Abstract
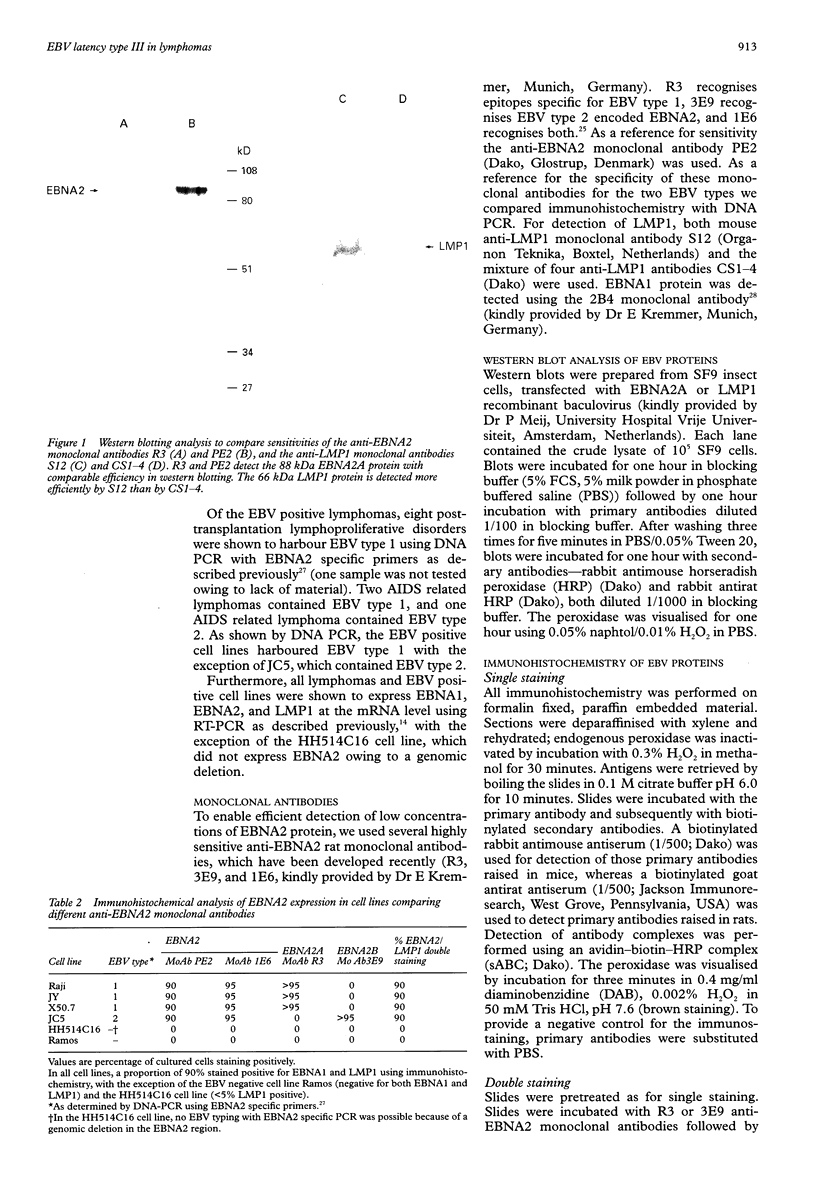
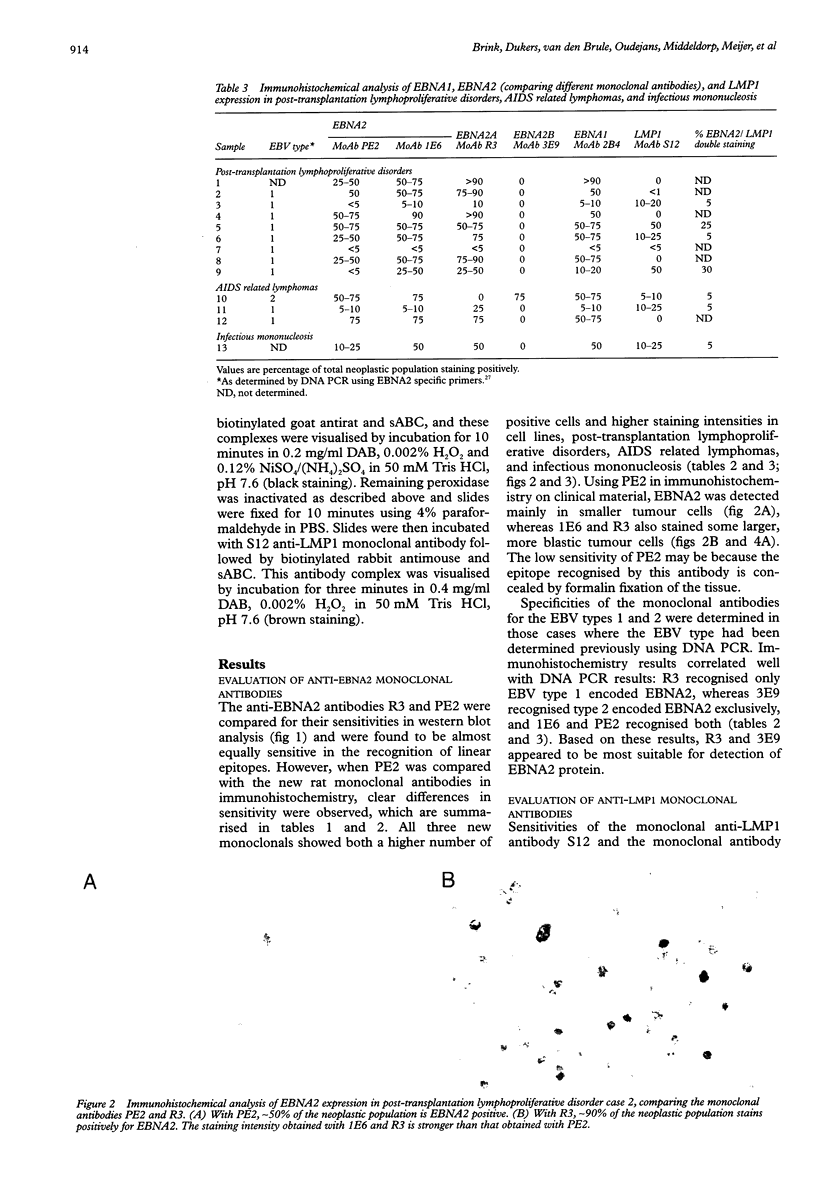
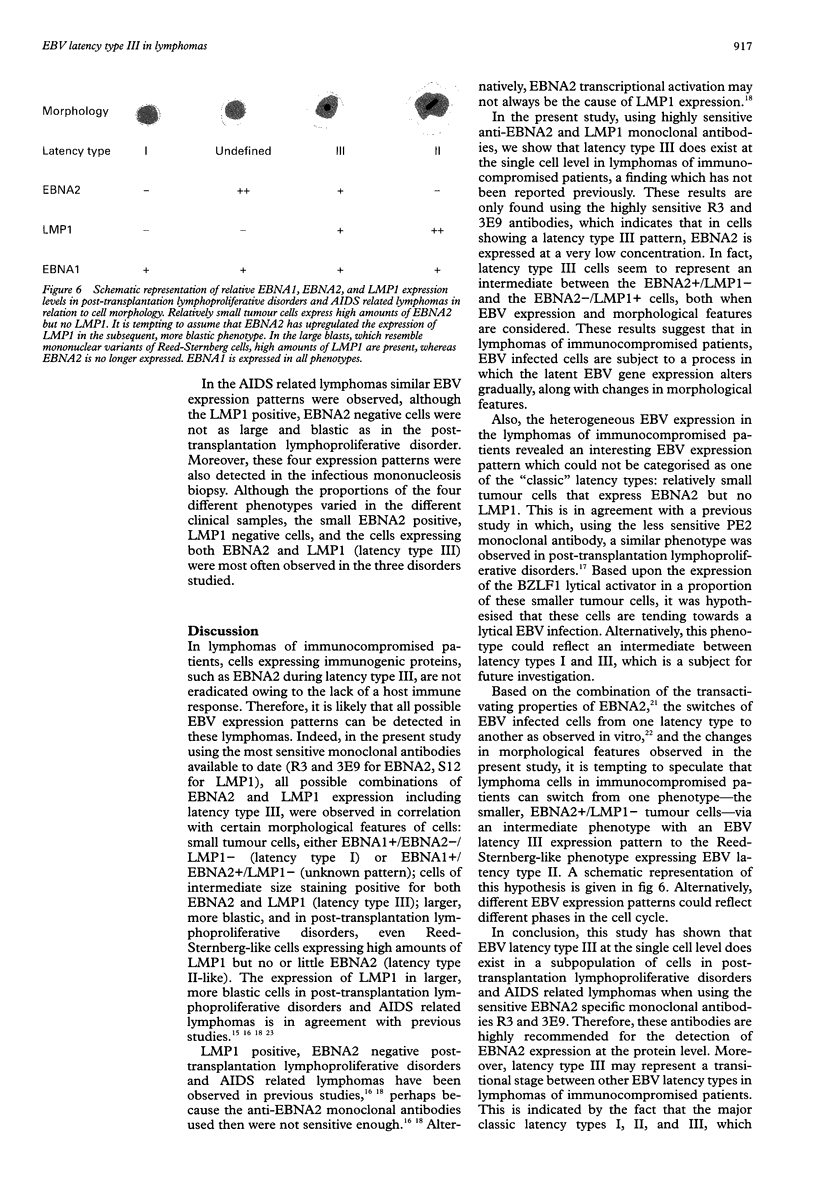
AIMS: To investigate the expression pattern of Epstein-Barr virus (EBV) latent genes at the single cell level in post-transplantation lymphoproliferative disorders and acquired immunodefiency syndrome (AIDS) related lymphomas, in relation to cellular morphology. METHODS: Nine post-transplantation lymphoproliferative disorders and three AIDS related lymphomas were subjected to immunohistochemistry using monoclonal antibodies specific for EBV nuclear antigen 1 (EBNA1) (2H4), EBNA2 (PE2 and the new rat anti-EBNA2 monoclonal antibodies 1E6, R3, and 3E9), and LMP1 (CS1-4 and S12). Double staining was performed combining R3 or 3E9 with S12. RESULTS: R3 and 3E9 anti-EBNA2 monoclonal antibodies were more sensitive than PE2, enabling the detection of more EBNA2 positive lymphoma cells. Both in post-transplantation lymphoproliferative disorders and AIDS related lymphomas, different expression patterns were detected at the single cell level. Smaller neoplastic cells were positive for EBNA2 but negative for LMP1. Larger and more blastic neoplastic cells, sometimes resembling Reed-Sternberg cells, were LMP1 positive but EBNA2 negative (EBV latency type II). Morphologically intermediate neoplastic cells coexpressing EBNA2 and LMP1 (EBV latency type III), were detected using R3 and 3E9, and formed a considerable part of the neoplastic population in four of nine post-transplantation lymphoproliferative disorders and two of three AIDS related lymphomas. All samples contained a subpopulation of small tumour cells positive exclusively for Epstein-Barr early RNA and EBNA1. The relation between cellular morphology and EBV expression patterns in this study was less pronounced in AIDS related lymphomas than in post-transplantation lymphoproliferative