MA, Dangi-Garimella S, et al. Contribution of Epithelial-to-Mesenchymal Transition and Cancer Stem Cells to Pancreatic Cancer Progression. Journal of Surgical Research. 2012;173:105–112. doi: 10.1016/j.jss.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhim AD. Pancreatology. Pancreatology. 2013;13:114–117. doi: 10.1016/j.pan.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feig C, Gopinathan A, Neesse A, et al. The Pancreas Cancer Microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stromnes IM, DelGiorno KE, Greenberg PD, et al. Stromal reengineering to treat pancreas cancer. Carcinogenesis. 2014;35:1451–1460. doi: 10.1093/carcin/bgu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of Carcinoma-Associated Fibroblastsand Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal Elements Actto Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato N, Fukushima N, Maehara N, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor–stromal interactions. Oncogene. 2003;22:5021–5030. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 14.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral Fibroblast SPARC Expression and Patient Outcome With Resectable Pancreatic Adenocarcinoma. Journal of Clinical Oncology. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 15.Hoff Von DD, Ramanathan RK, Borad MJ, et al. Gemcitabine Plus nab-Paclitaxel Is an Active Regimen in Patients With Advanced Pancreatic Cancer: A Phase I/II Trial. Journal of Clinical Oncology. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han W, Cao F, Chen MB, et al. Prognostic Value of SPARC in Patients with Pancreatic Cancer: A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0145803. doi: 10.1371/journal.pone.0145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ting DT, Wittner Ben S, Ligorio M, et al. Single-Cell RNA Sequencing Identifies Extracellular Matrix Gene Expression by Pancreatic Circulating Tumor Cells. CELREP. 2014;8:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121:4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichert M, Takano S, Burstin von J, et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes & Development. 2013;27:288–300. doi: 10.1101/gad.204453.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kar A, Gutierrez-Hartmann A. Molecular mechanisms of ETS transcription factor-mediated tumorigenesis. Critical Reviews in Biochemistry and Molecular Biology. 2013;48:522–543. doi: 10.3109/10409238.2013.838202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh S, Shin S, Janknecht R. ETV1, 4 and 5: An oncogenic subfamily of ETS transcription factors. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2012;1826:1–12. doi: 10.1016/j.bbcan.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chotteau-Lelievre A, Montesano R, Soriano J, et al. PEA3 transcription factors are expressed in tissues undergoing branching morphogenesis and promote formation of duct-like structures by mammary epithelial cells in vitro. Dev Biol. 2003;259:241–257. doi: 10.1016/s0012-1606(03)00182-9. [DOI] [PubMed] [Google Scholar]
- 23.Kobberup S, Nyeng P, Juhl K, et al. ETS-family genes in pancreatic development. Dev Dyn. 2007;236:3100–3110. doi: 10.1002/dvdy.21292. [DOI] [PubMed] [Google Scholar]
- 24.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 25.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neesse A, Frese KK, Chan DS, et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2013 doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichert M, Takano S, Heeg S, et al. Isolation, culture and genetic manipulation of mouse pancreatic ductal cells. Nat Protoc. 2013;8:1354–1365. doi: 10.1038/nprot.2013.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wescott MP, Rovira M, Reichert M, et al. Pancreatic ductal morphogenesis and the Pdx1 homeodomain transcription factor. Molecular Biology of the Cell. 2009;20:4838–4844. doi: 10.1091/mbc.E09-03-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burstin von J, Reichert M, Wescott MP, et al. The pancreatic and duodenal homeobox protein PDX-1 regulates the ductal specific keratin 19 through the degradation of MEIS1 and DNA binding. PLoS ONE. 2010;5:e12311. doi: 10.1371/journal.pone.0012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammad RM, Dugan MC, Mohamed AN, et al. Establishment of a human pancreatic tumor xenograft model: potential application for preclinical evaluation of novel therapeutic agents. Pancreas. 1998;16:19–25. doi: 10.1097/00006676-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kozono S, Ohuchida K, Eguchi D, et al. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Research. 2013;73:2345–2356. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 33.Edge S, Byrd DR, Compton CC, et al., editors. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 7. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama H, Nakanishi H, Kodera Y, et al. Biological significance of isolated tumor cells and micrometastasis in lymph nodes evaluated using a green fluorescent protein-tagged human gastric cancer cell line. Clin Cancer Res. 2006;12:361–368. doi: 10.1158/1078-0432.CCR-05-1963. [DOI] [PubMed] [Google Scholar]
- 35.Patel TD, Kramer I, Kucera J, et al. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- 36.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 37.Badea L, Herlea V, Dima SO, et al. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–2027. [PubMed] [Google Scholar]
