Abstract
Objective
The objective of the current study was to determine whether functional connectivity of the amygdala is altered in preschool-aged children with autism spectrum disorder (ASD) and to assess the clinical relevance of observed alterations in amygdala connectivity.
Method
We conducted a resting-state functional connectivity magnetic resonance imaging (MRI) study of the amygdala (and a parallel study of primary visual cortex) in 72 male children (mean age: 3.5 years; n=43 with ASD; n=29 age-matched controls).
Results
The ASD group showed significantly weaker connectivity between amygdala and several brain regions involved in social communication and repetitive behaviors, including bilateral medial prefrontal cortex, temporal lobes, and striatum (p < .05, corrected). Weaker connectivity between the amygdala and frontal and temporal lobes was significantly correlated with increased autism severity in the ASD group (p < .05). In a parallel analysis examining the functional connectivity of primary visual cortex, the ASD group showed significantly weaker connectivity between visual cortex and sensorimotor regions (p<.05, corrected). Weaker connectivity between visual cortex and sensorimotor regions was not correlated with core autism symptoms, but instead was correlated with increased sensory hypersensitivity in the visual/auditory domain (p<.05).
Conclusion
These findings indicate that preschool-aged children with ASD have disrupted functional connectivity between the amygdala and regions of the brain important for social communication and language, which may be clinically relevant since weaker connectivity was associated with increased autism severity. Moreover, whereas amygdala connectivity was associated with behavioral domains that are diagnostic of ASD, altered connectivity of primary visual cortex was related to sensory hypersensitivity.
Keywords: Autism, fMRI, Neuroimaging, Amygdala, Development
INTRODUCTION
The neuropathology of autism spectrum disorder (ASD) likely involves alterations in specific brain regions as well as connectivity patterns between multiple networks of brain regions. The connectivity theory of ASD has been of great interest in recent years,1 and increasing evidence suggests that abnormal white matter and connectivity patterns are hallmark features of the neuropathology of ASD.2-4 Resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) detects spontaneous low-frequency neural activity changes that are synchronized between brain regions belonging to a functional network.5,6 Recent studies provide evidence for altered resting-state functional connectivity in various brain networks in older children and adults with ASD,7,8 but only one rs-fcMRI study has examined very young children.2
The amygdala has been widely implicated in the neuropathology of ASD. Histological studies of postmortem brain tissue from patients with ASD have revealed neuronal abnormalities in the amygdala.9,10 Volumetric studies in preschool-aged children with ASD have consistently shown that the amygdala is abnormally enlarged,11-14 but little is known about amygdala function in very young children with ASD. Several recent studies have reported abnormal functional connectivity of the amygdala in older individuals with ASD,15-19 but there have been no studies of the functional connectivity of the amygdala in very young children, close in time to their clinical diagnosis of ASD.
We conducted an rs-fcMRI study of the amygdala in 72 preschool-aged children and evaluated whether amygdala connectivity was related to behavioral symptoms of ASD. As a comparison analysis to evaluate the specificity of functional connectivity alterations within multiple neural systems, we also assessed resting-state functional connectivity of the primary visual cortex. To our knowledge, this is the first study to examine the functional connectivity of the amygdala in preschool-aged children with ASD (3.5 years of age). This is an important period of rapid and dynamic brain growth, yet it precedes the age when behavioral treatments or medications may influence the consolidation and adaptation of neural networks, and thus the neural connectivity observed at this young age might more closely reflect the emerging diagnostic features of ASD.
METHOD
Participants
Participants were recruited through the University of California (UC) Davis MIND Institute, as part of the Autism Phenome Project. The sample for this study included 72 male children (ASD n=43; typically developing controls [TD] n=29; mean age 3.5 years). All participants were screened by a board-certified developmental behavioral pediatrician (author K.A.) and were free of seizures and the use of any psychotropic or behavioral medications. Children with ASD were screened and excluded for Fragile X syndrome. All children, both TD controls and children with ASD, were native English speakers, ambulatory, had no physical contraindications to MRI, vision or hearing problems, or known genetic disorders and/or other neurological conditions. For TD controls, inclusion criteria were developmental scores within two standard deviations on all scales of the Mullen Scales of Early Learning.20 In addition, TD children were screened and excluded for autism using the Social Communication Questionnaire (scores > 11).21 This study was approved by the UC Davis Institutional Review Board, and informed consent was obtained from the parent or guardian of each participant prior to imaging. Volumetric amygdala data from a subset of these participants has been reported on previously12,22.