disorders, because the AIDS related lymphomas were less polymorphic than the post-transplantation lymphoproliferative disorders. CONCLUSIONS: In post-transplantation lymphoproliferative disorders and AIDS related lymphomas, EBV latency type III can be detected by immunohistochemistry in a subpopulation of tumour cells using sensitive monoclonal antibodies R3 and 3E9. Our data suggest that EBV infected tumour cells in these lymphomas undergo gradual changes in the expression of EBV latent genes, and that these changes are associated with changes in cellular morphology.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfieri C., Birkenbach M., Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991 Apr;181(2):595–608. doi: 10.1016/0042-6822(91)90893-g. [DOI] [PubMed] [Google Scholar]
- Carbone A., Dolcetti R., Gloghini A., Maestro R., Vaccher E., di Luca D., Tirelli U., Boiocchi M. Immunophenotypic and molecular analyses of acquired immune deficiency syndrome-related and Epstein-Barr virus-associated lymphomas: a comparative study. Hum Pathol. 1996 Feb;27(2):133–146. doi: 10.1016/s0046-8177(96)90366-4. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Sadler R. H., Walling D. M., Su I. J., Hsieh H. C., Raab-Traub N. Epstein-Barr virus (EBV) gene expression in EBV-positive peripheral T-cell lymphomas. J Virol. 1993 Oct;67(10):6303–6308. doi: 10.1128/jvi.67.10.6303-6308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty R., Biddolph S. C., Kaklamanis L., Cary N., Stewart S., Giatromanolaki A., Gatter K. C. EBV latent membrane protein (LMP-1) and bcl-2 protein expression in Reed-Sternberg-like cells in post-transplant lymphoproliferative disorders. Histopathology. 1996 Mar;28(3):257–260. doi: 10.1046/j.1365-2559.1996.d01-425.x. [DOI] [PubMed] [Google Scholar]
