Abstract
Background
The most sensitive techniques for measuring minimal residual disease (MRD) in multiple myeloma (MM) currently require an invasive and costly bone marrow aspiration. These methods include immunohistochemistry, multicolor flow cytometry, real-time quantitative polymerase chain reaction (RQ-PCR), and next-generation sequencing. An ideal MM MRD test would be a serum-based test sensitive enough to detect low levels of immunoglobulin (Ig) secreted from multifocal lesions that magnetic resonance imaging (MRI) or PET/CT easily detects but routine bone marrow aspirate misses.
Methods
Patient serum with abundant M-protein prior to treatment is separated on a 1D-SDS-PAGE gel, and the Ig light-chain (LC) band is excised, trypsin digested, and analyzed on a Q Exactive mass spectrometer by LC-MS/MS. The peptide's abundance and sequence is utilized to identify tryptic peptides that map to complementary determining regions (CDRs) of Ig LCs. The CDR target tryptic peptides are utilized to monitor MRD in subsequent serum samples with prior affinity enrichment of the appropriate kappa or lambda class corresponding to the clone.
Results
A total of 67 patients were analyzed. Those with no detectable disease by immunohistochemistry (57 patients) and 6-color flow cytometry (10 patients) were included in the analysis. Of the 67 patients sampled, a target peptide that could be monitored was identified in 62 (93%). Of these 62 patients, there was detectable disease by LC-MS in 52 (90%) and 10 (100%) of the patients with no detectable disease by immunohistochemistry and 6-color flow cytometry, respectively.
Conclusions
The ability to measure disease in patients by LC-MS/MS that are negative by sensitive bone marrow–based methodologies indicates that a serum-based approach is a viable alternative. This method requires no bone marrow aspirate.
Keywords: bone marrow aspiration, mass spectrometry, minimal residual disease, multiple myeloma
Introduction
Multiple myeloma (MM) is characterized by amplification of a single plasma cell clone producing a monoclonal immunoglobulin (Ig). The clone proliferates in the bone marrow and can result in bone destruction. The monoclonal Ig produced by these cells can circulate as intact Ig or, in 20% of cases, as free LC (FLC) only with no heavy chain (1). The annual incidence of MM is 4–5/100,000 and accounts for 1% of all cases of cancer (2). MM is a cancer seen most frequently in older adults, with a median age of onset of 66 years (2).
In 97% of patients with MM, protein electrophoresis of serum or urine (the SPEP/UPEP test) results in a large spike (M-spike) appearing in the gamma, beta, or alpha2 region of the densitometer tracing corresponding to the Ig protein produced by the clone (1). Three percent of patients have no apparent M-protein in the serum or urine as measured by SPEP. There are numerous treatment options for patients with MM, including high-dose chemotherapy with hematopoietic cell support, but the disease is considered incurable. Treatments have improved considerably over the past decade, and a higher percentage of patients are achieving “complete response,” but virtually all these patients relapse, suggesting that either current complete response criteria are not sensitive enough to detect the deeper response required for an eventual cure or the therapies are not effective in killing bone marrow clones. With improving therapy, there needs to be an equally sensitive method to routinely identify these deeper responses. Minimal residual disease (MRD) is a measure used to estimate to the number of cancer cells remaining after treatment.
The International Myeloma Working Group has issued guidelines for determining the response to treatment and when a relapse has occurred (3, 4). A complete response (CR) has 1) no M-spike protein by immunofixation (IFE), 2) no soft tissue plasmacytomas, and 3) bone marrow plasma cells <5%. Stringent complete response (sCR) is defined as CR plus 1) a normal FLC ratio and 2) the absence of clonal cells in bone marrow by immunohistochemistry (IHC) or immunofluorescence (IF) (3). Most patients with MM after treatment have residual disease that is below the sensitivity of these assays and will eventually relapse (5).
To address this lack of sensitivity, more sensitive methods for measuring MRD have recently been developed. These include real-time quantitative polymerase chain reaction (RQ-PCR), multiparametric flow cytometry (MFC), and next-generation sequencing (NGS) (6, 7). Molecular complete response (mCR) is indicated when no identifiable allele-specific oligonucleotides by RQ-PCR are present, with a sensitivity of 10−5, in addition to sCR (3, 5). An immunophenotypic complete response (iCR) is indicated when there are no detectable phenotypically aberrant clonal plasma cells in >106 cells by >4-color MFCs, in addition to sCR (6). RQ-PCR is a highly sensitive technique but requires patient-specific primers (7–16). More recently, NGS has been found to be as sensitive as RQ-PCR and doesn't require the patient-specific reagents (7). RQ-PCR, MFC, and NGS are all sensitive, and in prospective studies, iCR and mCR are both predictive of survival outcomes (6, 17). With the incorporation of modern therapies for the treatment of MM when the remission response is more pronounced, sensitive detection of MRD by methods such as MFC, RQ-PCR, and NGS are required and will become more common. Currently, the most sensitive techniques available for measuring MRD are immunohistochemistry, RQ-PCR, MFC, and NGS, all of which require a bone marrow biopsy. This is an invasive procedure, and although considered safe, it is painful and more expensive (>$5,000) than a venous blood draw.
