Anaplastic lymphoma kinase-rearranged lung cancer is associated with significant response to crizotinib. However, resistance to crizotinib typically develops within 1-2 years. Preclinical studies and biopsies of resistant sites have demonstrated that diverse mechanisms underlie resistance. Insight into resistance mechanisms has facilitated development of next-generation inhibitors and led to therapeutic combination strategies, several of which are being tested in the clinic.
Abstract
In 2007, a chromosomal rearrangement resulting in a gene fusion leading to expression of a constitutively active anaplastic lymphoma kinase (ALK) fusion protein was identified as an oncogenic driver in non-small-cell lung cancer (NSCLC). ALK rearrangements are detected in 3%–7% of patients with NSCLC and are particularly enriched in younger patients with adenocarcinoma and a never or light smoking history. Fortuitously, crizotinib, a small molecule tyrosine kinase inhibitor initially developed to target cMET, was able to be repurposed for ALK-rearranged (ALK+) NSCLC. Despite dramatic and durable initial responses to crizotinib; however, the vast majority of patients will develop resistance within a few years. Diverse molecular mechanisms underlie resistance to crizotinib. This review will describe the clinical activity of crizotinib, review identified mechanisms of crizotinib resistance, and end with a survey of emerging therapeutic strategies aimed at overcoming crizotinib resistance.
introduction
Over the last decade, advances in molecular genetics have transformed our understanding of the pathogenesis of non-small-cell lung cancer (NSCLC). The discovery of a correlation between activating mutations in the epidermal growth factor receptor (EGFR) gene and responsiveness to EGFR-tyrosine kinase inhibitors (TKIs) not only led to establishment of these drugs as standard therapy for this molecular subgroup of patients but also marked the advent of genotype-directed therapies in NSCLC [1, 2]. This discovery subsequently fueled efforts to identify additional oncogenic drivers in NSCLC. In 2007, Soda and colleagues identified an inversion of chromosome 2 resulting in a fusion gene juxtaposing the 5′ end of the echinoderm microtubule-associated protein-like 4 (EML4) gene with the 3′ end of the anaplastic lymphoma kinase (ALK) gene. This fusion led to expression of a constitutively active novel ALK fusion protein with transforming activity [3].
It is now estimated that 3%–7% of patients with NSCLC will have an ALK rearrangement [4, 5]. The incidence of ALK rearrangements is increased in the subgroup of young patients with adenocarcinoma and a never or light smoking history [6, 7]. Simultaneous with the identification of patients with ALK-rearranged (ALK+) NSCLC, crizotinib, a new small molecule TKI with preclinical activity against ALK, had already entered the clinic and was being studied in a phase I trial, primarily designed for patients with aberrant activation of cMET. Early responses among patients with ALK+ NSCLC enrolled in this trial established ALK as a clinically validated molecular target.
In the years that have elapsed since the adoption of genotype-directed therapies in NSCLC, much has been learned about response and resistance to targeted therapeutics. Experiences with crizotinib resistance have been instrumental in illustrating the complex and dynamic nature of TKI resistance. Improved understanding of the molecular mechanisms underlying resistance has facilitated the development of next-generation TKIs. This review will describe the clinical activity of crizotinib, evaluate the known mechanisms of crizotinib resistance, and highlight established and emerging therapeutic strategies aimed at overcoming crizotinib resistance.
clinical activity of crizotinib
Crizotinib (PF-02341066) is a first-in-class, oral small molecule ATP competitive inhibitor initially developed as a cMET kinase inhibitor which was subsequently found to inhibit ALK and ROS1 kinases during biochemical characterization. This led to the enrollment of patients with ALK+ NSCLC in the original phase I trial investigating crizotinib in patients with various types of advanced cancer (PROFILE 1001). Due to promising clinical activity in two patients with ALK+ NSCLC who enrolled during the dose escalation phase, an expansion cohort was created. Based on an objective response rate (ORR) of 61% in the first 119 assessable patients in this trial, along with an ORR of 50% from the first 136 patients treated with crizotinib in the phase II trial (PROFILE 1005), crizotinib was granted accelerated approval by the FDA on 26 August 2011 [8–11].
Two phase III studies of crizotinib have been conducted. In the phase III trial of crizotinib versus single-agent chemotherapy in the second-line setting (PROFILE 1007), crizotinib significantly improved progression-free survival (PFS) from 3.0 to 7.7 months (hazard ratio [HR] 0.49, P < 0.001). ORR was also significantly higher with crizotinib at 65%, compared with 20% with chemotherapy [12]. In the phase III trial comparing upfront crizotinib to platinum-based combination chemotherapy (PROFILE 1014), crizotinib significantly improved PFS from 7.0 to 10.9 months (HR 0.45, P < 0.001). ORR with crizotinib was 74%, while ORR with chemotherapy was 45% [13]. In both phase III studies, crizotinib was well tolerated and was associated with a significantly greater improvement in quality of life compared with chemotherapy. Based on the positive data from PROFILE 1007, crizotinib was granted full approval by the FDA on 20 November 2013. Crizotinib was initially approved by the EMA as a second-line therapy before recent approval for use in the first-line setting on 24 November 2015. Crizotinib is also approved in many other countries for the treatment of patients with advanced, ALK+ NSCLC.