- Deacon E. M., Pallesen G., Niedobitek G., Crocker J., Brooks L., Rickinson A. B., Young L. S. Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med. 1993 Feb 1;177(2):339–349. doi: 10.1084/jem.177.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Zutter M. M., Minarovits J., Oosterveer M. A., Thomas E. D., Klein G., Ernberg I. Expression of Epstein-Barr virus-encoded growth-transformation-associated proteins in lymphoproliferations of bone-marrow transplant recipients. Int J Cancer. 1991 Jan 21;47(2):188–192. doi: 10.1002/ijc.2910470205. [DOI] [PubMed] [Google Scholar]
- Grässer F. A., Murray P. G., Kremmer E., Klein K., Remberger K., Feiden W., Reynolds G., Niedobitek G., Young L. S., Mueller-Lantzsch N. Monoclonal antibodies directed against the Epstein-Barr virus-encoded nuclear antigen 1 (EBNA1): immunohistologic detection of EBNA1 in the malignant cells of Hodgkin's disease. Blood. 1994 Dec 1;84(11):3792–3798. [PubMed] [Google Scholar]
- Hamilton-Dutoit S. J., Rea D., Raphael M., Sandvej K., Delecluse H. J., Gisselbrecht C., Marelle L., van Krieken H. J., Pallesen G. Epstein-Barr virus-latent gene expression and tumor cell phenotype in acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Correlation of lymphoma phenotype with three distinct patterns of viral latency. Am J Pathol. 1993 Oct;143(4):1072–1085. [PMC free article] [PubMed] [Google Scholar]
- Jiwa N. M., Kanavaros P., De Bruin P. C., van der Valk P., Horstman A., Vos W., Mullink H., Walboomers J. M., Meijer C. J. Presence of Epstein-Barr virus harbouring small and intermediate-sized cells in Hodgkin's disease. Is there a relationship with Reed-Sternberg cells? J Pathol. 1993 Jun;170(2):129–136. doi: 10.1002/path.1711700206. [DOI] [PubMed] [Google Scholar]