An ideal MM MRD test would be a serum-based test sensitive enough to detect low levels of Ig secreted. Such a serum-based test would be advantageous because it would be less invasive than a bone marrow aspirate/biopsy and because it would have the potential to detect the presence of patchy or focal disease that bone marrow aspirate might miss. Several groups have shown previously that clonotypic peptides specific to the complementarity determining region (CDR) of the M-protein obtained from serum can be used to monitor MRD (18–20). Additional work by Barnidge et al. (21) showed that the accurate molecular mass of LCs from intact M-proteins isolated from serum could be used to monitor MRD in MM patients who were negative by SPEP and IFE.
Based on the above, an analysis of specific CDR peptides unique to each patient's M-protein analyzed in a highly sensitive manner from a venous blood sample would suffice to monitor MRD. Each patient's clonal CDR-tryptic peptide(s) is a MRD signature for that patient. Preliminary data indicates that LC-MS/MS detection of CDR peptides in serum is sensitive enough to compete with both immunohistochemistry and MFC and would eliminate the need for bone marrow aspiration for sCR. Here, we outline a novel method to measure MM MRD by serum analysis of CDR peptides utilizing only Ig LCs isolated and identified from the M-spike protein in a venous blood sample without DNA sequence information.
Methods
Samples
Serum or plasma was obtained from an archive of samples collected under Mayo IRB 13-000220 from the same patient pre- and post-autologous stem cell transplant. The post-transplant serum and bone marrow aspirates were obtained within 5 days of each other.
Identification of Target Ig LC–Variable Region Peptides
Serum/plasma (0.5μL) was separated on a reducing gel, and the Ig LC band was excised and analyzed by LC-MS/MS on a Q Exactive mass spectrometer (see Supplemental methods for details).
Routine protein identification searches were performed using traditional database search strategies within PEAKS software (Bioinformatics Solutions Inc.), applying PEAKS denovo, database, PTM, and Spider search algorithms and utilizing a 4.5-ppm precursor mass tolerance and a 0.02-Da fragment ion tolerance. Carbamidomethylation was utilized as a fixed modification, and Met oxidation and deamidation of Asn/Gln were used as variable modifications. The augmented database of Dasari et al. (22) was utilized for the database searches.(21) Base peak ion chromatograms in XCalibur Qual Browser 2.2 for each high M-spike sample were then interrogated. Abundant ions were matched to their corresponding mass and retention time in the Spider search results. Resulting sequences corresponding to lambda or kappa LCs were then mapped to LC sequences utilizing IMGT/DomainGapAlign (22). Peptide sequences that contained all or part of CDR 1, 2, or 3 were considered to be diagnostic for each patient and were monitored in subsequent patient samples to measure MRD.
Plasma Cell Isolation, mRNA Extraction, and PCR Amplification of Ig LCs
Our proteomic de novo LC sequencing results were compared to Sanger-sequenced plasma cell LC mRNA isolated from a bone marrow aspirate (see Supplemental Methods for details). The Sanger LC mRNA sequence of each patient was aligned to the public repertoire of the Ig LC sequences using the ImmunoGeneTics V-QUEST search feature (23). The alignment was configured to use human Ig-variable region sequences and restricted to use only the V- and J- regions of the LCs. The alignment automatically detects the CDR regions and any insertions and deletions in the mRNA sequence. The edited mRNA sequence was translated into a mature, full-length protein sequence, which was considered the patient's M-protein sequence. Variable-region peptides obtained from the proteomics experiment were matched to these protein sequences using PEAKS software.
Measurement of MRD
Seventy-five μL of CaptureSelect anti-kappa or anti-lambda affinity matrix (LifeTechnologies, Grand Island, NY) was added to a 10-μm fritted microspin column and washed 3 times with PBS. The resin was resuspended in 200 μL of PBS and 50 μL of serum/plasma was added and incubated at 4°C overnight with gentle inversion. The bound Ig was washed 2 to 3 times with 200 μL of PBS and the material eluted with 100 μL 100 mM glycine pH 2.7 and collected in a microcentrifuge tube containing 6 μL 1M Tris pH 10. Ten microliters of this eluate was applied to a 10.5% to 14% Criterion precast SDS-polyacrylamide (SDS-PAGE) gel. After staining with BioSafe colloidal Coomassie, the LC band was excised and digested with trypsin and analyzed by nano-LC-MS/MS (See Supplemental materials). An extracted ion chromatogram corresponding to the parent ion mass of the CDR-tryptic peptide previously identified was prepared, and the integrated area and associated MS/MS spectra was compared to the original pre-transplant sample. Immunohistochemistry of bone marrow aspirates followed standard clinical practice. MFC was performed utilizing the method of Maurice et al.(24).