Diagnostic assessments for children with ASD included the Autism Diagnostic Interview–Revised23 and Autism Diagnostic Observation Schedule–Generic (ADOS-G),24,25 which was used to calculate the ADOS severity score, a standardized metric of quantifying ASD symptom severity that is relatively independent of age and verbal ability.26 Sensory processing was assessed using the Short Sensory Profile,27 a parent checklist measuring the child's sensory sensitivity in several domains (including visual/auditory sensitivity), with lower scores indicating greater hypersensitivity to sensory stimuli. Measures of cognitive ability for all participants (i.e., overall cognitive ability, verbal ability, and nonverbal ability) were derived using standard scores from the Mullen Scales of Early Learning20 and Differential Ability Scales.28
MRI Data Acquisition
Children were scanned during natural, nocturnal sleep29 at the UC Davis Imaging Research Center on a 3T Siemens TIM Trio MRI system using an eight-channel head coil. A high-resolution structural scan was acquired for anatomical parcellation and overlay of statistical maps (T1-weighted 3D magnetization-prepared rapid acquisition with gradient echo [MPRAGE]; 1 mm isotropic voxels; TR 2170 ms; TE 4.86 ms; FOV 256 mm; 192 sagittal slices). For each participant, a resting-state echo planar imaging blood oxygen level dependent (EPI-BOLD) sequence was acquired containing 300 whole-brain T2*-weighted volumes (37 interleaved axial slices per volume; 4 mm slice thickness; in-plane resolution 4 mm2; TR 2000 ms; TE 27 ms; flip angle 87°; FOV 256 mm).
The duration of sleep from the onset of sleep to the beginning of the resting-state BOLD scan was recorded for each participant. This measurement served as a proxy for determining sleep stage as an alternative to collecting polysomnography data during fMRI scanning, due to concerns that simultaneous acquisition of such data would increase the likelihood of children waking up. All scans were acquired within the first hour and a half after sleep onset. Several studies have demonstrated that young children at similar ages were reliably found to be in non-rapid eye movement sleep stage 3 (NREM3) within this time frame.30-32
MRI Analyses
Left and right amygdala were manually traced on T1-weighted images based on a study-specific anatomical protocol developed by our laboratory12 using Analyze software.33 Manual tracings from a subset of participants were derived from a previous study on amygdala volume12,22. The resulting regions of interest (ROIs) were used as seed regions for the functional connectivity analysis. For the comparison analysis of primary visual cortex, the seed ROI of primary visual cortex was anatomically parcellated using the FreeSurfer software package.34 All functional connectivity analyses were performed using Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/afni/) and FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl/). Of the 300 time points acquired for each participant, the first ten were discarded due to signal instability. EPI-BOLD resting-state scans were then pre-processed: time shifted (AFNI: 3dTshift), motion corrected (AFNI: 3dvolreg), spatially smoothed with an effective smoothness of Gaussian 6mm full width at half maximum (FWHM; AFNI: 3dmerge), band-pass filtered at 0.008 < f < 0.08 Hz5,35 (AFNI: 3dBandpass), and co-registered to the structural image (FSL: flirt). The structural ROIs were resampled (AFNI: 3dresample) to the functional time series (4mm isotropic voxels), smoothed with a 3mm kernel (AFNI: 3dmerge), and eroded to reduce partial voluming (AFNI: 3dcalc). The resulting ROI masks were visually inspected (FSLView) for anatomical accuracy in every participant to ensure that that no partial voluming had occurred (i.e., the voxels in the ROI mask were contained entirely within the anatomically defined ROI). Motion scrubbing/censoring was implemented to remove single frames that exceeded framewise displacement (FD) > .25mm, and not frames preceding or following, based on recommendations by Power et al. when using global signal regression.36,37
Several regressors were included in the individual model to remove motion and nuisance artifacts: 6 motion parameters (from AFNI: 3dvolreg), white matter and cerebrospinal fluid (CSF; from FSL: FAST), and temporal derivatives of each (computed using AFNI: 1d_tool.py). Given the current debate on the use of global signal regressor (GSR),36,38-40 we conducted our analyses both with GSR (extracted using AFNI: 3dmaskave) and without GSR and compared the results, which were strikingly similar. We therefore only present results with GSR in the main text (for analyses without GSR see Figures S1 and S2, available online). The nuisance regressors and their derivatives (6 motion parameters, white matter, CSF, GSR) were extracted from the time series prior to band-pass filtering; then the time series and nuisance signals were all bandpass filtered and entered simultaneously into the individual model.
