Abstract
Critical limb ischemia is a devastating manifestation of peripheral arterial disease with no effective strategies for improving morbidity and mortality outcomes. We tested the hypothesis that cellular mitochondrial function is a key component of limb pathology and that improving mitochondrial function represents a novel paradigm for therapy. BALB/c mice were treated with a therapeutic mitochondrial-targeting peptide (MTP-131) and subjected to limb ischemia (HLI). Compared to vehicle control, MTP-131 rescued limb muscle capillary density and blood flow (64.7±11% of contralateral vs. 39.9±4%), and improved muscle regeneration. MTP-131 also increased electron transport system flux across all conditions at HLI day-7. In vitro, primary muscle cells exposed to experimental ischemia demonstrated markedly reduced (~75%) cellular respiration, which was rescued by MTP-131 during a recovery period. Compared to muscle cells, endothelial cell (HUVEC) respiration was inherently protected from ischemia (~30% reduction), but was also enhanced by MTP-131. These findings demonstrate an important link between ischemic tissue bioenergetics and limb blood flow and indicate that the mitochondria may be a pharmaceutical target for therapeutic intervention during critical limb ischemia.
Keywords: ischemia, peripheral artery disease, critical limb ischemia, mitochondria
1. Introduction
Critical limb ischemia (CLI) patients have a risk of major amputation or death that approaches 40% in one year [1, 2], with few effective clinical treatment paradigms. Limb skeletal muscle plays a critical role in determining morbidity and mortality in peripheral arterial disease patients [3, 4] and the plastic nature of the ischemic skeletal muscle serves as a unique and potentially influential medium for the vascular network. As little is known about how ischemic limb metabolism contributes to the myopathic and angiogenic/arteriogenic responses, this represents an exciting area of discovery for therapeutic intervention. Limb muscle mitochondrial content is inversely related to PAD mortality [8] and PAD patients have abnormal mitochondrial function and increased oxidative stress-mediated damage [5–7]. In this report, we demonstrate the efficacy of therapeutically targeting mitochondria to improve limb perfusion recovery and myopathy using a murine model of CLI.
2. Materials and Methods
2.1 Animals
Experiments were conducted on adult (≥12 weeks) BALB/cJ male mice purchased from Jackson Laboratories. All animal experiments adhered to the Guide for the Care and Use of Laboratory Animals from the Institute for Laboratory Animal Research, National Research Council, Washington, D.C., National Academy Press, 1996, and any updates. All procedures were approved by the Institutional Animal Care and Use Committee of East Carolina University.
2.2 Materials
All reagents and chemicals were obtained from Sigma-Aldrich with the exception of Amplex Ultra Red (Invitrogen) and pronase (Calbiochem). All cell culture materials were obtained from Gibco (Life Technologies) unless otherwise stated.
2.3 Animal Models of Ischemic Peripheral Artery Disease
Acute hindlimb ischemia (HLI) [8] was induced by ligation and excision of the femoral artery from its origin just below the inguinal ligament by a single blinded investigator (JMM). The inferior epigastric, lateral circumflex, and superficial epigastric artery branches of the femoral artery were left intact, thereby preserving collateral perfusion to the limb. Mice were anesthetized via intraperitoneal (IP) injection of ketamine/xylazine.
2.4 Mitochondrial Therapy
Mice were administered either a mitochondrial-targeted therapy (MTP-131, generic name elamipretide) or saline control via IP injection. The dosage of MTP-131 was 1.5mg/kg body weight in sterile saline. IP injections began 24h prior to the onset of ischemia, and continued once daily until sacrifice for experiments in Figure 1, as well as Supplemental Figures 2 and 3. In Supplemental Figure 1, IP injections began 6 hours post surgery and continued once daily until sacrifice. A single investigator (CAS), who had no involvement with other in vivo analyses (LDPI, necrosis scoring), was responsible for treatments and daily animal handling.