clinical relapses on crizotinib
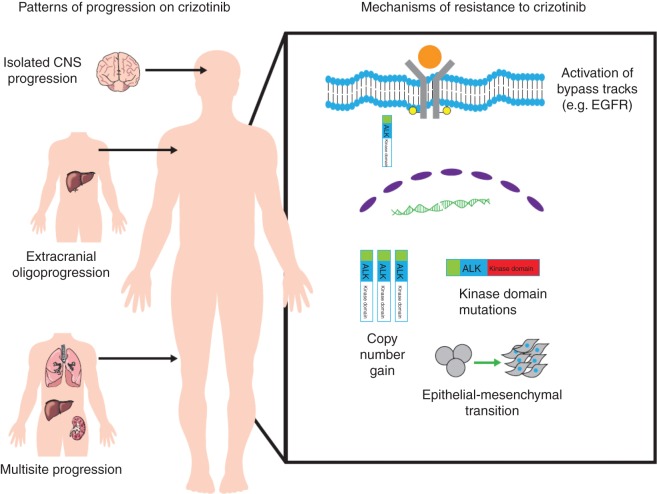
Patients with ALK+ NSCLC most often present with advanced disease involving multiple sites, particularly lymph nodes, pleural and pericardial surfaces, the brain, and liver [14]. Despite dramatic and typically durable responses, the vast majority of patients treated with crizotinib will develop disease progression. Most relapses occur within the first year of treatment, although prolonged responses lasting over 6 years can rarely be seen. For the majority of patients, disease progression after treatment with crizotinib will similarly involve multiple sites [10]. In a smaller proportion of patients, oligoprogression, or progression limited to a few metastatic sites, has been described. The following sections will review two patterns of progression that have emerged with increased experience with treating patients with crizotinib (Figure 1), and briefly discuss some early strategies that have been successful in addressing these unique patterns of treatment failure.
Figure 1.
Diverse mechanisms of resistance leading to systemic relapse can emerge in the setting of selective pressure exerted by crizotinib. Identified mechanisms of resistance are depicted on the right. Different patterns are seen during progression on crizotinib (depicted on the left). Progression typically involves multiple sites. Patients with ALK+ non-small-cell lung cancer who are treated with crizotinib are prone to central nervous system relapse, particularly isolated central nervous system relapse. A subgroup of patients will have oligoprogression, or relapse involving only limited sites.
central nervous system only relapses
Brain metastases are commonly present at diagnosis of ALK+ NSCLC and at the time of disease progression on crizotinib. In fact, brain metastases were present at baseline in 26% of patients enrolled on PROFILE 1014 [13]. Similarly, in one single-institution study, brain metastases were present in 23.8% and 58.4% of patients at the time of diagnosis and at 3 years despite treatment with crizotinib [15]. In patients with treated brain metastases enrolled on PROFILE 1014, there was a significant improvement in the intracranial disease control rate (DCR) and intracranial PFS in those treated with crizotinib compared with those treated with chemotherapy [16]. Unfortunately, despite significantly improved disease control with crizotinib compared with chemotherapy, central nervous system (CNS) progression is frequently observed [17, 18]. In a retrospective pooled analysis from the PROFILE 1005 and 1007 trials, median time to intracranial progression among patients with asymptomatic untreated brain metastases was 7 months compared with a 12.5-month median time to systemic progression [19]. In this pooled analysis, in patients with known brain metastases, the CNS was a site of new lesions or progression of non-target lesions in 70% of patients while on treatment with crizotinib. Notably, 20% of those without brain metastases at study enrollment developed brain metastases on crizotinib.
The predisposition toward CNS relapse as an initial site of failure has been largely attributed to pharmacokinetic shortcomings of crizotinib. In particular, crizotinib is a known substrate of P-glycoprotein, a drug efflux pump that limits accumulation of the drug in the CNS [20, 21]. In several studies, resuming crizotinib after local ablative therapies for brain metastases has been shown to be a feasible and effective strategy for ongoing extracranial disease control [22]. In the phase I PROFILE 1001 trial, of the 10 patients who continued crizotinib beyond CNS progression, the duration of treatment after progression ranged from 82 to >591 days [10]. Similarly, the median duration of treatment with crizotinib after progression for the 34 patients with CNS progression treated on PROFILE 1005 and 1007 was 19.3 weeks, with a range from 3.1 to 63.6 weeks. Of note, 27 of the 34 patients received local CNS therapies after progression before resuming treatment with crizotinib [19].
oligoprogression at extracranial sites
Although most patients who develop disease progression after treatment with crizotinib will progress at multiple sites, some patients will have discordant progression limited to a few sites. In these situations, brief interruption of crizotinib for local ablative therapy has been rationalised as an approach to eradicate emerging resistant clones before dissemination and to capitalise on continued ALK dependence at responding sites. Experience with this strategy has been limited to small, single-institution studies. While the rationale for this approach is compelling, randomised trials are indicated to determine whether such maneuvers truly impact the biological course of the disease.
In a single-institution study which included 14 patients with ALK+ NSCLC who had intracranial or extracranial oligoprogression on crizotinib, interruption of crizotinib for local ablative therapy to extracranial sites followed by continuation of crizotinib beyond progression was associated with an additional 7.0 months of extracranial PFS [23]. A follow-up study from the same group looking exclusively at 14 patients who developed extracranial-only oligoprogression on crizotinib treated with repeat local ablative therapies reported a 6- and 12-month actuarial local lesional control rate of 100% and 86% following local ablative therapies, respectively [24]. In those considered suitable for repeat local ablative therapy at progression, crizotinib was continued more than a year on average beyond initial diagnosis of extracranial progression. Experiences with treatment of oligoprogressive ALK+ NSCLC not only highlight the heterogeneity of the disease but also lend support for a multidisciplinary management approach in select patients.