- Jiwa N. M., Oudejans J. J., Dukers D. F., Vos W., Horstman A., van der Valk P., Middledorp J. M., Walboomers J. M., Meijer C. J. Immunohistochemical demonstration of different latent membrane protein-1 epitopes of Epstein-Barr virus in lymphoproliferative diseases. J Clin Pathol. 1995 May;48(5):438–442. doi: 10.1136/jcp.48.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. F., Shurin S., Abramowsky C., Tubbs R. R., Sciotto C. G., Wahl R., Sands J., Gottman D., Katz B. Z., Sklar J. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med. 1988 Mar 24;318(12):733–741. doi: 10.1056/NEJM198803243181203. [DOI] [PubMed] [Google Scholar]
- Kanavaros P., Briere J., Lescs M. C., Gaulard P. Epstein-Barr virus in non-Hodgkin's lymphomas of the upper respiratory tract: association with sinonasal localization and expression of NK and/or T-cell antigens by tumour cells. J Pathol. 1996 Mar;178(3):297–302. doi: 10.1002/(SICI)1096-9896(199603)178:3<297::AID-PATH469>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kerr B. M., Lear A. L., Rowe M., Croom-Carter D., Young L. S., Rookes S. M., Gallimore P. H., Rickinson A. B. Three transcriptionally distinct forms of Epstein-Barr virus latency in somatic cell hybrids: cell phenotype dependence of virus promoter usage. Virology. 1992 Mar;187(1):189–201. doi: 10.1016/0042-6822(92)90307-b. [DOI] [PubMed] [Google Scholar]
- Kremmer E., Kranz B. R., Hille A., Klein K., Eulitz M., Hoffmann-Fezer G., Feiden W., Herrmann K., Delecluse H. J., Delsol G. Rat monoclonal antibodies differentiating between the Epstein-Barr virus nuclear antigens 2A (EBNA2A) and 2B (EBNA2B). Virology. 1995 Apr 1;208(1):336–342. doi: 10.1006/viro.1995.1157. [DOI] [PubMed] [Google Scholar]
- Oudejans J. J., Jiwa M., van den Brule A. J., Grässer F. A., Horstman A., Vos W., Kluin P. M., van der Valk P., Walboomers J. M., Meijer C. J. Detection of heterogeneous Epstein-Barr virus gene expression patterns within individual post-transplantation lymphoproliferative disorders. Am J Pathol. 1995 Oct;147(4):923–933. [PMC free article] [PubMed] [Google Scholar]