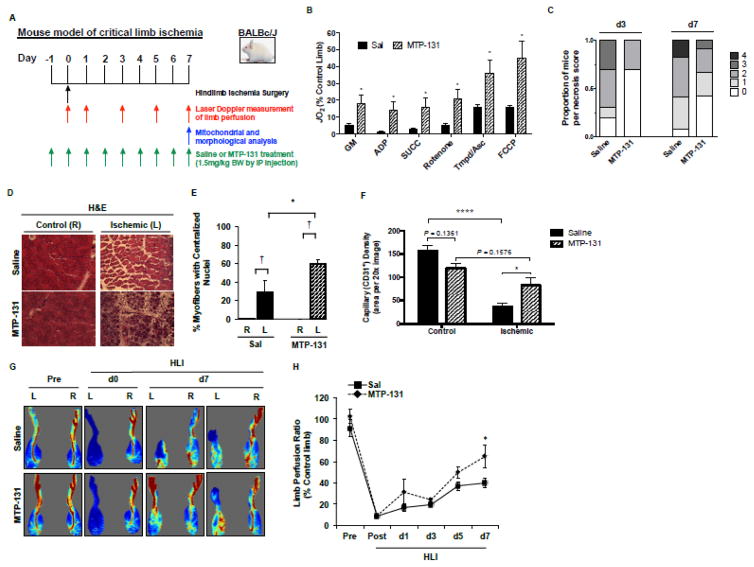
Figure 1. Effects of pre-surgery MTP-131 therapy on limb necrosis, perfusion and vascular density, and skeletal muscle mitochondrial function.
MTP-131 or Saline was administered by IP-injection 24-hours prior to the onset of acute hindlimb ischemia (HLI), and once daily until sacrifice. A. Graphical display of experimental time course. B. Skeletal muscle mitochondrial respiratory function (expressed as a percentage of the non-ischemic control limb) HLI d7 (n=10/group). C. Distribution of limb necrosis score after 3- and 7-day HLI (n=16/group). D. Representative H&E sections demonstrating TA morphology after HLI d7. R, right (non-surgical limb); L, left (surgical limb). E. Quantitation of regenerating TA myofibers (centralized nuclei) at HLI d7 (n=10/group). † P<0.05 vs. contralateral control (R). * P<0.05 vs. Saline Ischemia (L). F. Capillary density (CD31 positive immunofluorescence) in the control and ischemic TA muscle at HLI d7 (n=10/group). * P<0.05; **** P<0.0001. G. Representative images from laser Doppler perfusion imaging in mice before (Pre), immediately post-op (d0), and HLI d7. H. Quantitation of whole limb perfusion, presented as a ratio of ischemic (L) to non-ischemic (R) limb perfusion (n=16/group). * P<0.05 vs. Saline.
2.5 Necrosis Score
The extent of necrosis, if any, was recorded postoperatively using a semiquantitative scale by a single blinded investigator (JMM) [8]: grade 0, no necrosis in ischemic limb; grade I, necrosis limited to toes; grade II, necrosis extending to dorsum pedis; grade III, necrosis extending to crus; and grade IV, necrosis extending to thigh or complete limb necrosis.
2.6 Assessment of Limb Perfusion
Laser Doppler perfusion image (LDPI) scanning and image analysis was performed by a single blinded investigator (TER) using a Moor Instruments LDI2-High Resolution (830nM) System (Moor, Axminster, UK).
2.7 Preparation of isolated skeletal muscle mitochondria
Skeletal muscle mitochondria were isolated by a blinded investigator (TER) from the plantar flexor (i.e. gastrocnemius and soleus) muscles of both control (right) and surgery (left) hindlimbs. To ensure sufficient mitochondrial yield, muscle was pooled from two animals. Dissected muscle was washed in mitochondrial isolation medium (MIM: 300mM sucrose, 10mM HEPES, 1mM EGTA), minced on ice, then trypsinized for two minutes. Trypsinized muscle was homogenized on ice and centrifuged at 800xg to pellet non-mitochondrial myofibrillar proteins, nuclei, and other cellular components. The resulting supernatant was centrifuged at 12,000xg to pellet mitochondria. The final mitochondrial pellet was resuspended in MIM and stored on ice until analysis (less than 1 hr). Mitochondrial protein content was determined by BCA protein assay (Pierce).