Results
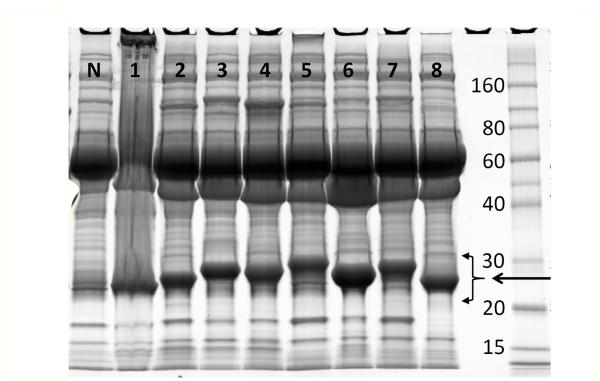
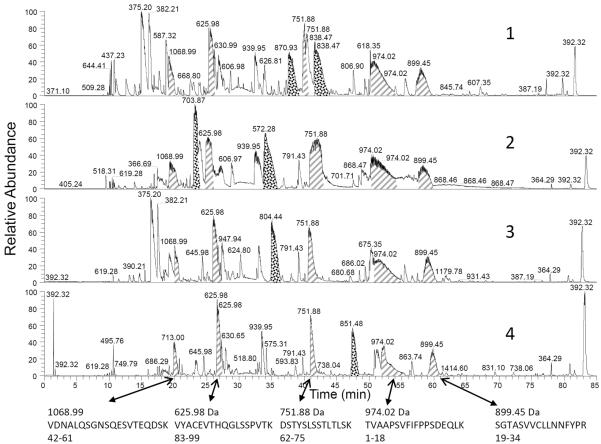
Eight MM patients (4 kappa, 4 lambda) with plasma cell mRNA Sanger sequencing and associated serum/plasma samples were initially analyzed. Figure 1 shows serum/plasma (0.5 uL) from these patients with large M-spike separated by polyacrylamide gel electrophoresis. The bands corresponding to Ig LC were isolated, reduced, alkylated, and analyzed by nanoLC-MS/MS. Figure 2 shows the base peak ion chromatogram of the 4 kappa patients (see Supplemental Figure 1 for lambda). In each case, high-intensity peaks corresponding to constant- (or framework-) region–tryptic peptides can be identified (PEAKS) in each patient (hash) with similar retention times and identical precursor mass. Additionally, close observation of these chromatograms indicates unidentified peptide peaks with intensity comparable to the constant- and framework-region peptides but with variable retention times. Utilizing the Spider function within PEAKS, these peptides (stippled) were matched by homology to CDR-tryptic peptides in the IMGT database using DomainGapAlign and to the sequences obtained by translating Sanger-sequenced plasma cell–isolated mRNA from the same patients. Supplemental Figure 2 indicates the 8 LC sequences obtained for 8 MM patients. The CDR 1, 2, and 3 regions (mapped by DomainGapAlign) are in red font and the tryptic peptides corresponding to CDR-variable regions are bold.
Figure 1.
MM patient serum samples (1–8) and normal (N) separated by 1D SDS-PAGE and stained with Coomassie Blue. Monoclonal LC bands are indicated by the intense staining compared to the normal sample between the arrows from 20–30 kDa.
Figure 2.
Four base-peak ion chromatograms of kappa MM light-chain–tryptic digests. Constant-region peptides are noted by the hash fill and arrows. Variable region peptides are stippled. Peaks are labeled with the base-peak mass. Unannotated peaks most frequently correspond to LC framework sequences, apolipoprotein A-1, trypsin autolysis products, and albumin sequences.