Mean time series of the left and right amygdala ROIs were extracted separately from each individual (AFNI: 3dmaskave) and correlated with all other voxels in the brain (AFNI: 3dDeconvolve). Single-subject-level connectivity maps for each amygdala seed were entered into one-sample t-tests for within-group analyses and two-sample independent t-tests for group comparison (AFNI: 3dttest++) in standard Montreal Neurological Institute (MNI) space. The uncorrected voxel-wise p-value thresholds used prior to family-wise error (FWE) cluster-size correction was p=.00001 for within-group analyses and p=.01 for between-group analyses. To adjust for multiple comparisons, cluster size significance—2 voxels for within-group and 32 voxels for between-group—was determined by Monte Carlo alpha simulations (AFNI: 3dClustSim) for a corrected significance threshold of p < .05 (AFNI: 3dmerge).
RESULTS
Participant demographic, diagnostic, and behavioral measures are presented in Table 1. There were no group differences in age. As expected, overall cognitive ability, verbal ability, and nonverbal ability were significantly higher in TD controls (p < .001).
Table 1.
Group Comparison of Participant Characteristics, Motion Parameters, Framewise Displacement (FD), and Sleep Duration
| Mean (SD) | |||
|---|---|---|---|
| ASD | TD | p Value | |
| N | 43 | 29 | |
| Age (years) | 3.5 (.79) | 3.6 (.86) | .46 |
| Overall cognitive ability | 70.6 (18.4) | 103.2 (12.0) | <.001 |
| Verbal ability | 69.1 (19.6) | 102.3 (9.7) | <.001 |
| Nonverbal ability | 72.1 (19.2) | 104.1 (16.6) | <.001 |
| ADOS Severity | 8.0 (1.36) | n/a | n/a |
| Short sensory profile visual/auditory sensitivity | 17.72 (5.09) | 19.21 (3.47) | .36 |
| Motion Parameters (in mm) | |||
| Overall motion (RMSD) | 0.05 (.02) | 0.052 (.02) | .68 |
| dL | 0.009 (.00) | 0.01 (.00) | .74 |
| dP | 0.027 (.01) | 0.027 (.01) | .84 |
| dS | 0.041 (.02) | 0.041 (.02) | .95 |
| Pitch | 0.020 (.02) | 0.019 (.01) | .92 |
| Roll | 0.009 (.01) | 0.009 (.00) | .73 |
| Yaw | 0.012 (.01) | 0.012 (.01) | .87 |
| FD | 0.054 (.03) | 0.049 (.03) | .46 |
| # Time points remaining after motion scrubbing frames w/ FD > .25mm (out of 290) | 287.9 (4.7) | 285.9 (8.2) | .20 |
| Duration of sleep prior to scan (mins.) | 64.6 (19.7) | 67.7 (18.3) | .50 |
Note: Standard scores for overall cognitive, verbal, nonverbal ability have Mean=100, SD=15. ADOS = Autism Diagnostic Observation Schedule; ASD = Autism spectrum disorder; RMSD = root-mean-square displacement; TD = typically developing controls.
We employed several important control procedures to rule out artifacts that may contribute to any observed group differences in connectivity patterns. Since each participant was scanned during natural nocturnal sleep, virtually no motion artifact was found in either group (i.e., very few time points needed to be scrubbed/removed due to FD greater than 0.25mm),36 and the groups did not differ in the number of time points scrubbed (Table 1). Even so, stringent motion correction procedures were implemented, and the groups were well matched (p > .70) on a variety of motion parameters (see Table 1), including all 6 motion directions and overall motion over the entire scan.36,41 Thus, group differences in connectivity could not be attributed to motion artifact. Given the current debate on the use of global signal regressor,36,38-40 we conducted our analyses both with GSR (Figures 1 and 2) and without GSR (see Figures S1 and S2, available online), and the findings were strikingly similar. This lack of difference in the use of GSR suggests that the data acquired in this study is of such high quality (i.e., no motion) that the white matter and CSF regressors accounted for all remaining variance attributed to noise (i.e., no significant variance remained for the GSR to regress). Notably, the groups also did not differ in the duration of sleep prior to the resting-state scan (p = .50; Table 1). This suggests that the stage of sleep did not differ between the two groups and did not contribute to group differences in functional connectivity.