mechanisms of resistance to crizotinib
The vast majority of patients with ALK+ NSCLC will initially respond to first-line treatment with crizotinib. In fact, in PROFILE 1014, the phase III trial comparing upfront crizotinib to chemotherapy, the DCR with crizotinib was 91% [13]. The mechanisms underlying innate or de novo lack of response to therapy, often referred to as intrinsic resistance, are not well understood. In those with initial response, the median duration of response to upfront treatment is 11.3 months [13]. This review will focus on mechanisms underlying acquired resistance (Figure 1) or progression that occurs after initial disease stabilization or shrinkage with treatment.
secondary resistance mutations in ALK
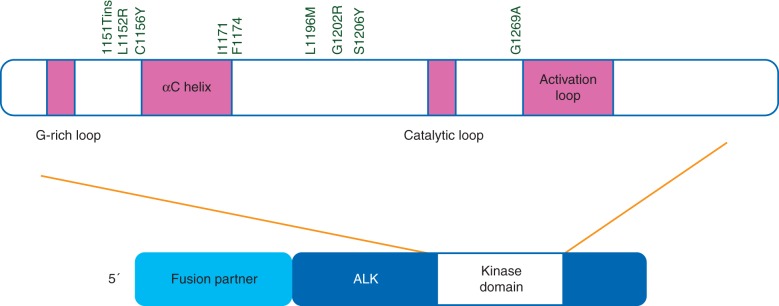
Initial insight into mechanisms of resistance to crizotinib was provided when Choi and colleagues identified two independently acquired ALK kinase domain mutations, L1196M and C1156Y, through deep sequencing of pre-treatment and post-progression biopsies in a patient who developed resistance after 5 months of crizotinib [25]. Structural modeling and comparison with other receptor tyrosine kinases revealed that the L1196M mutation most likely corresponds to a gatekeeper residue, or a residue located in the ATP-binding pocket of a protein kinase that when mutated causes a change in the structure of the kinase that prevents TKI binding. Since the initial report, at least eight additional secondary resistance mutations involving the kinase domain have been identified from efforts using patient samples at progression, patient-derived cell lines, and ALK cell lines made resistant in vitro (Figure 2). These include G1269A, F1174, 1151Tins, L1152R, S1206Y, I1171T, G1202R, and D1203N [26–29].
Figure 2.
The anaplastic lymphoma (ALK) receptor tyrosine kinase comprises an extracellular ligand-binding domain (residues 19–1038), transmembrane domain (residues 1039–1059), and intracellular domain (residues 1060–1620). The tyrosine kinase domain is located in the cytoplasm and spans residues 1116–1392. The kinase domain includes a glycine rich (G-rich) loop (residues 1123–1128), an αC helix (residues 1157–1173), a catalytic loop (residues 1246–1251), and an activation loop (residues 1271–1288). The identified acquired secondary ALK kinase mutations conferring resistance to crizotinib are located between the G-rich loop and the activation loop. Structural modeling of these mutations suggests that crizotinib resistance is caused by increased catalytic activity of ALK or diminished affinity of crizotinib for mutant ALK.
In the two largest published case series of patients with available post-progression biopsy samples amenable to molecular characterization, secondary resistance mutations were reported to occur in between 22% and 36% of patients [26, 29]. In the series by Doebele and colleagues, 11 patients underwent repeat biopsy at disease progression. Four patients were noted to have secondary ALK kinase domain mutations. Notably, in the four cases, there was not sufficient tissue in the pre-treatment biopsy to determine whether the mutations were present before treatment. In the series of 18 patients who underwent biopsy after relapse on crizotinib reported by Katayama and colleagues, four patients were found to have secondary ALK kinase domain mutations. Sequencing of the pre-treatment biopsies in three of these patients determined that the resistance mutations were not detectable before treatment. For those mutations where the biochemical impact has been elucidated, resistance is thought to be mediated by either increasing catalytic activity of ALK or by diminishing affinity of crizotinib for mutant ALK, primarily through steric hindrance [30].
ALK copy number alterations
In a cell line model of crizotinib resistance, resistance to intermediate doses of crizotinib was mediated by amplification of EML4-ALK [31]. Although these cells later developed the L1196M mutation as they became progressively more resistant, highly sensitive PCR assays were used to verify that the initial amplification event occurred before acquisition of a resistance mutation, suggesting a stepwise progression. Two subsequent studies using patient biopsies at progression have verified that ALK copy number gain can serve as a mechanism of resistance to crizotinib [26, 29]. While copy number gain in some cases coexisted with acquisition of secondary resistance mutations, in at least one case copy number gain alone was sufficient to confer resistance [29]. In all of these cases, resistance arose through selective copy number gain or amplification of ALK rather than polysomy.
bypass tracks
Approximately two-thirds of patients who develop resistance to crizotinib will not have identifiable secondary resistance mutations or ALK copy number alterations. In these cases, the most commonly implicated mechanism of crizotinib resistance involves aberrant activation of alternate kinases leading to ALK-independent growth (Table 1). Activation of EGFR is the most commonly reported means of bypassing ALK signaling [28, 29]. However, activation of other kinases has also been described. Katayama and colleagues reported two cases of crizotinib resistance mediated by acquired KIT amplification [29]. In both cases, KIT amplification was accompanied by other resistance mechanisms, specifically EGFR activation and a secondary ALK mutation, supporting the notion that multiple resistance mechanisms can be activated in an individual. In cell line models, treatment with imatinib, a small molecule inhibitor of KIT, was able to overcome resistance. Lovly and colleagues demonstrated IGF-1R activation in crizotinib-resistant tumor samples and the combination of IGF1-R activation and increased IGF-1 ligand levels in cell lines [34]. Combination treatment with crizotinib and monoclonal antibodies against IGF-1R worked synergistically to inhibit growth in cell lines and in mouse models. The second-generation ALK inhibitor, ceritinib, which inhibits ALK and has some activity against IGF-1R, was also able to overcome this resistance in preclinical models [38]. However, in clinical practice, toxicity likely limits dosing ceritinib at a level capable of effectively inhibiting IGF-1R.