2.8 Mitochondrial respiration measurements
High-resolution O2 consumption measurements were conducted by blinded investigators (TER and RJA) at 37 C in buffer Z (105 mM K-MES, 30 mM KCl, 1 mM EGTA, 10 mM K2HPO4, 5 mM MgCl2-6H2O, 0.5 mg/ml BSA, pH 7.1), supplemented with creatine monohydrate (20 mM), using the OROBOROS O2K Oxygraph. A substrate inhibitor titration protocol was run as follows: 2mM Malate + 10mM Glutamate (State 2 respiration), followed by the addition of 4mM ADP to initiate State 3 respiration supported by Complex I substrates; convergent electron flow was initiated with 10mM Succinate; 10μM Rotenone was added to inhibit Complex I, followed by 10μM Cytochrome C to test the integrity of the mitochondrial membrane; Complex IV supported respiration was examined using N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) at 0.5mM with 2mM Ascorbate (to limit auto-oxidation of TMPD) and 5μM of Antimycin A (to inhibit reverse electron flow); finally, uncoupled respiration was assessed with 0.5μM Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP). All respiration measurements were performed in duplicate and the average taken for statistical analysis. The rate of respiration was normalized to mitochondrial protein loaded.
2.9 Histological and Immunofluorescence Microscopy
The tibialis anterior muscle (TA) was harvested at the time of sacrifice and placed in OCT (optimal cutting temperature medium) and frozen in liquid nitrogen cooled isopentane. 8μm sections were cut on a CM3050S cryotome (Leica). For histological analysis, sections were stained with Hematoxylin and Eosin, images were acquired at 20× magnification and centralized nuclei were quantified using NIH ImageJ. For immunofluorescence staining, sections were fixed in 50/50 acetone/methanol. Fixed sections were blocked in 5% goat serum in 1X PBS. Sections were incubated with Rat anti-Mouse CD31 (AbD Serotec MCA-2388), Mouse IgG anti smooth muscle actin (Sigma 074M4814V), and Rabbit anti dystrophin (Thermo Scientific RB-9024) primary antibodies. Washes were performed using 1X PBS. Sections were incubated in Alexa fluor 488 goat anti-rat igG (Invitrogen A-11006), Alexa fluor 568 goat anti-mouse IgG2a (Invitrogen A-21134), and Alexa fluor 647 goat anti-rabbit IgG highly cross adsorbed (Invitrogen A-21245) secondary antibodies. Sections were mounted using Vectashield hard set mounting medium with DAPI (Vector Laboratories H-1500). Images were obtained using an Evos FL auto cell imaging system (Life Technologies AMAFD1000). 20X images of each section were taken at similar topographical positions (n = 3 each: anterolateral, central, and posteromedial). ImageJ was used to overlay a grid on each image and a cell counter plugin was used to count discrete positive CD31 signal in each grid. All images were coded and randomized for blinded analysis.
2.10 Primary Muscle Progenitor Cell Isolation and Culture
Primary murine muscle precursor cells (mouse myoblasts) derived from peripheral hindlimb muscles as previously described [8]. After myoblast purification (1hr pre-plating on an uncoated flask to allow fibroblast adherence), cells were maintained on collagen coated T150 flasks. After reaching approximately 70% confluence, MPCs were then plated in pre-warmed growth media (GM: Hams F10 with 20% FBS and 1% Penicillin/Streptomycin/Amphotericin B) on culture-ware coated with entactin/collagen/laminin and allowed to reach approximately 90% confluence. Confluent MPCs were then switched to differentiation medium (DM: DMEM with 4.5g/L glucose, supplemented with 2% HoS and 1% Penicillin/Streptomycin/Amphotericin B and supplemented with 0.1% insulin/transferrin/selenium). DM was changed every 24-hours.
2.11 Human Umbilical Vein Endothelial Cell Culture
HUVECs were purchased from ATCC and cultured on 0.1% gelatin coated dishes in endothelial cell growth medium (GM: EBM2 with EGM-2MV bullet kit, supplemented with 20%FBS and 1% Penicillin/Streptomycin/Amphotericin B).