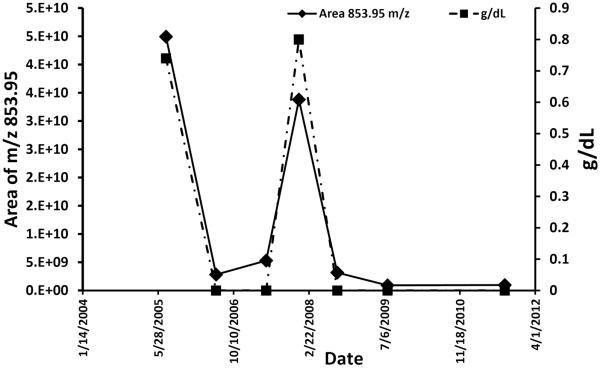
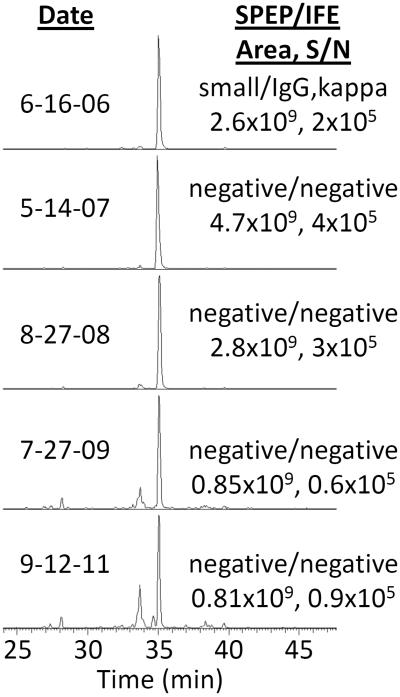
The mass/retention time pairs corresponding to CDR-variable region-tryptic peptides can be utilized to monitor MRD in subsequent serum samples, thus eliminating the need for a bone marrow aspirate. As proof of principle, we monitored the CDR-tryptic peptides from 2 additional patients with serial blood samples over the course of treatment for MM after an initial affinity purification of the appropriate Ig class (kappa or lambda). Figure 3 and Supplemental Figure 5 compare the SPEP-reported concentration and the LC-MS–integrated area of CDR-variable region-tryptic peptides from 2 patients over time. The LC-MS/MS methodology recapitulates the SPEP results. However, 5 of the time points yielded either small (6/16/2006) or no M-protein by SPEP and IFE (Figure 3). In contrast, Figure 4 shows the extracted ion chromatogram of the 853.95 m/z ion of the CDR peptide (see Supplemental Figure 3 for CDR confirmation) from the same patient samples at the 5 time points indicated in Figure 3. The signal-to-noise (S/N) and area counts indicate that the new MS method has a considerably higher sensitivity to detect disease, even though 4 time points were negative by SPEP/IFE. Importantly, it follows the disease trend reported by SPEP.
Figure 3.
Time course of MRD measured by SPEP (squares/dashed) and LC-MS/MS (diamond/solid) from the same patient.
Figure 4.
Extracted ion chromatogram of m/z 853.95 for a CDR peptide from a MM patient at various time points. Each chromatogram normalized to 100%. Integrated MS1 peak area and S/N, SPEP, and IFE results are noted in the figure and correspond to the time points in Figure 3. Note the extremely strong signal at all time-points examined when IFE is negative
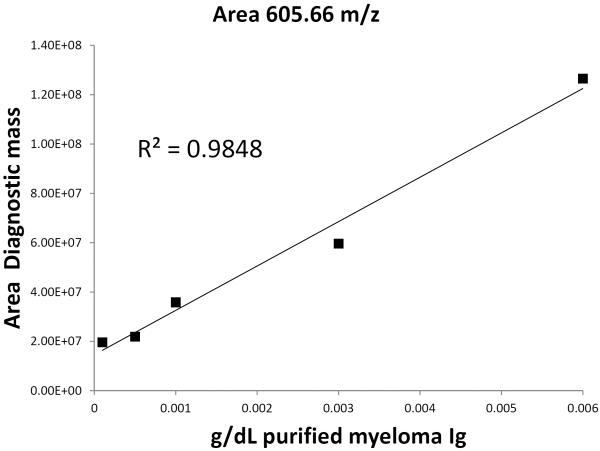
To measure the potential sensitivity of this workflow, we purified a kappa Ig from a high M-spike patient and prepared a dilution curve into normal serum. Figure 5 shows the linearity of the workflow when the area under the curve from a CDR-region peptide was integrated and plotted against concentration using the MRD measurement technique outlined here. The lowest concentration prepared was 0.0001 g/dL, where the S/N was still ≥1,000. In contrast, the SPEP LOQ is ~0.2 g/dL. The protocol described here yielded an assay >2,000 times more sensitive than SPEP without any optimization.
Figure 5.
Dilution curve of purified M-spike protein spiked into normal serum at various concentrations and analyzed by the method proposed here. Our lowest concentration was 0.0001 g/dL and still had a signal-to-noise ratio of 1000.
We evaluated how large of an M-spike was necessary to be able to identify a target CDR-tryptic peptide from blood. Supplemental Figure 4 is a scatter plot of 49 high M-protein (baseline) patient samples versus SPEP-protein concentration, indicating a threshold of ≥0.8 g/dL M-protein for target peptide identification.
We next analyzed 62 patients with a baseline (high M-protein) and serum sample that had been acquired ≤5 days from bone marrow aspiration with either negative serum and urine IFE and <5% plasma cells in bone marrow by IHC (52 patients) or no detectable plasma cells by 6-color flow cytometry (10 patients). Target peptides were identified in the baseline sample and subsequently monitored in the sample acquired with no detectable disease by IHC and MFC. All of these samples had no detectable MRD by the reference method. However, when the serum was analyzed by LC-MS/MS, utilizing the target peptides identified from the high M-spike sample, ≥92% of the IHC and MFC samples had detectable MS signal still indicating the presence of disease (47 patients and 10 patients, respectively).