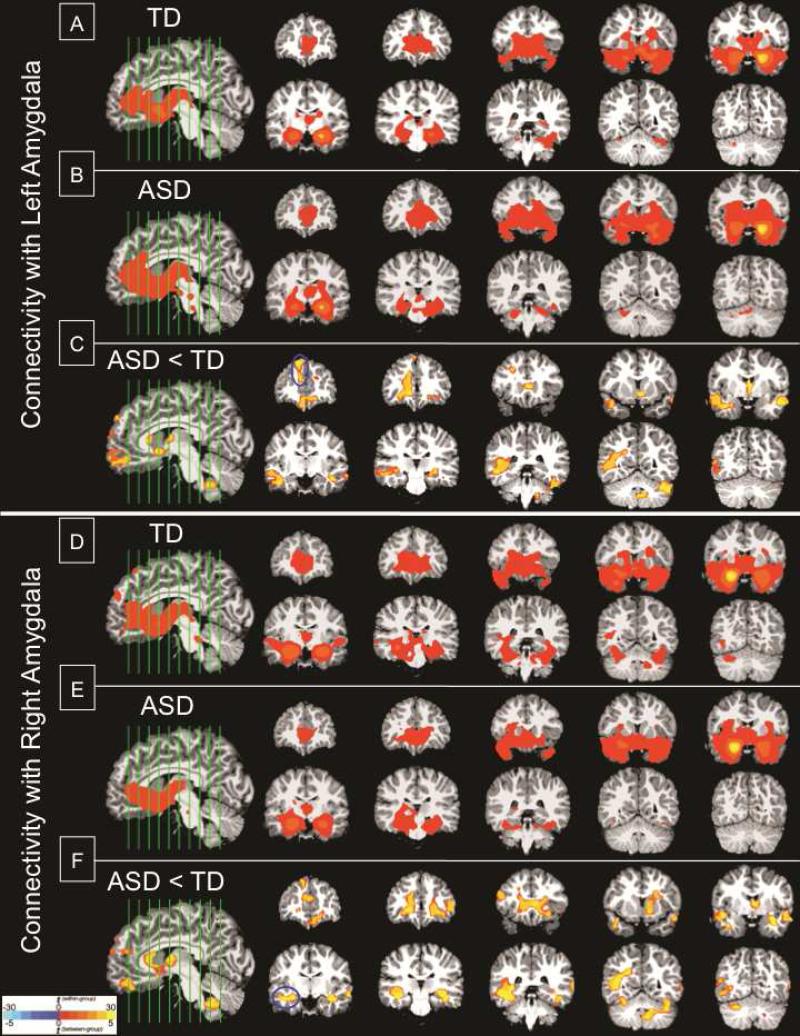
Figure 1.
Functional connectivity maps with the amygdala. Top panel (A-C): Significant clusters of connectivity with left amygdala seed. (A) Within-group typical development (TD); (B) Within-group autism spectrum disorder (ASD); (C) Between-group difference ASD<TD. Bottom panel (D-F): Significant clusters of connectivity with right amygdala seed. (D) Within-group TD; (E) Within-group ASD; (F) Between-group difference ASD<TD. Note: All significant clusters are p<.05, corrected; clusters are overlaid on representative 3.5-year-old structural brain image; and are shown in radiological convention (Left=Right). Blue circles mark the peak location of the clusters used in behavioral correlations.
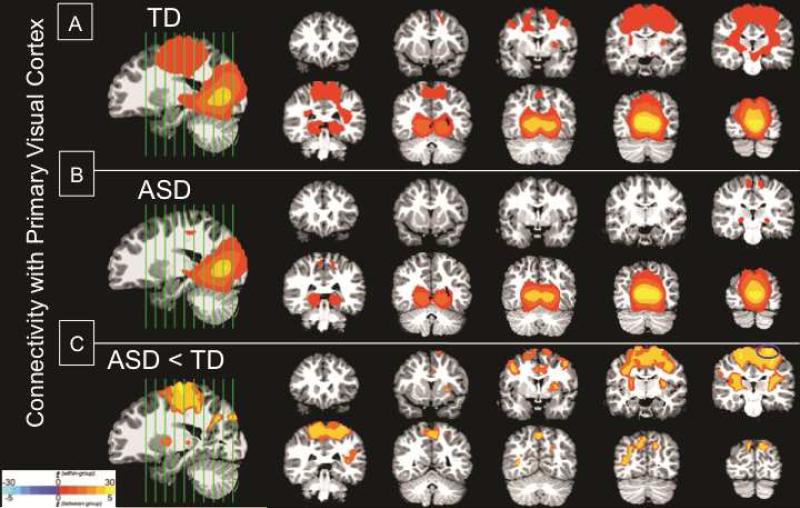
Figure 2.