- Oudejans J. J., van den Brule A. J., Jiwa N. M., de Bruin P. C., Ossenkoppele G. J., van der Valk P., Walboomers J. M., Meijer C. J. BHRF1, the Epstein-Barr virus (EBV) homologue of the BCL-2 protooncogene, is transcribed in EBV-associated B-cell lymphomas and in reactive lymphocytes. Blood. 1995 Sep 1;86(5):1893–1902. [PubMed] [Google Scholar]
- Pallesen G., Hamilton-Dutoit S. J., Rowe M., Lisse I., Ralfkiaer E., Sandvej K., Young L. S. Expression of Epstein-Barr virus replicative proteins in AIDS-related non-Hodgkin's lymphoma cells. J Pathol. 1991 Dec;165(4):289–299. doi: 10.1002/path.1711650404. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Sakamoto K., Saemundsen A. K., Sullivan J. L., Synnerholm A. C., Anvret M., Pritchard J., Sloper C., Sieff C., Pincott J. Documentation of Epstein-Barr virus infection in immunodeficient patients with life-threatening lymphoproliferative diseases by clinical, virological, and immunopathological studies. Cancer Res. 1981 Nov;41(11 Pt 1):4226–4236. [PubMed] [Google Scholar]
- Rea D., Fourcade C., Leblond V., Rowe M., Joab I., Edelman L., Bitker M. O., Gandjbakhch I., Suberbielle C., Farcet J. P. Patterns of Epstein-Barr virus latent and replicative gene expression in Epstein-Barr virus B cell lymphoproliferative disorders after organ transplantation. Transplantation. 1994 Aug 15;58(3):317–324. [PubMed] [Google Scholar]
- Rowe M., Lear A. L., Croom-Carter D., Davies A. H., Rickinson A. B. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992 Jan;66(1):122–131. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin P. C., Jiwa M., Oudejans J. J., van der Valk P., van Heerde P., Sabourin J. C., Csanaky G., Gaulard P., Noorduyn A. L., Willemze R. Presence of Epstein-Barr virus in extranodal T-cell lymphomas: differences in relation to site. Blood. 1994 Mar 15;83(6):1612–1618. [PubMed] [Google Scholar]