2.12 In vitro Hypoxia and Nutrient Deprivation
We utilized a previously described in vitro model of hypoxia and nutrient deprivation to examine the response in primary cells and mitochondria [8]. For cell experiments, the cells were placed in Hank’s buffered saline solution (HBSS) in a cakepan hypoxia chamber flushed for nitrogen for ~10 min.
2.13 Primary cell respiration measurements
Primary muscle and endothelial cell respiration was assessed using the Seahorse XF24 Analyzer. Cells were placed in assay media containing 2.5mM Glucose, 10mM Pyruvate, and 2mM GlutaMax approximately 30 minutes prior to the start of the experiment, and allowed to equilibrate. Respiration was measured with sequential additions of 1μg/ml oligomycin (to inhibit ATP synthase), 1μM FCCP (to uncouple respiration), and 2.5μM Antimycin-A (to inhibit electron flow by blocking Complex III). All cell respiration experiments were performed using identical cell seeding densities (150,000 cells/well for myoblasts) and corrected for background rates of oxygen consumption. Seahorse experiments were performed by a single blinded investigator (TER) who was unaware of groups/treatments.
2.14 Statistics
Data are presented as mean ± SEM. a priori two sided t-tests were performed to determine differences in the control (R) limbs of the vehicle and peptide groups. Statistical analysis was performed using unpaired Student’s t test or one-way ANOVA with Tukey’s multiple comparison’s test for analysis of significance among groups. Repeated-measures ANOVA was used for analysis for LDPI data. The level of significance was set at P < 0.05.
3. Results
Ischemia robustly attenuated BALB/c muscle mitochondrial respiratory function, which was rescued by MTP-131 (Figure 1A; expressed as a percentage of contralateral control limb). MTP-131 also altered the distribution of BALB/c limb tissue necrosis (Figure 1B), and improved ischemic muscle regeneration at HLI day-7 (Figure 1C,D). Laser Doppler perfusion imaging verified improved whole limb blood flow by MTP-131 (64.7±11% versus 39.9±4%; expressed as a percentage of contralateral control limb) (Figure 1E,F) and rescued capillary density (Figure 1G) at HLI d7. Importantly, there were no detectable effects of MTP-131 on mitochondrial respiration, limb perfusion, or vascular density in the non-ischemic control limb (vs. vehicle control limb). In an attempt to more closely mimic patients in the clinic, we also administered MTP-131 after the onset of ischemia. Post-surgery delivery of MTP-131 improved limb perfusion recovery and muscle regeneration, as well as modest increases in mitochondrial respiratory function (Supplemental Figure 1). However, the magnitude of improvement in these outcomes was not as large as when MTP-131 was delivered prior to ischemia. This apparent difference in pre-clinical efficacy is likely due to limitations of drug availability to the ischemic limb when administered post femoral artery ligation and excision.
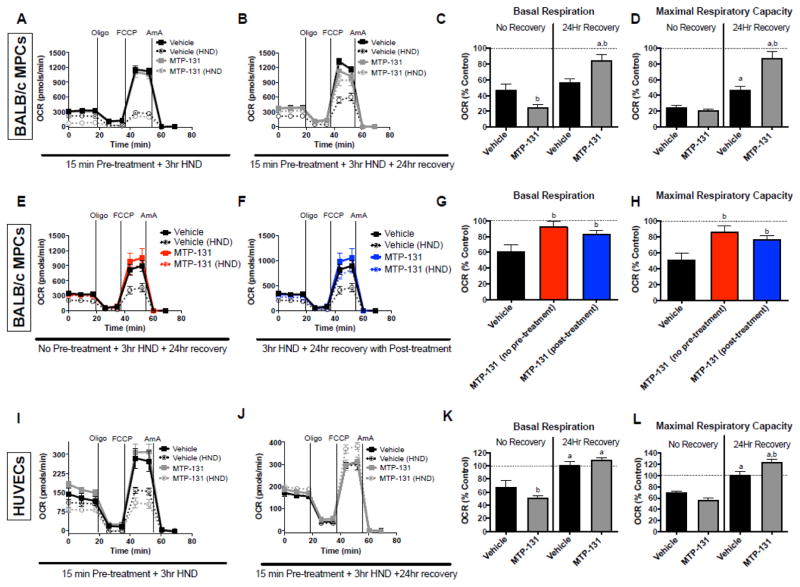
We next isolated primary muscle progenitor cells from BALB/c hindlimbs. After differentiation, cultures were subjected to experimental ischemia with or without MTP-131 and cellular respiration was assessed. Myotubes displayed a marked reduction (~75%) in maximal respiratory capacity following ischemia, which was largely rescued by MTP-131 treatment during 24-hours of recovery (Figure 2A–D). To mimic the in vivo experiments, we also performed in vitro assays in primary myotubes in which MTP-131 was administered during ischemia (no pre-treatment) as well as after ischemia (during the 24h recovery period). In both treatment scenarios MTP-131 rescued primary muscle cell respiratory function (Figure 2E–H). Interestingly, endothelial cells (HUVECs) displayed only a modest (~30%) reduction in maximal respiratory capacity following 3-hour ischemia, which was intrinsically recovered by 24-hours of normoxia (Figure 2I–L). MTP-131 increased the maximal respiratory capacity of HUVEC cells above baseline during ischemia recovery (Figure 2J,L).