Table 1.
Bypass pathways identified as mediators of crizotinib resistance
| Pathway/signal | Mechanism of activation | Potential combinations/agentsa | Reference |
|---|---|---|---|
| EGFR | Ligand secretion (EGF, amphiregulin, neuregulin 1) | Crizotinib + dacomitinib Crizotinib + gefitinib Crizotinib + erlotinib |
[28, 29, 32, 33] |
| cKIT | Amplification, ligand secretion (stem cell factor) | Crizotinib + imatinib | [29] |
| IGF-IR | Ligand secretion (IGF-1) | Crizotinib + linsitinib Ceritinibb |
[34] |
| HER2/HER3 | Ligand secretion (EGF, neuregulin 1) | Crizotinib + lapatinib | [32, 35] |
| SRC | The mechanism is unknown. ALK inhibition is speculated to lead to SRC upregulation via release of a negative regulatory signal | Crizotinib + saracatinib Crizotinib + dasatinib |
[36] |
| P2Y purinergic receptors | Activation of protein kinase C | Crizotinib + sotrastaurin | [35] |
| MAPK | Wild-type KRAS copy number gain, downregulation of DUSP6 | Crizotinib + trametinib | [37] |
aSimilar combinations are active in preclinical models and have not been validated in clinical trials.
bNote: toxicity limits dosing ceritinib in patients at a level necessary to inhibit IGF-1R.
Advanced genetic and pharmacologic screening techniques have been instrumental in uncovering additional activated bypass tracks that may mediate crizotinib resistance. Using pharmacologic screens, Crystal and colleagues demonstrated activation of SRC family kinases in six of nine patient-derived resistant cell lines and posited that ALK inhibition may lead to release of a negative regulatory signal for SRC [36]. Regression of tumors in a mouse xenograft model and attenuated growth of cell lines was seen with the combination of an SRC family kinase inhibitor and an ALK inhibitor. Using an open-reading frame (ORF) library to identify transcripts whose overexpression led to resistance to crizotinib after introduction into an ALK-dependent cell line, Wilson and colleagues identified a potential role for protein kinase C activation via increased expression of P2Y purinergic G-protein-coupled receptors as a mediator of crizotinib resistance [35]. Resistance was able to be reversed by treatment with crizotinib and sotrastaurin, a pan-protein kinase C inhibitor. Protein kinase C activation, however, has yet to be demonstrated in cancers that have developed resistance to crizotinib.
paracrine factors driving resistance
Cancers are dependent on the complex signaling networks between tumor cells and stromal cells in their surrounding microenvironment for promotion of growth and survival. In models of crizotinib resistance, paracrine signaling acts to promote growth of cancer cells by activating downstream signaling cascades in an ALK-independent fashion. As discussed above, experiments by Katayama and colleagues suggest that stromal secretion of stem cell factor can lead to activation of its receptor KIT which may promote crizotinib resistance [29]. EGFR ligands, however, are the most commonly implicated paracrine mediators of crizotinib resistance.
Work by Yamada and colleagues demonstrated that EGFR ligands produced by endothelial cells reduced ALK cell line sensitivity to crizotinib by reversal of inhibition of ALK-mediated phosphorylation of downstream targets Akt and Erk1/2 [39]. In two separate experiments using cell lines derived from patients with crizotinib-resistant ALK+ NSCLC, increased secretion of the ligands EGF and amphiregulin led to activation of EGFR in the absence of activating mutations or amplification. EGFR activation was identified as the primary mechanism of resistance to crizotinib in both cases [28, 32]. Upregulation of amphiregulin leading to increased phosphorylation of EGFR has also been detected as a mechanism of resistance in cell lines that develop resistance upon chronic exposure to ALK inhibitors [29]. In all of these experiments, concurrent treatment with ALK and EGFR inhibitors was able to attenuate growth of resistant cells.
Ligand activation of the HER2/HER3 axis has also been identified as a potential mechanism of resistance to treatment with crizotinib. In the ORF library screen described previously, Wilson and colleagues found that the ORF that most strongly induced resistance in cell lines, including a patient-derived cell line, was neuregulin-1, an HER3 ligand [35]. Akin to EGFR ligands, neuregulin was shown to reactivate signaling downstream of ALK. Enrichment of HER2 expression in ALK+ lung tumors with acquired resistance to crizotinib compared with crizotinib-naive tumors was confirmed with RNA sequencing. The combination of an ALK inhibitor and lapatinib, an HER2 inhibitor, was able to resensitise cells that developed resistance after exposure to recombinant neuregulin peptide. The role of neuregulin in crizotinib resistance has been confirmed in separate studies using native and patient-derived cell lines [29, 33].