- 38.Lunardi S, Muschel RJ, Brunner TB. Cancer Letters. Cancer Letters. 2014;343:147–155. doi: 10.1016/j.canlet.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 39.Mahadevan D, Hoff Von DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Molecular Cancer Therapeutics. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 40.Itano N, Sawai T, Yoshida M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 41.Kultti A, Zhao C, Singha NC, et al. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. Biomed Res Int. 2014;2014:817613. doi: 10.1155/2014/817613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng XB, Sato N, Kohi S, et al. Prognostic Impact of Hyaluronan and Its Regulators in Pancreatic Ductal Adenocarcinoma Lo AWI, ed. PLoS ONE. 2013;8:e80765. [Google Scholar]
- 43.Yuen HF, Chan YK, Grills C, et al. Polyomavirus enhancer activator 3 protein promotes breast cancer metastatic progression through Snail-induced epithelial-mesenchymal transition. J Pathol. 2011;224:78–89. doi: 10.1002/path.2859. [DOI] [PubMed] [Google Scholar]
- 44.Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015:1–18. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu GC, Kimmelman AC, Hezel AF, et al. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 48.Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hingorani SR, Harris WP, Beck JT, et al. Final results of a phase Ib study of gemcitabine plus PEGPH20 in patients with stage IV previously untreated pancreatic cancer. J Clin Oncol. 2015 [Google Scholar]
- 50.Pop MS, Stransky N, Garvie CW, et al. A small molecule that binds and inhibits the ETV1 transcription factor oncoprotein. Molecular Cancer Therapeutics. 2014 doi: 10.1158/1535-7163.MCT-13-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: (A) Immunohistochemistry for Etv1 in human low- and high- grade PanIN from a Tissue Microarray (TMA). Etv1 is expressed in 88% (15/17) of the low-grade PanIN and 100% (5/5) of the high-grade PanIN with similar staining grades in both cohorts. Staining is predominantly nuclear with occasional cytoplasmic staining also seen. (B) Immunohistochemistry for Etv1 in matched human primary PDAC and paired metastasis from a rapid autopsy cohort TMA. Etv1 is expressed in 88%(14/16) of the examined primary and in 94% (15/16) matched metastatic lesions with a similar distribution of staining intensity. Staining is predominantly nuclear with occasional cytoplasmic staining also seen. (C) Immunohistochemistry for Etv1 in the tumor areas and matched tumor associated stromal areas of human PDAC. Etv1 is expressed in both compartments in all examined tumors (7/7). (D) Immunohistochemistry for Etv1 in matched mouse primary PDAC (KPfC) and paired liver metastasis. Etv1 is expressed in 100% (5/5) of the examined primary and in 80% (4/5) matched liver metastatic lesions. (E) Etv1 expression in normal human pancreas vs. PDAC. Data from www.oncomine.org. Data from 39 samples analyzed using the Human Genome U133 Plus 2.0 Array (37). A 5.12 fold increase in Etv1 expression was found in human PDAC in comparison to matched normal controls (p<6.96×10-16).
Supplemental Figure 2: (A) Quantitative PCR for Etv1 in wild type, control and lentivirally transduced mEtv1 over-expressing cell lines: (Left) Stepwise increase in mEtv1 gene expression by qPCR in comparing wild type pancreatic ductal cells (PDC) with parental cell lines from KC (PanIN) and KPfC (PDAC) animals. There is a 15- fold increase in Etv1 between PDC and PanIN, and a 184-fold increase in Etv1 between PDC and PDAC cell lines. (Right) A consistent Etv1- overexpression of 6-9 fold after lentiviral transduction was seen across cell lines as compared to respective controls. *p<0.001 (B) Western blot for FLAG-M1 in KPfC, KPfC mEtv1, KPfC Sparc-/- Control, KPfC Sparc-/- mEtv1, KPfCY Control, and KPfCY mEtv1 cell lines used for orthotopic xenograft experiments. Respective parental cell lines were lentivirally transduced with Etv1-FLAG or empty vector (Control). β-actin served as loading control. (C) Treatment of KPfC PDAC-cells with the MEK-inhibitor U0126 resulted a significantly decreased expression of Etv1 compared to DMSO control. *p<0.001.
Supplemental Figure 3: (A) Ki67 positive cells per high power field in KPfCY Control and KPfCY mEtv1 tumors. There is no significant difference seen. (B) TUNEL positive cells per high power field of KPfCY Control and KPfCY mEtv1 tumors. There is no significant difference seen. (C) Growth curves of KPfC Control and KPfC mEtv1 cells in vitro. WST-1 assay demonstrates an increase in proliferation between KPfC Control and KPfC mEtv1 cells, with control cells proliferating at a slightly higher rate. (D) Grading of Ascites in orthotopic transplantation experiments with KPfCY Control and KPfCY mEtv1 tumors. Mice with mEtv1 overexpressing tumors displayed significantly more frequent and higher-grade ascites. (E) Quantification of metastatic events in the lungs of animals undergoing orthotopic transplantation with KPfCY control and KPfCY Etv1. There were significantly more frequent lung metastasis in the Etv1- overexpressing animals in comparison to their controls. All metastatic events seen in the lungs were isolated tumor cells. (F) Matched H&E and Immunofluorescence staining for YFP of a representative lung metastasis from a KPfCY mEtv1 xenograft. YFP positive cells from a KPfCY Etv1 tumor have formed a lung metastasis.