Functional connectivity maps with the primary visual cortex: significant clusters of connectivity with visual cortex seed. (A) Within-group typical development (TD); (B) Within-group autism spectrum disorder (ASD); (C) Between-group difference ASD<TD. Note: All significant clusters are p<.05, corrected; clusters are overlaid on representative 3.5-year-old structural brain image; and are shown in radiological convention (Left=Right). Blue circles mark the peak location of the cluster used in behavioral correlations.
Functional Connectivity With Amygdala
The functional connectivity maps for the left and right amygdala are shown in Figure 1 (see Tables S1 and S2 for detailed descriptions of the clusters, available online). Both ASD and TD groups exhibited connectivity between the amygdala and the striatum, bilateral medial temporal lobes, posterior ventral temporal lobes, and medial prefrontal cortex (p < .05, corrected) (Figure 1A-B, Figure 1D-E). However, direct between-group comparison revealed that the ASD group had significantly weaker connectivity between the amygdala and several brain regions involved in social communication and repetitive behaviors, including the medial prefrontal cortex, bilateral temporal lobe, striatum, thalamus, cingulate cortex, and cerebellum (p < .05, corrected) (Figure 1C and Figure 1F).
Comparison Analysis: Functional Connectivity With Primary Visual Cortex
In order to evaluate whether the patterns of connectivity differences were specific to the amygdala, rather than reflecting global connectivity differences across multiple brain systems, we performed a comparison study using primary visual cortex (V1) as the seed ROI for a functional connectivity analysis. Results for the functional connectivity analysis with V1 are shown in Figure 2 (see Table S3 for detailed descriptions of the clusters, available online). While the ASD group showed limited V1 connectivity primarily within the occipital lobe (Figure 2B), the TD group showed additional connectivity between V1 and regions outside the occipital lobe, including the striatum, primary motor, and primary somatosensory cortices (Figure 2A). Direct between-group comparisons confirmed that the ASD group showed significantly weaker connectivity between V1 and sensorimotor regions, including pre- and post-central gyrus, striatum, and thalamus (Figure 2C).
Behavioral Correlations With Functional Connectivity
We tested whether connectivity between the amygdala and clusters showing significant group differences was associated with autism severity, as measured by the ADOS severity score of each participant with ASD. For the clusters with the greatest between-group difference (i.e., highest t-value) in left and right amygdala connectivity, weaker functional connectivity was associated with increased ADOS severity scores in the ASD group. For the left amygdala, this cluster was centered in the right superior medial frontal gyrus (Pearson r = −0.32; p= .04), and for the right amygdala, the cluster was centered in the right middle temporal gyrus (r = −0.33; p= .04) (See Tables S1 and S2 for additional anatomical details for the clusters, available online). These associations were significant (p < .05) even when controlling for overall cognitive ability, which suggests that weaker connectivity between the amygdala and frontal and temporal lobes had a specific relation to the severity of autism symptoms, above and beyond levels of overall cognitive functioning.
We also tested whether connectivity between V1 and the cluster with greatest between-group difference (i.e., sensorimotor regions; see Table S3, available online) was associated with sensory functioning (as measured by the Short Sensory Profile in both ASD and TD participants), while co-varying for overall cognitive ability and differences between groups. Across both groups, weaker connectivity between primary visual cortex and sensorimotor regions was significantly correlated with greater hypersensitivity (lower scores) in the visual/auditory domain (r = 0.39; p= .04; co-varying for group and cognitive ability). We also conducted this analysis within the ASD group (in parallel to the approach with the ADOS analysis above) and found this significant association held for the ASD group alone (r = 0.59; p= .02; co-varying for cognitive ability).
Conversely, V1 connectivity with sensorimotor regions was not related to autism severity in the ASD group (r = −0.07; p = .65; co-varying for overall cognitive ability), and amygdala connectivity with frontal lobe (r = 0.01; p = .72) and temporal lobe clusters (r = 0.03; p =.75) was not related to visual-auditory hypersensitivity across both groups (co-varying for group and cognitive ability) or within the ASD group alone.