epithelial–mesenchymal transition
Epithelial–mesenchymal transition (EMT), a cellular reprogramming resulting in the morphologic change from an epithelial shape to a more spindled appearance, is associated with enhanced migratory and invasive capacity. ALK+ NSCLC may be more likely to express EMT markers than other molecular subgroups of NSCLC [40]. Kobayashi and colleagues reported a case of a patient who developed sarcomatoid changes in a progressing lesion associated with high-level ALK gene amplification and loss of epithelial markers after 7 months of treatment with crizotinib [41]. Cell line studies have confirmed a potential role for EMT as an independent mechanism of resistance to crizotinib. Kim and colleagues were able to induce reversible EMT in an ALK cell line and correlated this phenotypic change with reversible crizotinib resistance [42]. Hypoxia may promote resistance to crizotinib by upregulating EMT-related genes in ALK+ cell lines [43]. Unfortunately, little is known about the frequency of EMT in ALK+ lung cancer. Moreover, EMT has not been validated in patients as a stand-alone mechanism of crizotinib resistance. As EMT may play an increasingly important role in treatment resistance for patients treated with more potent next-generation ALK inhibitors, additional studies looking at EMT in patient-derived samples are necessary.
therapeutic strategies to overcome resistance to crizotinib
The enthusiasm generated by crizotinib's marked clinical activity in ALK+ NSCLC has been somewhat tempered by the recognition that patients eventually relapse on crizotinib due to acquired resistance. In fact, the original report of secondary resistance mutations leading to crizotinib resistance was published simultaneously with the initial results of the phase I crizotinib trial [25]. As described above, it has since been recognised that diverse mechanisms of resistance can emerge due to the selection pressure exerted by crizotinib. Understanding the fundamental processes underlying these diverse mechanisms of resistance is essential to developing strategies to overcome them.
Since the initial approval of crizotinib in 2011, several other ALK inhibitors have been developed (Table 2). These inhibitors are more potent than crizotinib and are capable of overcoming the gatekeeper L1196M mutation and others, depending on the specific inhibitor. Not surprisingly, with these increases in potency, copy number gain has not been described as a mechanism of resistance to any of these newer ALK inhibitors. The next-generation ALK inhibitors are generally active in crizotinib-resistant patients (Table 3), with an ORR ranging from 48% to 71% and a median PFS ranging from 6.9 to 13.4 months [44, 46–48]. Ceritinib and alectinib, two next-generation ALK inhibitors, have been approved by the FDA for use after progression on crizotinib. Ceritinib has also been approved by the EMA for the same indication. In the international, multicenter Phase I ASCEND-1 trial which enrolled 163 crizotinib-pretreated patients and 83 crizotinib-naive patients, the ORR and median PFS for patients treated with ceritinib in the crizotinib-pretreated group were 56% and 6.9 months, respectively [44]. In two phase II studies of alectinib in crizotinib-resistant patients (NP28761 and NP28673), the ORR and median PFS were ∼50% and 8–9 months, respectively [46, 47]. Brigatinib another next-generation ALK inhibitor, received breakthrough therapy designation by the FDA based on an ORR of 71% and median PFS of 13.4 months in crizotinib-pretreated patients [48]. The next-generation agents appear to be particularly effective in the ALK inhibitor-naive setting with ORR ranging from 66.3% to 100% and median PFS of at least 18 months. A phase III study (NCT02075840) comparing alectinib with crizotinib as first-line treatment is currently underway.
Table 2.
Next-generation ALK inhibitors
| Next-generation ALK inhibitors | Manufacturer | Approval status | Ongoing trials |
|---|---|---|---|
| Alectinib | Genentech | Approved | Phase 3 study of alectinib versus crizotinib in treatment-naive patients [NCT02075840] |
| Brigatinib | Ariad | Breakthrough Therapy Designation* | Phase 1/2 study of brigatinib [NCT01449461] Phase 2 randomised study testing two doses of brigatinib in patients previously treated with crizotinib (ALTA) [NCT02094573] Phase 3 study of brigatinib versus crizotinib in ALK inhibitor-naive patients (ALTA-1L) [NCT02737501] |
| Ceritinib | Pfizer | Approveda,b | Phase 2 study of ceritinib in crizotinib-naive patients [NCT01685138] Phase 3 study of ceritinib versus chemotherapy in treatment-naive patients [NCT01828099] Phase 3 study of ceritinib versus chemotherapy in patients previously treated with chemotherapy and crizotinib [NCT01828112] |
| Ensartinib | Xcovery | Investigational | Phase 1/2 study of ensartinib in patients with advanced solid tumors [NCT01625234] |
| Entrectinib | Ignyta | Investigational | Phase 1/2a study of entrectinib in patients with advanced solid tumors harboring NTRK, ROS1, or ALK alterations [NCT02097810] Phase 2 basket study of entrectinib in patients with solid tumors harboring NTRK, ROS1, or ALK rearrangements [NCT02568267] |
| Lorlatinib | Pfizer | Investigational | Phase 1/2 Study of lorlatinib in patients with ALK or ROS-rearranged lung cancer [NCT01970865] |
ALK, anaplastic lymphoma kinase, aApproved by the United States Food and Drug Administration, bApproved by the European Medicines Agency.
Table 3.