Of the 62 patients negative for MRD by IHC and MFC, 45, 68, and 29 target peptides were associated with CDR 1, 2, and 3, respectively. Patients with 2 or more CDR-target peptides comprised 64% of all patients. In regard to uniqueness, of the 143 CDR-tryptic peptides identified, 51% were unique, 13% were found in 1 additional patient, 17% in 2 to 9 additional patients, and 19% in 10 to 49 patients. Sequence uniqueness by CDR was 60%, 47%, and 45% for CDR 1, 2, and 3, respectively.
Seven additional patients met our ≥0.8 g/dL M-protein threshold, but no target peptide was identified. Four patients had SPEP values between 0.8 and 1.1 g/dL, indicating that our previously defined threshold of 0.8 g/dL was too low for all patients. Two patients had SPEP values of 4.49 and 2.8 g/dL, but inspection of the SDS-PAGE gels indicated discrepancies between SPEP and our stained gels (visual inspection). One patient (3.6 g/dL) had abundant prominent constant/framework peptides but no abundant-variable tryptic peptide. Digestion with GluC yielded similar results for this patient (data not shown).
Discussion
Our data indicates that high-resolution (≥120,000) mass spectrometers (e.g., Q Exactive, Thermo Scientific) are ideal for de novo peptide sequencing. Our results indicate that LC CDR-region tryptic peptides can be identified from serum (<1 μL) after isolation of the LC band from a 1D-SDS-PAGE gel separation. This is not surprising, as several vendors are offering complete de novo sequencing of immunoglobulins directly from purified proteins without genomic sequence information (25–27).
The protocol described here does not seek complete sequencing of the LC clone, which could be afforded only after digestion with multiple proteases, but only to identify a unique LC CDR-tryptic peptide distinctive to the plasma cell clone for subsequent monitoring. The M-spike concentration must be above the polyclonal background for a CDR-tryptic peptide to be identified from the polyclonal background. We have preliminarily identified this threshold as >0.8 g/dL in an analysis of 49 MM patients. However, 7 additional patients met our ≥0.8 g/dL M-protein threshold, but target peptides were not identified. Four patients had SPEP values between 0.8 and 1.1 g/dL, indicating that our previously defined threshold of 0.8 g/dL is too low for all patients. Two patients had SPEP values of 4.49 and 2.8 g/dL, but inspection of the SDS-PAGE gels indicated discrepancies between SPEP and our stained gels (visual inspection). One patient (3.6 g/dL) had abundant prominent constant/framework peptides but no abundant-variable tryptic peptide. Digestion with GluC (cleaves at Glu) yielded similar results (data not shown).
Once the peptide has been identified for a given patient, the technique can detect a target CDR peptide down to at least 0.0001 g/dL based upon our dilution curve. A lower threshold would be possible by additional purification, but this has not been examined, as the current protocol is already more sensitive than IHC and MCF. The CDR-tryptic peptide is identified by a combination of 1) relative ion abundance as compared to constant-region peptides (either kappa or lambda), 2) sequence information as provided by Spider software within PEAKS, and either a 3) BLAST homology search to ascertain homology with known LC-variable region sequences or 4) submission of a LC-variable protein sequence to IMGT DomainGapAlign. We are currently working with commercial software (Pinnacle; Optys Tech Corp), which has integrated most of these tasks.
Once treatment for MM has commenced, the M-spike necessarily disappears into the polyclonal background until relapse. In a recent study by Song et al. (29) of 126 patients, an almost equal number presented with focused versus diffuse/variegated MM (28, 29). Whether the disease is diffuse/variegated or focal at diagnosis or, more importantly, at supposed remission, our technique should allow for detection potentially beyond what a routine iliac crest aspirate can provide. This fact, plus additional sensitivity of LC-MS, may contribute to our ability to accurately diagnose disease in ≥90% of patients reported to be in CR by serum and urine IFE and IHC and/or MFC of the bone marrow. These observations point to a major advantage of the methodology outlined here in that, just like MRI, both focal and diffuse/variegated MM should be detected when the plasma cell Ig clone is secreted into the circulation and requires neither initial nor subsequent bone marrow aspirates.
In all 8 patients with Sanger sequence data, a tryptic peptide covering at least 1 CDR was identified by proteomics. Supplemental Table 1 shows juxtaposed CDR-tryptic sequences obtained by proteomics (bottom) and DNA (top). Subtle differences between DNA sequences and the MS2-interpreted spectra are noted. Identification of CDR region–tryptic peptides from all 8 DNA-sequenced, 52 IHC, and 10 MFC patients was accomplished without knowledge of the DNA-derived sequences, indicating that workflow is robust.