Post Hoc Analyses of Amygdala Volume
Given the evidence of volumetric differences in the amygdala in young children with ASD, we tested whether amygdala volume was related to the observed differences in functional connectivity. Amygdala volumes were derived from the T1-weighted manual tracings. In the present sample, there were marginally significant group differences in amygdala volume only in the right hemisphere (ASD M[SD] = 1.58cm3 [0.17]; TD M[SD]=1.49cm3 [.17]; p=.09), but not in the left (ASD M[SD]=1.45cm3 [0.18]; TD M[SD]=1.37cm3 [0.14]; p=.38), after controlling for overall brain volume and age. Amygdala volume was not correlated with amygdala connectivity in any clusters from the between-group results (all Pearson correlations p > .20) in either diagnostic group.
DISCUSSION
We found that preschool-aged children with ASD have altered functional connectivity of the amygdala compared to age-matched children with TD, particularly between the amygdala and areas important for social communication and repetitive behaviors, including the prefrontal cortex, temporal lobes, and striatum. While our study focused on the amygdala because of its known pathology in young children with ASD, we also sought to determine whether connectivity differences were specific to the amygdala. We therefore performed a comparison functional connectivity analysis using primary visual cortex (V1) as the seed ROI to determine whether connectivity differences were specific to certain neural systems. Similar to the amygdala findings, the ASD group showed overall weaker connectivity of V1, but the regional patterns of reduced connectivity were entirely different, occurring exclusively in sensorimotor regions, rather than the frontal and temporal lobes. While our findings add to the growing literature showing altered functional connectivity of visual cortex in ASD42-44, it is interesting to note that the neural circuitry involved in altered V1 connectivity is completely different than what we observed in the amygdala. This suggests that distinct behavioral associations may arise from the different patterns of altered connectivity.
Indeed, in addition to contrasting patterns of abnormal functional connectivity in ASD for amygdala and V1, we also found that connectivity differences for these two regions were accompanied by specific and distinct behavioral associations. While weaker connectivity between the amygdala and frontal lobe was associated with increased severity of the core diagnostic features of ASD, abnormal connectivity between visual cortex and sensorimotor regions was associated with sensory hypersensitivity. These findings highlight the importance of evaluating connectivity abnormalities in multiple neural systems, in order to dissociate their relationships with different functional domains of behavior in early childhood. This may be of particular importance in ASD, in which the interpretation of functional connectivity differences seen in the literature may not simply be stronger or weaker connectivity across the brain, but rather disrupted connectivity in specific functional networks.
To our knowledge, this is the first study to evaluate the functional connectivity of the amygdala in preschool-age children of any population (either ASD or TD). Compared to typically developing controls, children with ASD had significantly weaker connectivity with several areas, including the medial prefrontal cortex (mPFC) and striatum. Several resting-state fcMRI studies have demonstrated aberrant striatal connectivity in school-age children with ASD,45,46 including specifically between the striatum and amygdala46, and fMRI studies have shown abnormal function of the striatum.47,48 Abnormal connectivity and function of the striatum have been linked to altered social reward processing in ASD (for review, see 49). The mPFC regulates emotional responses triggered by the amygdala by providing contextual and experiential input to the amygdala, which in turn uses this information to interpret social stimuli and prepare behavioral and emotional responses.50,51 Abnormal function of the amygdala mPFC in older children with ASD has been related to an exaggerated response of the amygdala to faces19 and has been linked to alterations in social reward and social motivation.52 In the present study of children at an early age close in time to diagnosis, we found that weaker connectivity between the amygdala and mPFC was associated with increased autism severity. Thus, disrupted connectivity between the amygdala and mPFC during early development may reflect poorly coordinated co-activation between regions that underlie core features of ASD, particularly social-communicative impairments that may worsen over development.
One prominent framework of developmental changes in connectivity in ASD suggests that “young” individuals with ASD have over-connectivity that transitions to under-connectivity later in development.53-55 While our findings of under-connectivity in preschool-aged children may seem inconsistent with this theory, it is worth noting that the existing framework is based on data in which school-age children (7-12 years of age) have been considered “young” but do not extend down to preschool-aged children. Our findings are consistent with the single other rsfcMRI study of preschool-aged children with ASD, which reports under-connectivity at this age.2 Studies utilizing diffusion tensor imaging also indicate that white matter connectivity in toddlers4,56 does not resemble that of school age, which raises the importance of studying development from the earliest ages at a time near the age of diagnosis and following individuals longitudinally across the lifespan (for review, see 57).