Figure 2. Effects of MTP-131 on primary myotube and HUVEC cellular respiratory function.
Cellular respiration was assessed using the Seahorse XF24 analyzer in both control (normoxia with normal culture media) and HND (hypoxia with HBSS) muscle and endothelial cells. A. Primary BALB/c muscle cells (vehicle vs. MTP-131) were exposed to 3-hr HND, after which cellular respiration was assessed immediately following using a sequential protocol with additions of 1μg/ml oligomycin (ATP synthase inhibitor), 1μM FCCP (chemical uncoupler), and 2.5μM antimycin A (mitochondrial complex III inhibitor). B. Vehicle-treated primary muscle cells and MTP-131 primary muscle cells were subjected to 3-hr HND and allowed to recover in normal media for 24 hours before assaying cell respiration. C. Basal respiration rates (prior to addition of oligomycin) expressed as a percentage of the control (normoxia) cells with no recovery (from panel A) and 24h recovery (from panel B). D. Maximal respiratory capacity (addition of FCCP) expressed as a percentage of the control (normoxia) cells with no recovery (from panel A) and 24h recovery (from panel B). E. BALB/c primary muscle cells were treated with MTP-131 and immediately subjected to 3-hr HND (no pre-treatment) and allowed to recovery 24 hours before cell respiration assay. F. BALB/c primary muscle cells were subjected to 3-hr HND and then treated with MTP-131 after HND (post-treatment) and allowed to recovery 24 hours before cell respiration assay. G. Basal respiration rates (prior to addition of oligomycin) expressed as a percentage of the control (normoxia) cells (from panels E and F) and 24h recovery. H. Maximal respiratory capacity (addition of FCCP) expressed as a percentage of the control (normoxia) cells (from panels E and F). I. Human endothelial cells (vehicle vs. MTP-131) were exposed to 3-hr HND, after which cellular respiration was assessed immediately following using a sequential protocol with additions of 1μg/ml oligomycin (ATP synthase inhibitor), 1μM FCCP (chemical uncoupler), and 2.5μM antimycin A (mitochondrial complex III inhibitor). J. Vehicle-treated HUVECs and MTP-131 treated HUVECs were subjected to 3-hr HND and allowed to recover in normal media for 24 hours before assaying cell respiration. K. Basal respiration rates (prior to addition of oligomycin) expressed as a percentage of the control (normoxia) cells with no recovery (from panel E) and 24h recovery (from panel F). L. Maximal respiratory capacity (addition of FCCP) expressed as a percentage of the control (normoxia) cells with no recovery (from panel E) and 24h recovery (from panel F). a P<0.05 vs. no recovery (time effect). bP<0.05 vs. vehicle-treated control (drug effect).