Next-generation ALK inhibitor efficacy in crizotinib-resistant patients
| Ceritinib |
Alectinib |
Brigatinib | |||
|---|---|---|---|---|---|
| ASCEND-1 [44] | ASCEND-2 [45] | NP28763 [46] | NP28761 [47] | Phase I/II [48] | |
| Number of patients | 163 | 140 | 138 | 87 | 70 |
| Objective response rate (%) | 56 | 38 | 50 | 48 | 71 |
| Disease control rate (%) | 74.2 | 77.1 | 78.7 | 80 | 87 |
| Median PFS (months) | 6.9 | 5.7 | 8.9 | 8.1 | 13.4 |
| Median duration of response (months) | 8.3 | 9.7 | 11.2 | 13.5 | — |
Most patients who develop disease progression on crizotinib will respond to treatment with a next-generation ALK inhibitor, even in the absence of a detectable resistance mutation [49]. These structurally diverse next-generation ALK inhibitors are characterised by variable ability to overcome different secondary resistance mutations. Ceritinib was able to overcome secondary mutations in patients enrolled in the phase I ASCEND trial but additional testing using preclinical in vitro and in vivo models showed that ceritinib has limited ability to overcome the G1202R and F1174C mutations [50]. Ceritinib, however, is able to overcome distinct resistance mutations that emerge after treatment with alectinib, specifically those involving the I1171 residue [51, 52]. The second-generation ALK inhibitors, as a group, are characterised by an inability to overcome the ALK G1202R mutation [53, 54]. However, the newest next-generation inhibitor lorlatinib (PF-06463922) has shown superior potency compared with other ALK inhibitors against known resistance mutations in preclinical models and is the only known ALK inhibitor to overcome G1202R [55]. Interestingly, a recent case study demonstrated that crizotinib is active against an acquired compound mutation, C1156Y + L1198F, that leads to resistance to next-generation drugs, including lorlatinib [56]. With expansion of the cadre of available ALK inhibitors, it is anticipated that informed sequential selection of ALK inhibitors based on resistance mutation profile will emerge as a standard practice.
The higher potency and improved blood–brain barrier penetration of these newer agents has translated into improved CNS control. In a pooled analysis of the phase II studies of alectinib in crizotinib-resistant ALK+ NSCLC (NP28761 and NP28673), the intracranial ORR and DCR in patients with brain metastases was 64% and 90% in those with measurable CNS disease [57]. In these studies, the median duration of CNS response was ∼10–11 months [46, 47]. In a retrospective analysis of 15 assessable patients with measurable CNS disease treated with brigatinib, the intracranial ORR and DCR was reported at 53% and 86%, respectively [48]. Lorlatinib has also been shown to have CNS activity in preclinical and early clinical studies [55, 58]. The initial impressive CNS response seen with these agents may challenge the role of crizotinib as an initial therapeutic strategy in the 26% of patients who present with brain metastasis.
In patients with resistance due to activation of bypass tracks, combination therapy with ALK inhibitors and agents targeting the activated receptor appears to be effective in preclinical studies as described above. As the bypass tracks converge upon activation of downstream effectors of the MAPK pathway, it is not surprising that the combination of ALK inhibitors and inhibitors of the MAPK pathway shows promising antitumor activity in preclinical studies [37]. Interestingly, some of the more promising ALK inhibitors, alectinib and lorlatinib, derive their potency from greater specificity for ALK and therefore have limited effect on other kinases. It is plausible that with treatment with multiple next-generation ALK inhibitors, escape from growth inhibition via bypass tracks or EMT may become a more prominent resistance mechanism than resistance mutations. Indeed, there have already been reports of MET activation leading to resistance to alectinib [59, 60].
Treatment with other non-TKI agents has also shown promise in patients with ALK+ NSCLC. Inhibition of HSP90, a molecular chaperone that is central to regulation of folding, stability, and function of ‘client’ proteins including ALK and EGFR, has been adopted as a therapeutic strategy to promote proteosomal degradation and disruption of signals that promote tumor growth and survival. HSP90 inhibitors have been shown to potently suppress growth in preclinical models of acquired resistance in the setting of ALK amplification or resistance mutations [31, 61, 62]. The experience with HSP90 inhibitor monotherapy has been mixed. A phase II trial of ganetespib monotherapy in patients with NSCLC noted clinical activity in a small group of crizotinib-naive patients with ALK+ NSCLC [63]. However, in a more recent phase II trial of AUY922 monotherapy in ALK+ patients who had progressed on prior ALK TKIs, no objective responses were seen in six assessable patients. [64]. Although the small number of ALK+ NSCLC patients enrolled on these trials limits drawing firm conclusions, the results suggest that HSP90 inhibition may not represent a viable therapeutic strategy in the TKI-resistant setting.
Recent data from Ota et al. demonstrating upregulation of PD-L1 expression in ALK+ cell lines and NSCLC specimens suggest that checkpoint inhibitors could be a promising strategy in this patient population [65]. However, there is some evidence that despite variable PD-L1 expression in ALK+ lung cancer, treatment with an ALK TKI does not lead to recruitment of CD8+ tumor infiltrating lymphocytes. [66]. Moreover, checkpoint inhibition has been shown to be less effective in never-smokers, a population enriched for ALK rearrangements [67]. This suggests that there may be some limitation with simply combining ALK TKIs with checkpoint inhibitors, and trial designs investigating optimal sequencing of these agents may be necessary. Several trials combining ALK inhibitors and checkpoint inhibitors are underway.
conclusion
The first demonstration of crizotinib's clinical activity in ALK+ lung cancer was a pivotal discovery for a population of patients with an otherwise dismal prognosis. Since the introduction of crizotinib into clinical practice, it has become apparent that the vast majority of patients will invariably relapse due to resistance. As discussed above, there are multiple and diverse resistance mechanisms that underlie relapses on crizotinib. Biopsies at progression have contributed immensely to our understanding of the dynamics of treatment resistance. Relying solely on molecular characterization of tumors derived from diagnostic biopsies provides an insufficient blueprint from which to predict a patient's clinical course. Considering the dynamic evolution of tumors throughout treatment, repeat surveillance of a tumor's molecular signature, particularly in the setting of treatment resistance, is necessary.