Given the dearth of fcMRI studies in preschool-aged children, our findings are also worth consideration in the context of the developmental trajectories in typical development. Interestingly, the typically developing control group exhibited amygdala functional connectivity patterns similar to what is seen in healthy adults58 with robust connectivity between the amygdala and the mPFC, striatum, bilateral medial temporal lobes, and posterior ventral temporal lobes. Resting-state fcMRI studies in typically developing individuals suggest that amygdala connectivity changes with age. Recent evidence suggests that school-age children have weaker amygdala connectivity with various regions, including mPFC, compared to adults.59,60 While this growing literature has focused on school-age children through adulthood in typical development, previous studies do not include evaluation of younger, preschool-aged children, and our findings indicate that amygdala–medial prefrontal cortex connectivity is reliably detected by three years of age in typically developing children. It is important to consider potential differences between resting state functional connectivity during sleep, as data were acquired in the current study, versus while awake, as data have been acquired in other studies of older children and adults. It is also possible that the developmental trajectory of functional connectivity may not follow a linear pattern from early to middle childhood and into adulthood. Additional longitudinal studies are needed to fully understand the developmental time course of amygdala connectivity.
Our study focused on a period of development (~3.5 years) that coincides with the average age of ASD diagnosis, before behavioral adaptation and treatment effects may influence the connectivity patterns of the amygdala. Our overall findings that preschool-aged children with ASD have significantly weaker amygdala connectivity may represent a shift in the developmental time course in ASD that leads to altered connectivity compared to typically developing controls. It is possible that aberrant connectivity in ASD may be related to the different time course of amygdala growth. Evidence from cross-sectional structural MRI studies have shown that the amygdala undergoes an abnormal course of development in ASD that includes a period of early enlargement in the preschool years11-14 followed by a slower growth trajectory in preadolescence.13 While we did not observe direct correlations between amygdala volume and connectivity in the present study, future studies are needed to examine whether different subgroups of amygdala growth12 may be related to longitudinal development of amygdala functional connectivity.
The findings presented in this study further our understanding of the role of the amygdala in the neuropathology of autism. Volumetric abnormalities of the amygdala have consistently been reported in young children with autism,11-14 and our findings indicate that amygdala functional connectivity is also disrupted. Preschool-aged children with ASD had weaker functional connectivity between the amygdala and regions important for social communication and language. Furthermore, connectivity related to the severity of core autism symptoms can be dissociated from connectivity related to sensory difficulties. While additional studies are clearly needed, these findings raise the potential for future treatment studies aimed at normalizing amygdala function and connectivity and decreasing social communication deficits in young children with ASD.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute of Mental Health (1R01MH089626-01, U24MH081810, 1R00MH085099-01A1, U54 HD079125) and the UC Davis MIND Institute.
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors are particularly grateful to all the families and children who participated in the Autism Phenome Project at the UC Davis MIND Institute. Thank you to the numerous staff of the Autism Phenome Project for assistance in the logistics of family visits, data collection, and acquisition of MRI data. Thank you to Bradley Schlaggar, MD, PhD, of Washington University School of Medicine, for helpful suggestions and comments on the functional connectivity methods used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
This study was presented as an abstract at the International Meeting for Autism Research, May 2013, Spain.
Disclosure: Drs. Shen, Johnson, Angkustsiri, Rogers, Müller, Amaral, Nordahl, Ms. Li, Mr. Keown, and Mr. Lee report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Mark D. Shen, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
Ms. Deana D. Li, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
Mr. Christopher L. Keown, Brain Development Imaging Laboratory at San Diego State University, San Diego, CA.; University of California, San Diego, La Jolla, CA.
Mr. Aaron Lee, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
Dr. Ryan T. Johnson, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
Dr. Kathleen Angkustsiri, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
Dr. Sally J. Rogers, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
Dr. Ralph-Axel Müller, Brain Development Imaging Laboratory at San Diego State University, San Diego, CA..
Dr. David G. Amaral, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
Dr. Christine Wu Nordahl, MIND Institute and the University of California at Davis School of Medicine in Sacramento, CA..