4. Discussion
PAD is often associated with skeletal muscle myopathies including reduced muscle strength and exercise intolerance, reduced mitochondrial enzyme activity, and increased markers of oxidative stress which have been related to morbidity and mortality in these patients [3, 7, 9, 10]. Current treatment paradigms are focused on re-establishing blood flow to the limb through surgical or therapeutic neovascularization approaches, but these have limited success at reducing long-term morbidity and mortality [11]. In the clinical realm, a clear need exists for novel therapeutic treatments that are cytoprotective, easy to administer, and can prevent/lessen limb necrosis. Mitochondria are an attractive therapeutic for several reasons: 1) reduced ATP generation can disrupt ion homeostasis and contribute to the activation of the mitochondrial permeability transition pore and initiation of apoptosis, and 2) substantial degenerative and regenerative processes in the ischemic limb require efficient electron transport system flux to satisfy the high demand for ATP and the efficient performance of these tasks.
A number of previous reports have demonstrated the beneficial effects of MTP-131 on ischemic pathology in pre-clinical models of ischemic diseases in other organ/tissue systems including cardiac [12–16] and kidney [17–19] ischemia/reperfusion. In addition, mitochondrial-targeted peptides such as SS-31 or MTP-131 have also shown beneficial effects in non-ischemic disease models [20–24]. MTP-131 is an analogue of SS-31 [25], that accumulates specifically in mitochondria in a membrane potential-independent manner [12, 26]. MTP-131 has been repeatedly shown to reduce production/emission of reactive oxygen species [15, 26, 27] although the underlying mechanism is independent of direct scavenging [12]. MTP-131 exerts its effects through interactions with cardiolipin, which result in stabilization of the inner mitochondrial membrane and its resident electron transport system proteins [13, 28, 29] thereby improving the function of the electron transport system while consequently reducing electron leak [30].
Within the ischemic limb, multiple cell types are involved in the complex regulation of the microenvironment. Little is known about how each individual cell type responds to ischemia/hypoxia or how the individual responses contribute directly or indirectly (i.e. paracrine signaling) to limb pathology. We have previously shown that there is a cell type specific susceptibility to ischemia [8], and extend these findings to mitochondrial respiratory function, revealing that skeletal muscle cell respiratory capacity is substantially reduced during experimental ischemia, whereas endothelial cell respiratory capacity is only modestly reduced with experimental ischemia and recovered rapidly. Importantly, primary myotube respiratory capacity could be rescued with mitochondrial-targeted treatment with MTP-131.
Together, these findings suggest that MTP-131 treatment in vivo may be exerting most of its effects through skeletal muscle mitochondria. Interestingly, MTP-131 therapy improved mitochondrial respiration only when muscle and endothelial cells were allowed to recover from ischemia. This finding mirrors previous studies[12], which demonstrated cardioprotection of this peptide specifically during the reperfusion phase of ischemia-reperfusion. Importantly, MTP-131 demonstrated efficacy both in vivo and in vitro even when delivered after the ischemic event, which may be most similar to PAD/CLI patients that often arrive at the clinic vascular insufficiency. In summary, the current work demonstrates the efficacy of mitochondrial-targeted therapy to improve ischemic limb pathology and provides pre-clinical support for the further investigation of limb tissue bioenergetics as a means to improve PAD outcomes.
Supplementary Material
Highlights.
Critical limb ischemia results in severe skeletal muscle mitochondrial myopathy.
Mitochondrial dysfunction is more severe in muscle compared to endothelial cells.
Mitochondrial-targeted peptide therapy improves recovery from limb ischemia.
Acknowledgments
T.E.R. is supported by NIH/NHLBI F32HL129632. J.M.M. is supported by NIH/NHLBI R00HL103797 and R01HL125695, D.A.B. is supported by NIH/NHLBI R01HL123647 and R15HL122292201. P.D.N. is supported by NIH/NIDDK R01 DK096907. This study was not funded by Stealth BioTherapeutics.