Next-generation ALK TKIs have made significant strides toward overcoming some of the limitations of crizotinib, particularly potency and CNS penetration. With these improvements, a shift in the distribution of mechanisms of resistance is anticipated. For example, biopsies at progression on these next-generation agents demonstrate that the spectrum of resistance mutations is narrower, with enrichment for certain mutations like ALK G1202R [50]. As only lorlatinib has demonstrated potency against all clinically identified resistance mutations, appropriate sequencing of TKIs will need to be context specific and based on detected mutations. Sequencing strategies must also be flexible rather than linear, as in some cases revisiting previous agents may be the optimal approach [56]. Sequential therapy already has shown promising results, with a multi-institutional series reporting a combined PFS of 17.4 months and impressive median overall survival of 49.4 months with sequential crizotinib and ceritinib [68]. With adoption of next-generation inhibitors, it is expected that resistance will be increasingly driven by ALK-independent processes, including activation of bypass pathways and EMT. It is unclear whether sequential ALK TKIs will be efficacious in patients who develop resistance to these agents who do not have detectable resistance mutations. As such, it is imperative that combination strategies be developed in parallel. Intercalated or intermittent administration of agents may need to be explored when designing combination studies in order to maximize efficacy and minimise toxicity.
funding
This work was supported by the National Cancer Institute (5R01CA164273 to A.T.S.) and the National Foundation for Cancer Research.
disclosure
A.S. has served as a consultant for and received honoraria from Pfizer, Novartis, Genentech, Roche, Ariad, Ignyta, Blueprint Medicines, Taiho, Daiichi Sankyo, and EMD Serono.
acknowledgements
Medical writing support was provided by ACUMED® (Tytherington, UK), an Ashfield company, part of UDG Healthcare plc, and was funded by Pfizer.
references
- 1.Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561–566. [DOI] [PubMed] [Google Scholar]
- 4.Rikova K, Guo A, Zeng Q et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007; 13: 1190–1203. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi K, Choi YL, Soda M et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008; 14: 6618–6624. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Yeap BY, Mino-Kenudson M et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009; 27: 4247–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gainor JF, Varghese AM, Ou SH et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013; 19: 4273–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou SH, Bartlett CH, Mino-Kenudson N, Cui J, Iafrate AJ.. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 2012; 17: 1351–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak EL, Bang YJ, Camidge DR et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010; 363: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camidge DR, Bang YJ, Kwak EL et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012; 13: 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik SM, Maher VE, Bijwaard KE et al. U.S. Food and Drug Administration approval: crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res 2014; 20: 2029–2034. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385–2394. [DOI] [PubMed] [Google Scholar]
- 13.Solomon BJ, Mok T, Kim DW et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371: 2167–2177. [DOI] [PubMed] [Google Scholar]
- 14.Doebele RC, Lu X, Sumey C et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012; 118: 4502–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangachari D, Yamaguchi N, VanderLaan PA et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015; 88: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon BJ, Cappuzzo F, Felip E et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol 2016. In press. [DOI] [PubMed] [Google Scholar]
- 17.Chun SG, Choe KS, Iyengar P, Yordi JS, Timmerman RD.. Isolated central nervous system progression on Crizotinib: an Achilles heel of non-small cell lung cancer with EML4-ALK translocation? Cancer Biol Ther 2012; 13: 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT.. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol 2013; 8: 1570–1573. [DOI] [PubMed] [Google Scholar]
- 19.Costa DB, Shaw AT, Ou SH et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 2015; 33: 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa DB, Kobayashi S, Pandya SS et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011; 29: e443–e445. [DOI] [PubMed] [Google Scholar]
- 21.Tang SC, Nguyen LN, Sparidans RW et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer 2014; 134: 1484–1494. [DOI] [PubMed] [Google Scholar]
- 22.Takeda M, Okamoto I, Nakagawa K. Clinical impact of continued crizotinib administration after isolated central nervous system progression in patients with lung cancer positive for ALK rearrangement. J Thorac Oncol 2013; 8: 654–657. [DOI] [PubMed] [Google Scholar]
- 23.Weickhardt AJ, Scheier B, Burke JM et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012; 7: 1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gan GN, Weickhardt AJ, Scheier B et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys 2014; 88: 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi YL, Soda M, Yamashita Y et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010; 363: 1734–1739. [DOI] [PubMed] [Google Scholar]
- 26.Doebele RC, Pilling AB, Aisner DL et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012; 18: 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyokawa G, Hirai F, Inamasu E et al. Secondary mutations at I1171 in the ALK gene confer resistance to both Crizotinib and Alectinib. J Thorac Oncol 2014; 9: e86–e87. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Koivunen J, Ogino A et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res 2011; 71: 6051–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama R, Shaw AT, Khan TM et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med 2012; 4: 120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bresler SC, Weiser DA, Huwe PJ et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell 2014; 26: 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katayama R, Khan TM, Benes C et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci USA 2011; 108: 7535–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanizaki J, Okamoto I, Okabe T et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clin Cancer Res 2012; 18: 6219–6226. [DOI] [PubMed] [Google Scholar]