REFERENCES
- 1.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Dinstein I, Pierce K, Eyler L, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudie JD, Hernandez LM, Brown JA, et al. Autism-Associated Promoter Variant in MET Impacts Functional and Structural Brain Networks. Neuron. 2012;75:904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff JJ, Gu H, Gerig G, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 6.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. Journal of Neuroscience. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardinale RC, Shih P, Fishman I, Ford LM, Müller R-A. Pervasive Rightward Asymmetry Shifts of Functional Networks in Autism Spectrum Disorder. JAMA Psychiatry. 2013;70:975. doi: 10.1001/jamapsychiatry.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishman I, Keown CL, Lincoln AJ, Pineda JA, Müller R-A. Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry. 2014;71:751–760. doi: 10.1001/jamapsychiatry.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 1993;11:175–187. [PubMed] [Google Scholar]
- 10.Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. Journal of Neuroscience. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosconi MW, Cody Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordahl CW, Scholz R, Yang X, et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012;69:53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biological Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 15.Ebisch SJH, Gallese V, Willems RM, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32:1013–1028. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135:2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagen von dem EAH, Stoyanova RS, Baron-Cohen S, Calder AJ. Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 2013;8:694–701. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudie JD, Shehzad Z, Hernandez LM, et al. Reduced Functional Integration and Segregation of Distributed Neural Systems Underlying Social and Emotional Information Processing in Autism Spectrum Disorders. Cerebral Cortex. 2012;22:1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala Habituation and Prefrontal Functional Connectivity in Youth With Autism Spectrum Disorders. J Am Acad Child Adolesc Psychiatry. 2013;52:84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullen EM. Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- 21.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 22.Breece E, Paciotti B, Nordahl CW, et al. Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brain Behav Immun. 2013;31:69–75. doi: 10.1016/j.bbi.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord C, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 24.DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. J Autism Dev Disord. 1995;25(4):355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- 25.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 26.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn W. Short Sensory Profile. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 28.Elliott CD. Differential Ability Scales: Administration and Scoring Manual. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- 29.Nordahl CW, Simon TJ, Zierhut C, Solomon M, Rogers SJ, Amaral DG. Brief report: methods for acquiring structural MRI data in very young children with autism without the use of sedation. J Autism Dev Disord. 2007;38(8):1581–1590. doi: 10.1007/s10803-007-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bes F, Schulz H, Navelet Y, Salzarulo P. The distribution of slow-wave sleep across the night: a comparison for infants, children, and adults. Sleep. 1991;14(1):5–12. doi: 10.1093/sleep/14.1.5. [DOI] [PubMed] [Google Scholar]
- 31.Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res. 2001;10(3):165–172. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 32.Manning JH, Courchesne E, Fox PT. Intrinsic connectivity network mapping in young children during natural sleep. Neuroimage. 2013;83(C):288–293. doi: 10.1016/j.neuroimage.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13(6):433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- 34.Fischl B, Salat DH, Busa E, et al. Whole Brain Segmentation:: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 35.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 36.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84(C):320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105(C):536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller R-A. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biological Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uddin LQ, Supekar K, Lynch CJ, et al. Salience Network–Based Classification and Prediction of Symptom Severity in Children With Autism. JAMA Psychiatry. 2013;70:869–79. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller R-A. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25(3):916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Washington SD, Gordon EM, Brar J, et al. Dysmaturation of the default mode network in autism. Hum Brain Mapp. 2013;35(4):1284–1296. doi: 10.1002/hbm.22252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Martino A, Kelly C, Grzadzinski R, et al. Aberrant striatal functional connectivity in children with autism. BPS. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padmanabhan A, Lynn A, Foran W, Luna B, O'Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci. 2013;7(814):1–19. doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delmonte S, Balsters JH, McGrath J, et al. Social and monetary reward processing in autism spectrum disorders. Mol Autism. 2012;3(7):1–13. doi: 10.1186/2040-2392-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012;7:160–72. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4(1):19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 51.Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 52.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. NeuroImage: Clinical. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supekar K, Uddin LQ, Khouzam A, et al. Brain Hyperconnectivity in Children with Autism and its Links to Social Deficits. Cell Reports. 2013;5(3):738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7(458):1–11. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bashat DB, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–7. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 57.Wolff JJ, Piven J. Neurodevelopmental disorders: Accelerating progress in autism through developmental research. Nature Reviews Neuroscience. 2014;10(8):431–432. doi: 10.1038/nrneurol.2014.126. [DOI] [PubMed] [Google Scholar]
- 58.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabard-Durnam LJ, Flannery J, Goff B, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin S, Young CB, Supekar K. Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci U S A. 2012;109:7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.