Supplemental Figure 4: (A) Schematic diagram of the Sparc promoter. Green boxes indicate predicted Etv1 binding sites. The promoter fragment upstream of the 5′ untranslated region was subcloned into the pGL3 Vector for Luciferase reporter analysis. (B) KPfCY mice (n=3) were sacrificed and primary tumors were first sorted by FACS for YFP+ fraction. The YFP+ fraction was then sorted into ECAD+ and ECAD- fractions and RNA isolated. Quantitative PCR demonstrates significantly increased Etv1 and Sparc in the YFP+/Ecad- fraction of 2/3 and 3/3 of these mice, respectively. (C) Histology of tumors used for the isolation of YFP+, ECad+/ECad- cell fractions in (B). (D) Quantitative PCR for EMT-related genes in KC control and KC mEtv1 cells. KC mEtv1 cells display a significant upregulation in classical regulators of EMT as well as matrix metalloproteases. (E) Invasion assay of KC control and KC mEtv1 cells using BD BioCoat™Matrigel Invasion Chambers (8μm Pore size). The invasive capacity of KC mEtv1 cells is significantly increased compared to KC control. (F) Knockdown of Etv1 by siRNA leads to a significant downregulation of Zeb1 in KPfC-cells. The invasive capacity of KPfC-cells is significantly reduced by knockdown of Etv1.
Supplemental Figure 5: (A) TGF-β-induced EMT in KC cells. Expression of Etv1 is significantly increased with induction of EMT in parallel with Snail, Zeb1, Zeb2, N-cad, followed by a decrease in Etv1 and these markers with withdrawal of TGF-β. (B) Loss of Sparc abrogates the increased expression of the EMT-markers Snail, Zeb1 and Zeb2 mediated by Etv1 in KPfC-cells. (C) Immunofluorescence staining for dTom (green) and Sparc (red) and E-cadherin (white, lower panel) in KPfC control, KPfC mEtv1 and KPfC Sparc-/- mEtv1 pancreatic orthotopic xenograft primary tumors. Co-localization of dTom and Sparc is present in the KPfC mEtv1 tumors (arrows indicating cells colocalizing), very low in the KPfC control (arrows indicating lack of colocalization) and absent KPfC Sparc-/- Etv1 tumors (arrows indicating lack of colocalization). KPfC mEtv1 xenografts that show co-localization of dTom and Sparc are negative for E-cadherin. Host-derived Sparc is detected in the KPfC Sparc-/- mEtv1 tumors in the dTom negative tumor associated stroma. (D) Quantification of frequency and grade of ascites in KPfC Control, KPfC mEtv1, KPfC Sparc-/- mEtv1 Orthotopic Transplantation Experiment. The increased frequency and grade of ascites observed with KPfC mEtv1 overexpressing xenografts is abrogated by the loss of Sparc.
Supplemental Figure 6: (A) Immunofluorescence staining for YFP (green) and αSMA (red) in KPfCY control and KPfCY mEtv1 tumors. Automated semi-quantitation is shown adjacent. Etv1 overexpressing tumors do not display increased α-SMA expression. (B) Number of GFAP-positive cells per high power field in KPfCY control and KPfCY mEtv1 tumors. No significant difference between KPfCY control and KPfCY mEtv1 tumors was observed. (C) Immunofluorescence staining for YFP (green) and lysyl-oxidase (LOX) (red) in KPfCY control and KPfCY mEtv1 tumors. Etv1 overexpressing tumors do not show increased expression of lysyl-oxidase. (D) Immuno-fluorescence staining for YFP (green) and Collagen I (red) in KPfCY control and KPfCY mEtv1 tumors. Etv1 overexpressing tumors do not show increased expression of Collagen I. (E) Quantitative PCR for Collagen I, III, and FAP in KPfCY Control and KPfCY mEtv1 cell lines shows no significant difference.
Supplemental Figure 7: (A) Left Panel: Expression of Has2 in KPfC - and KPfC Sparc-/- -cells (left panel). There was no significant difference in Has2 between these two cell lines. Right Panel: Expression of Sparc in KPfC and KPfC-cells overexpressing Has2. KPfC mHas2 cells had a 4.7 fold increase in Has2 in comparison to respective parental control (data not shown). There was no significant difference in Has2 between these two cell lines. (B+C) Staining for hyaluronic acid with the molecular probe HTI601 in KPfC control, KPfC mEtv1 and KPfC Sparc-/- mEtv1 tumors and quantification. KPfC mEtv1 xenografts display significantly larger hyaluronic acid positive areas than KPfC control, similar to findings with KPfCY control and KPfCY mEtv1. The increase in hyaluronic acid with Etv1 overexpression is completely abrogated by the loss of Sparc. (D) Correlation of tumor volume and hyaluronic acid positive area in KPfC Control (green dots), KPfC mEtv1 (red dots), and KPfC Sparc-/- mEtv1 (yellow dots).
Supplemental Table 1: Primer Sequences
Supplemental Table 2: Predicted Ets-binding sites in ECM components of PDAC