Abbreviations
- HLI
hindlimb ischemia
- HUVECs
human umbilical vein endothelial cells
- PAD
peripheral arterial disease
Footnotes
Disclosures: D.A.B. has served as a consultant for Stealth BioTherapeutics, and has received research funding from Stealth BioTherapeutics for work not related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirsch AT, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Taylor SM, et al. Comparison of interventional outcomes according to preoperative indication: a single center analysis of 2,240 limb revascularizations. J Am Coll Surg. 2009;208(5):770–8. doi: 10.1016/j.jamcollsurg.2009.01.025. discussion 778–80. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, et al. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. J Am Coll Cardiol. 2012;59(13):1159–67. doi: 10.1016/j.jacc.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain A, et al. Declining walking impairment questionnaire scores are associated with subsequent increased mortality in peripheral artery disease. J Am Coll Cardiol. 2013;61(17):1820–9. doi: 10.1016/j.jacc.2013.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat HK, et al. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation. 1999;99(6):807–12. doi: 10.1161/01.cir.99.6.807. [DOI] [PubMed] [Google Scholar]
- 6.Brass EP, Wang H, Hiatt WR. Multiple skeletal muscle mitochondrial DNA deletions in patients with unilateral peripheral arterial disease. Vasc Med. 2000;5(4):225–30. [PubMed] [Google Scholar]
- 7.Thompson JR, et al. Protein Concentration and Mitochondrial Content in the Gastrocnemius Predicts Mortality Rates in Patients With Peripheral Arterial Disease. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 8.McClung JM, et al. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am J Pathol. 2012;180(5):2156–69. doi: 10.1016/j.ajpath.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pipinos, et al. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med. 2006;41(2):262–9. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Leeper NJ, et al. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57(3):728–33. doi: 10.1016/j.jvs.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammer A, Steiner S. Gene therapy for therapeutic angiogenesis in peripheral arterial disease - a systematic review and meta-analysis of randomized, controlled trials. Vasa. 2013;42(5):331–9. doi: 10.1024/0301-1526/a000298. [DOI] [PubMed] [Google Scholar]
- 12.Brown DA, et al. Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. J Cardiovasc Pharmacol Ther. 2014;19(1):121–32. doi: 10.1177/1074248413508003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DA, Sabbah HN, Shaikh SR. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther. 2013;140(3):258–66. doi: 10.1016/j.pharmthera.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Dai W, et al. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 2014;64(6):543–53. doi: 10.1097/FJC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 15.Kloner RA, et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective Peptide. J Am Heart Assoc. 2012;1(3):e001644. doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sloan RC, et al. Mitochondrial permeability transition in the diabetic heart: Contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol. 2012;52(5):1009–18. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Birk AV, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250–61. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, et al. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol. 2014;306(9):F970–80. doi: 10.1152/ajprenal.00697.2013. [DOI] [PubMed] [Google Scholar]
- 19.Szeto HH, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22(6):1041–52. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min K, et al. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol (1985) 2011;111(5):1459–66. doi: 10.1152/japplphysiol.00591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai DF, et al. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58(1):73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, et al. Mitochondria-targeted antioxidant peptide SS31 attenuates high glucose-induced injury on human retinal endothelial cells. Biochem Biophys Res Commun. 2011;404(1):349–56. doi: 10.1016/j.bbrc.2010.11.122. [DOI] [PubMed] [Google Scholar]
- 23.Reddy TP, et al. Toxicity of neurons treated with herbicides and neuroprotection by mitochondria-targeted antioxidant SS31. Int J Environ Res Public Health. 2011;8(1):203–21. doi: 10.3390/ijerph8010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam NM, et al. A mitochondrial therapeutic reverses visual decline in mouse models of diabetes. Dis Model Mech. 2015;8(7):701–10. doi: 10.1242/dmm.020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10(3):601–19. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 26.Zhao K, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279(33):34682–90. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 27.Zhao K, et al. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70(12):1796–806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Birk AV, et al. Targeting Mitochondrial Cardiolipin and the Cytochrome C/Cardiolipin Complex to Promote Electron Transport and Optimize Mitochondrial Atp Synthesis. Br J Pharmacol. 2013 doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbah HN, et al. Chronic Therapy With Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ Heart Fail. 2016;9(2):e002206. doi: 10.1161/CIRCHEARTFAILURE.115.002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szeto HH, Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther. 2014;96(6):672–83. doi: 10.1038/clpt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.