A sensitive blood-based test for MRD in MM, as shown here, would have a considerable impact in reducing invasive bone marrow aspirations and the associated costs of serial bone marrow sampling. The results shown here indicate that the test will have greater sensitivity than SPEP, IFE, IHC, and MFC in detecting MRD. In the analysis of our IHC and MFC samples, all but 5 IHC samples still had detectable disease by LC-MS/MS, where IHC and MFC indicated no disease. Our initial results are profound but require additional validation in order to serve as a viable clinical test. The following paragraphs indicate the questions that will need to be answered in order to translate the methodology into the clinical laboratory.
First, is a single tryptic peptide sufficiently unique to characterize the clone, or is >1 peptide required for monitoring? Only after evaluating more patients will this be ascertained. A “unique” tryptic peptide covering the CDR 3 region would offer the most reliable unique variable-region peptide, since it results from the recombination of the VJ regions. In our dataset, by abundance we identified more CDR 2>CDR 1>CDR 3 target peptides. By sequence, CDR 1 peptides were more unique. The production of an appropriate CDR 3 target-tryptic peptide is dependent on the lysine and arginine locations in the LC sequence. Though the majority of CDR sequences identified were unique (54%) and not found in the rest of our patient cohort, it stands to reason that recombination events would produce identical copies of the same sequence in the polyclonal background. Multiple targets can be monitored in modern LC-MS/MS systems and would provide precision in this era of “precision medicine” (30). Where 2 or more targets were identified, all were always present in the post-treatment samples. Additionally, although this work focused on the LC, it could easily have been expanded to include heavy-chain CDR peptides or alternate proteases to capture ≥2 CDR peptides. Alternatively, like Barnidge et al. (20), the clone could have been identified by Sanger or NGS DNA sequencing and appropriate peptides/proteases subsequently identified a priori only requiring a single bone marrow aspiration.
Second, does a test with an informatically and computationally intensive algorithm fit in a clinical laboratory? We have successfully performed the database searches and de novo searches on both cluster-based and dual processor workstations. After all the data is collected, it must be collated and analyzed to accept only potential target peptides that reach a minimum abundance threshold relative to the constant-region peptides. Some type of homology search (BLAST) must be performed to confirm that the de novo sequences belong to the LC variable–region protein family and then a CDR region. Pinnacle (Optys Tech Corp.) currently provides the majority of this functionality.
Third, what threshold defines CR by LC-MS/MS? No clone was present in only 5 patients out of all those analyzed in the CR samples by IHC and MFC. Possible solutions might include the following: 1) Defining a threshold ratio between the target peptide and an exogenous peptide standard. 2) Defining a threshold ratio between the target peptide and a constant-region peptide. 3) Having a signal below a defined signal-to-noise ratio. We are currently in the process of ascertaining a robust threshold.
Lastly, the workflow outlined here was developed in a research laboratory. Translation to a clinical laboratory requires optimization and elimination of tedious sample-handling steps (e.g., electrophoresis and gel band analysis). However, the data shown here was accomplished with no optimization, utilizing only the tools at hand. Simple modifications, such as solution digests of intact Igs isolated by ammonium sulfate fractionation, may provide comparable results.
Our previous work demonstrated that LCs derived from intact M-proteins can be used to successfully identify a monoclonal LC in serum at levels <0.8 g/dL (20). Both the intact method and the clonotypic-peptide–monitoring method outlined here show great promise for measuring MRD in MM, and neither requires a single bone marrow aspirate. Further optimization, validation, and comparison of these methods to the current gold standard techniques will further our knowledge of where this workflow fits in the clinician's tool kit for measuring MRD in MM patients.
Supplementary Material
Table 1.
Distribution of CDR of the 131 CDR tryptic peptides identified from MM Patients.
| CDR | n | Unique, %a |
|---|---|---|
| 1 | 41 | 66 |
| 2 | 63 | 52 |
| 3 | 27 | 41 |
Sequence no found in any other patient, including the polyclonal background.
Table 2.
Additional patient in whom the same sequence was found in all patients, including the polyclonal background.
| Additional patients, n | % |
|---|---|
| 0 | 54 |
| 1 | 12 |
| 2–9 | 15 |
| 10–49 | 18 |
Acknowledgements
The authors wish to acknowledge the help of Diana L. Ayerhart in manuscript preparation. The authors also wish to thank Ben Madden for acquiring the LC-MS/MS data, Christine Charlesworth for running the SDS-PAGE gels, and the rest of the Mayo Proteomics Core technical staff for all their assistance.
