Abstract
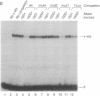
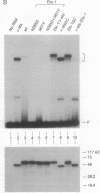
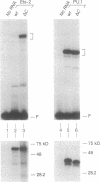
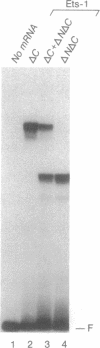
The mb-1 gene is expressed only during the early stages of B-lymphocyte differentiation. Here we show that the mb-1 proximal promoter region contains a functionally important binding site for members of the ETS family of DNA-binding proteins. We found that both the E26 virus-encoded v-ets and the myeloid/B-cell-specific factor PU.1 bind efficiently to this site in vitro. By contrast, Ets-1, the lymphocyte-specific cellular homologue of v-ets, and the related, more ubiquitously expressed Ets-2 protein interacted weakly with this binding site. DNA binding by both Ets-1 and Ets-2, however, could be increased 20- to 50-fold by deleting as few as 16 carboxyl-terminal amino acids. The inhibitory carboxyl-terminal amino acid sequence is highly conserved between Ets-1 and Ets-2 but is not present in either v-ets or PU.1. Replacement of the carboxyl-terminal amino acids of v-ets with those of Ets-1 decreased DNA binding by v-ets drastically. Cotranslation of Ets-1 transcripts encoding proteins of different lengths suggested that Ets-1 binds DNA as a monomer. Therefore, the carboxyl-terminal inhibitory domain appears to interfere directly with DNA binding and not with homodimerization. Finally, the functional relevance of ETS factor binding to the mb-1 promoter site was evidenced by the stimulation of transcription through this site by a v-myb-v-ets fusion protein. Together, these data suggest that one or more ETS family factors are involved in the regulation of mb-1 gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat N. K., Fisher R. J., Fujiwara S., Ascione R., Papas T. S. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. doi: 10.1073/pnas.84.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulukos K. E., Pognonec P., Begue A., Galibert F., Gesquière J. C., Stéhelin D., Ghysdael J. Identification in chickens of an evolutionarily conserved cellular ets-2 gene (c-ets-2) encoding nuclear proteins related to the products of the c-ets proto-oncogene. EMBO J. 1988 Mar;7(3):697–705. doi: 10.1002/j.1460-2075.1988.tb02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C. Capturing antigen receptor components. Curr Biol. 1991 Feb;1(1):25–27. doi: 10.1016/0960-9822(91)90117-f. [DOI] [PubMed] [Google Scholar]
- Chen J. H. Cloning, sequencing, and expression of mouse c-ets-1 cDNA in baculovirus expression system. Oncogene Res. 1990;5(4):277–285. [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Fisher C. L., Ghysdael J., Cambier J. C. Ligation of membrane Ig leads to calcium-mediated phosphorylation of the proto-oncogene product, Ets-1. J Immunol. 1991 Mar 15;146(6):1743–1749. [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Dernis D., Leprince D., Stehelin D. Identification and preferential expression in thymic and bursal lymphocytes of a c-ets oncogene-encoded Mr 54,000 cytoplasmic protein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1714–1718. doi: 10.1073/pnas.83.6.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Hagman J., Travis A., Grosschedl R. A novel lineage-specific nuclear factor regulates mb-1 gene transcription at the early stages of B cell differentiation. EMBO J. 1991 Nov;10(11):3409–3417. doi: 10.1002/j.1460-2075.1991.tb04905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipskind R. A., Rao V. N., Mueller C. G., Reddy E. S., Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991 Dec 19;354(6354):531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Bhat N. K., Gottschalk L. R., Lindsten T., Thompson C. B., Papas T. S., Leiden J. M. Sequence-specific binding of human Ets-1 to the T cell receptor alpha gene enhancer. Science. 1990 Nov 9;250(4982):814–818. doi: 10.1126/science.2237431. [DOI] [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Sippel A. E. The highly conserved amino-terminal region of the protein encoded by the v-myb oncogene functions as a DNA-binding domain. EMBO J. 1987 Sep;6(9):2719–2725. doi: 10.1002/j.1460-2075.1987.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Fisher R. J., Fujiwara S., Jorcyk C., Bhat N. K., Seth A., Papas T. S. Isoforms of the human ets-1 protein: generation by alternative splicing and differential phosphorylation. Oncogene. 1990 May;5(5):675–681. [PubMed] [Google Scholar]
- LaMarco K., Thompson C. C., Byers B. P., Walton E. M., McKnight S. L. Identification of Ets- and notch-related subunits in GA binding protein. Science. 1991 Aug 16;253(5021):789–792. doi: 10.1126/science.1876836. [DOI] [PubMed] [Google Scholar]
- Leiden J. M. Transcriptional regulation during T-cell development: the alpha TCR gene as a molecular model. Immunol Today. 1992 Jan;13(1):22–30. doi: 10.1016/0167-5699(92)90200-q. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gesquiere J. C., Stehelin D. The chicken cellular progenitor of the v-ets oncogene, p68c-ets-1, is a nuclear DNA-binding protein not expressed in lymphoid cells of the spleen. Oncogene Res. 1990;5(4):255–265. [PubMed] [Google Scholar]
- Lim F., Kraut N., Framptom J., Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992 Feb;11(2):643–652. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Piette J., Yaniv M., Tang W. J., Folk W. R. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5839–5843. doi: 10.1073/pnas.85.16.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias P., Müller M. M., Schreiber E., Rusconi S., Schaffner W. Eukaryotic expression vectors for the analysis of mutant proteins. Nucleic Acids Res. 1989 Aug 11;17(15):6418–6418. doi: 10.1093/nar/17.15.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Pognonec P., Boulukos K. E., Gesquière J. C., Stéhelin D., Ghysdael J. Mitogenic stimulation of thymocytes results in the calcium-dependent phosphorylation of c-ets-1 proteins. EMBO J. 1988 Apr;7(4):977–983. doi: 10.1002/j.1460-2075.1988.tb02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol. 1992 Jan;12(1):368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis A., Amsterdam A., Belanger C., Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Travis A., Hagman J., Grosschedl R. Heterogeneously initiated transcription from the pre-B- and B-cell-specific mb-1 promoter: analysis of the requirement for upstream factor-binding sites and initiation site sequences. Mol Cell Biol. 1991 Nov;11(11):5756–5766. doi: 10.1128/mcb.11.11.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Lapis P., Lautenberger J. A., Schweinfest C. W., Papas T. S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K., Bishop J. M. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989 Jul 14;58(1):85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]