- 33.Kimura M, Endo M, Inoue T et al. Analysis of ERBB ligand-induced resistance mechanism to crizotinib by primary culture of lung adenocarcinoma with EML4-ALK fusion gene. J Thorac Oncol 2015; 10: 527–530. [DOI] [PubMed] [Google Scholar]
- 34.Lovly CM, McDonald NT, Chen H et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med 2014; 20: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson FH, Johannessen CM, Piccioni F et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015; 27: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crystal AS, Shaw AT, Sequist LV et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014; 346: 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hrustanovic G, Olivas V, Pazarentzos E et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med 2015; 21: 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsilje TH, Pei W, Chen B et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013; 56: 5675–5690. [DOI] [PubMed] [Google Scholar]
- 39.Yamada T, Takeuchi S, Nakade J et al. Paracrine receptor activation by microenvironment triggers bypass survival signals and ALK inhibitor resistance in EML4-ALK lung cancer cells. Clin Cancer Res 2012; 18: 3592–3602. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Jang SJ, Chung DH et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One 2013; 8: e76999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi Y, Sakao Y, Ito S et al. Transformation to sarcomatoid carcinoma in ALK-rearranged adenocarcinoma, which developed acquired resistance to crizotinib and received subsequent chemotherapies. J Thorac Oncol 2013; 8: e75–e78. [DOI] [PubMed] [Google Scholar]
- 42.Kim HR, Kim WS, Choi YJ et al. Epithelial-mesenchymal transition leads to crizotinib resistance in H2228 lung cancer cells with EML4-ALK translocation. Mol Oncol 2013; 7: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kogita A, Togashi Y, Hayashi H et al. Hypoxia induces resistance to ALK inhibitors in the H3122 non-small cell lung cancer cell line with an ALK rearrangement via epithelial-mesenchymal transition. Int J Oncol 2014; 45: 1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim DW, Mehra R, Tan DS et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016; 17: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crino L, Ahn MJ, De Marinis F et al. Ceritinib in patients with ALK-rearranged non-small cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol 2016. in press. [DOI] [PubMed] [Google Scholar]
- 46.Ou SH, Ahn JS, De Petris L et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol 2015; 34: 661–668. [DOI] [PubMed] [Google Scholar]
- 47.Shaw AT, Gandhi L, Gadgeel S et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016; 17: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gettinger SN, Bazhenova L, Salgia R et al. Brigatinib (AP26113) Efficacy and safety in ALK+ NSCLC: phase 1/2 trial results. J Thorac Oncol 2015; 10(Suppl. 2): S66–S890.26710300 [Google Scholar]
- 49.Shaw AT, Kim DW, Mehra R et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014; 370: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friboulet L, Li N, Katayama R et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014; 4: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katayama R, Friboulet L, Koike S et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res 2014; 20: 5686–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ou SH, Greenbowe J, Khan ZU et al. I1171 missense mutation (particularly I1171N) is a common resistance mutation in ALK-positive NSCLC patients who have progressive disease while on alectinib and is sensitive to ceritinib. Lung Cancer 2015; 88: 231–234. [DOI] [PubMed] [Google Scholar]
- 53.Fontana D, Ceccon M, Gambacorti-Passerini C, Mologni L.. Activity of second-generation ALK inhibitors against crizotinib-resistant mutants in an NPM-ALK model compared to EML4-ALK. Cancer Med 2015; 4: 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ou SH, Azada M, Hsiang DJ et al. Next-generation sequencing reveals a novel NSCLC ALK F1174V mutation and confirms ALK G1202R mutation confers high-level resistance to alectinib (CH5424802/RO5424802) in ALK-rearranged NSCLC patients who progressed on crizotinib. J Thorac Oncol 2014; 9: 549–553. [DOI] [PubMed] [Google Scholar]
- 55.Zou HY, Friboulet L, Kodack DP et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell 2015; 28: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw AT, Friboulet L, Leshchiner I et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med 2016; 374: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gadgeel S, Shaw A, Govindan R et al. Pooled analysis of CNS responses to alectinib in two studies of pre-treated ALK+ NSCLC. J Thorac Oncol 2015; 10(Suppl 2): S66–S890.26710300 [Google Scholar]
- 58.Johnson TW, Richardson PF, Bailey S et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17,tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacylotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 2014; 57: 4720–4744. [DOI] [PubMed] [Google Scholar]
- 59.Kogita A, Togashi Y, Hayashi H et al. Activated MET acts as a salvage signal after treatment with alectinib, a selective ALK inhibitor, in ALK-positive non-small cell lung cancer. Int J Oncol 2015; 46: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 60.Tanimoto A, Yamada T, Nanjo S et al. Receptor ligand-triggered resistance to alectinib and its circumvention by Hsp90 inhibition in EML4-ALK lung cancer cells. Oncotarget 2014; 5: 4920–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Z, Akbay E, Mikse O et al. Co-clinical trials demonstrate superiority of crizotinib to chemotherapy in ALK-rearranged non-small cell lung cancer and predict strategies to overcome resistance. Clin Cancer Res 2014; 20: 1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sang J, Acquaviva J, Friedland JC et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov 2013; 3: 430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Socinski MA, Goldman J, El-Hariry I et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res 2013; 19: 3068–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gainor JF, Marcoux JP, Rabin M et al. A phase II trial of AUY922, a heat shock protein 90 (HSP90) inhibitor, in ALK-positive lung cancer patients previously treated with ALK inhibitors. J Thorac Oncol 2015; 10(Suppl 2): S66–S890.26710300 [Google Scholar]
- 65.Ota K, Azuma K, Kawahara A et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res 2015; 21: 4014–4021. [DOI] [PubMed] [Google Scholar]
- 66.Gainor JF, Shaw AT, Sequist LV et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer (NSCLC): a retrospective analysis. Clin Cancer Res 2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 68.Gainor JF, Tan DS, De Pas T et al. Progression-free and overall survival in ALK-positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin Cancer Res 2015; 21: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]