Author Disclosure/Conflict of Interest: Research reported in this manuscript was supported by The National Cancer Center of the National Institutes of Health (NIH) under award R21CA187462 and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS) (H.R.B). The manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Support was also provided by Gordon C. and Elizabeth W. Gilroy (H.R.B), the JABBS Foundation, the Robert A. Kyle Hematologic Malignancies Program, and the Mayo Clinic Center for Individualized Medicine (H.R.B).
Abbreviations
- CDR
complementarity determining region
- CR
complete response
- FLC
free light chain
- iCR
immunophenotypic complete response
- IF
immunofluorescence
- IFE
immunofixation
- Ig
immunoglobulin
- IHC
immunohistochemistry
- LC
light chain
- mCR
molecular complete response
- MFC
multiparametric flow cytometry
- MM
multiple myeloma
- MRD
minimal residual disease
- MRI
magnetic resonance imaging
- NGS
next generation sequencing
- RQ-PCR
real-time quantitative polymerase chain reaction
- sCR
stringent complete response
- S/N
signal-to-noise
- SPEP
serum protein electrophoresis
Human Genes Discussed
- IGKC
immunoglobulin kappa constant
- IGKV
immunoglobulin kappa variable
- IGLC
immunoglobulin lambda constant
- IGLV
immunoglobulin lambda variable
Footnotes
Some of this work was presented in abstract form at the American Society of Hematology 55th Annual Meeting, New Orleans, LA, December 9, 2013.
Contributions: H.R.B and R.C.T performed the experiments. S.D. and H.R.B. analyzed the data. A.D., J.R.M., D.F.J, and R.C.T. provided samples. H.R.B., S.D., A.D., M.R-A., R.C.T., D.F.J, D.R.B., and D.L.M. designed the experiments. D.R.B. and D.L.M. introduced the initial ideas for MRD from serum. H.R.B made the figures and wrote the paper.
Conflict-of-Interest Disclosure: The authors declare no competing financial interests.
References
- 1.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 5.Hart AJ, Jagasia MH, Kim AS, Mosse CA, Savani BN, Kassim A. Minimal residual disease in myeloma: Are we there yet? Biol Blood Marrow Transplant. 2012;18:1790–9. doi: 10.1016/j.bbmt.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Paiva B, Vidriales MB, Cervero J, Mateo G, Perez JJ, Montalban MA, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017–23. doi: 10.1182/blood-2008-05-159624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladetto M, Bruggemann M, Monitillo L, Ferrero S, Pepin F, Drandi D, et al. Next-generation sequencing and real-time quantitative pcr for minimal residual disease detection in b-cell disorders. Leukemia. 2014;28:1299–307. doi: 10.1038/leu.2013.375. [DOI] [PubMed] [Google Scholar]
- 8.Cavo M, Terragna C, Martinelli G, Ronconi S, Zamagni E, Tosi P, et al. Molecular monitoring of minimal residual disease in patients in long-term complete remission after allogeneic stem cell transplantation for multiple myeloma. Blood. 2000;96:355–7. [PubMed] [Google Scholar]
- 9.Martinelli G, Terragna C, Zamagni E, Ronconi S, Tosi P, Lemoli RM, et al. Molecular remission after allogeneic or autologous transplantation of hematopoietic stem cells for multiple myeloma. J Clin Oncol. 2000;18:2273–81. doi: 10.1200/JCO.2000.18.11.2273. [DOI] [PubMed] [Google Scholar]
- 10.Martinelli G, Terragna C, Zamagni E, Ronconi S, Tosi P, Lemoli R, et al. Polymerase chain reaction-based detection of minimal residual disease in multiple myeloma patients receiving allogeneic stem cell transplantation. Haematologica. 2000;85:930–4. [PubMed] [Google Scholar]
- 11.Corradini P, Voena C, Tarella C, Astolfi M, Ladetto M, Palumbo A, et al. Molecular and clinical remissions in multiple myeloma: Role of autologous and allogeneic transplantation of hematopoietic cells. J Clin Oncol. 1999;17:208–15. doi: 10.1200/JCO.1999.17.1.208. [DOI] [PubMed] [Google Scholar]
- 12.Swedin A, Lenhoff S, Olofsson T, Thuresson B, Westin J. Clinical utility of immunoglobulin heavy chain gene rearrangement identification for tumour cell detection in multiple myeloma. Br J Haematol. 1998;103:1145–51. doi: 10.1046/j.1365-2141.1998.01075.x. [DOI] [PubMed] [Google Scholar]
- 13.Bjorkstrand B, Ljungman P, Bird JM, Samson D, Gahrton G. Double high-dose chemoradiotherapy with autologous stem cell transplantation can induce molecular remissions in multiple myeloma. Bone Marrow Transplant. 1995;15:367–71. [PubMed] [Google Scholar]
- 14.Corradini P, Voena C, Astolfi M, Ladetto M, Tarella C, Boccadoro M, Pileri A. High-dose sequential chemoradiotherapy in multiple myeloma: Residual tumor cells are detectable in bone marrow and peripheral blood cell harvests and after autografting. Blood. 1995;85:1596–602. [PubMed] [Google Scholar]
- 15.Sarasquete ME, Garcia-Sanz R, Gonzalez D, Martinez J, Mateo G, Martinez P, et al. Minimal residual disease monitoring in multiple myeloma: A comparison between allelic-specific oligonucleotide real-time quantitative polymerase chain reaction and flow cytometry. Haematologica. 2005;90:1365–72. [PubMed] [Google Scholar]
- 16.Hadzidimitriou A, Stamatopoulos K, Belessi C, Lalayianni C, Stavroyianni N, Smilevska T, et al. Immunoglobulin genes in multiple myeloma: Expressed and non-expressed repertoires, heavy and light chain pairings and somatic mutation patterns in a series of 101 cases. Haematologica. 2006;91:781–7. [PubMed] [Google Scholar]
- 17.Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: Impact on outcome in the medical research council myeloma ix study. J Clin Oncol. 2013;31:2540–7. doi: 10.1200/JCO.2012.46.2119. [DOI] [PubMed] [Google Scholar]
- 18.Dekker LJ, Zeneyedpour L, Brouwer E, van Duijn MM, Sillevis Smitt PA, Luider TM. An antibody-based biomarker discovery method by mass spectrometry sequencing of complementarity determining regions. Anal Bioanal Chem. 2011;399:1081–91. doi: 10.1007/s00216-010-4361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remily-Wood ER, Benson K, Baz RC, Chen YA, Hussein M, Hartley-Brown MA, et al. Quantification of peptides from immunoglobulin constant and variable regions by lc-mrm ms for assessment of multiple myeloma patients. Proteomics Clin Appl. 2014;8:783–95. doi: 10.1002/prca.201300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnidge DR, Tschumper RC, Theis JD, Snyder MR, Jelinek DF, Katzmann JA, et al. Monitoring m-proteins in patients with multiple myeloma using heavy-chain variable region clonotypic peptides and lc–ms/ms. J Proteome Res. 2014;13:1905–10. doi: 10.1021/pr5000544. [DOI] [PubMed] [Google Scholar]
- 21.Dasari S, Theis JD, Vrana JA, Meureta OM, Quint PS, Muppa P, et al. Proteomic detection of immunoglobulin light chain variable region peptides from amyloidosis patient biopsies. J Proteome Res. 2015;14:1957–67. doi: 10.1021/acs.jproteome.5b00015. [DOI] [PubMed] [Google Scholar]
- 22.Ehrenmann F, Kaas Q, Lefranc MP. Imgt/3dstructure-db and imgt/domaingapalign: A database and a tool for immunoglobulins or antibodies, t cell receptors, mhc, igsf and mhcsf. Nucleic Acids Res. 2010;38:D301–7. doi: 10.1093/nar/gkp946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brochet X, Lefranc MP, Giudicelli V. Imgt/v-quest: The highly customized and integrated system for ig and tr standardized v-j and v-d-j sequence analysis. Nucleic Acids Res. 2008;36:W503–8. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morice WG, Hanson CA, Kumar S, Frederick LA, Lesnick CE, Greipp PR. Novel multi-parameter flow cytometry sensitively detects phenotypically distinct plasma cell subsets in plasma cell proliferative disorders. Leukemia. 2007;21:2043–6. doi: 10.1038/sj.leu.2404712. [DOI] [PubMed] [Google Scholar]
- 25.Creative-Biolabs [Accessed April 1, 2014];Next generation antibody seqeuncing. http://www.creative-biolabs.com/next-generation-antibody-sequencing.html.
- 26.Bioinformatics Solutions I [Accessed April, 1 2014];Champs--antibody sequencing service. http://www.bioinfor.com/peaks/products/champs.html.
- 27.MabPrex I [Accessed April 1, 2014];De novo antibody variable region sequencing. http://mabprex.com/de-novo-antibody-sequencing/
- 28.Song MK, Chung JS, Lee JJ, Min CK, Ahn S, Lee SM, et al. Magnetic resonance imaging pattern of bone marrow involvement as a new predictive parameter of disease progression in newly diagnosed patients with multiple myeloma eligible for autologous stem cell transplantation. Br J Haematol. 2014 doi: 10.1111/bjh.12820. [DOI] [PubMed] [Google Scholar]
- 29.Waheed S, Mitchell A, Usmani S, Epstein J, Yaccoby S, Nair B, et al. Standard and novel imaging methods for multiple myeloma: Correlates with prognostic laboratory variables including gene expression profiling data. Haematologica. 2013;98:71–8. doi: 10.3324/haematol.2012.066555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin R. Precision medicine: The future or simply politics? JAMA. 2015;313:1089–91. doi: 10.1001/jama.2015.0957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.