Abstract
Rationale
The endogenous oxytocin system has emerged as an inhibitor of drug-seeking and stress in preclinical models.
Objectives
The goal of this study was to examine whether systemic oxytocin administration attenuated methamphetamine (METH)–seeking in rats pre-exposed to a predator odor threat.
Methods
In Experiment 1, rats were exposed for five days to the predator odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), or saline before METH self-administration began. After extinction training, rats were injected with 1 mg/kg, ip oxytocin (OXT) or saline 30 min before a cue-induced reinstatement test followed by re-extinction and a TMT–induced reinstatement test. In Experiment 2, TMT pre-exposure was followed by 10 days of 1 mg/kg OXT or saline injections before METH self-administration, extinction, and a TMT-induced reinstatement test.
Results
In Experiment 1, TMT pre-exposed rats that were injected with saline 30 min before reinstatement exhibited greater drug-seeking induced by conditioned cues or TMT than that exhibited by saline pre-exposed rats. A single injection of OXT 30 min before reinstatement suppressed METH-seeking in both saline- and TMT pre-exposed rats. In Experiment 2, TMT pre-exposed rats that received saline injections for 10d prior to METH self-administration exhibited enhanced drug-seeking induced by TMT during stress-induced reinstatement. OXT injections for 10d prior to METH self-administration blocked only the stress-induced exacerbation of drug-seeking in TMT pre-exposed rats.
Conclusions
These results support further research on the development of oxytocin as a novel therapeutic that has enduring effects on drug-seeking exacerbated by stress.
Keywords: Addiction, Methamphetamine, Oxytocin, PTSD, Predator odor, Reinstatement, Self-administration, Stress, Substance use disorder
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating anxiety disorder with three major symptom clusters: persistent avoidance of trauma-related stimuli, hyperarousal, and intrusive re-experiencing of the traumatic event (American Psychiatric Association, 2013). PTSD is often complicated by the presence of concurrent substance use disorder (SUD). Individuals with PTSD report a rate of substance dependence that is 2-3 times higher than that of those without PTSD (Cottler et al. 1992; Jacobsen et al. 2001; Kessler et al. 1995). Moreover, clinical studies have demonstrated that methamphetamine (METH) is twice as likely to be used in individuals with concurrent PTSD than in trauma-exposed individuals without PTSD (Smith et al. 2010). These studies are consistent with a wider body of literature reporting a strong relationship between PTSD and substance abuse (Back et al. 2000; Brady 2001; Cottler et al. 1992; Khoury et al. 2010).
Treatment of PTSD symptoms has been reported to have a greater impact on improving SUD symptoms than vice versa (Hien et al. 2010). During the past decade, the endogenous neuropeptide, oxytocin (OXT), has been shown to exert potent anxiolytic, anti-fear, and anti-stress effects in humans (Heinrichs and Domes 2008; Kirsch et al. 2005) and in animals (Neumann and Landgraf 2012; Ring et al. 2006; Viviani et al. 2011). Based on these and other findings, intra-nasal OXT has been promoted as a potential early intervention to prevent the development of PTSD after trauma in humans (Frijling et al. 2014; Frijling et al. 2015). Similarly, based on preclinical studies, OXT has emerged as a potential treatment for substance use disorders (McGregor and Bowen 2012; Sarnyai 2011). Systemic administration of OXT inhibits METH-induced hyperactivity and conditioned place preference (Baracz et al. 2012; Carson et al. 2010; Qi et al. 2009) and dose-dependently decreases the motivation to self-administer METH on a progressive ratio schedule (Carson et al. 2010). Furthermore, OXT decreases drug-seeking induced by a METH prime or the anxiogenic drug, yohimbine (Carson et al. 2010; Cox et al. 2013). However, the effects of OXT in a preclinical model of repeated stress combined with drug self-administration (SA) and seeking has not been investigated previously.
Preclinical models of PTSD pathology involve exposing rodents to various stressors in order to elicit behavioral and biological responses that simulate those observed in humans (Pitman et al. 2012; Goswami et al. 2013). A history of stress can alter responses to substances of abuse. Recent studies have demonstrated that footshock-induced stress increased alcohol consumption (Meyer et al. 2013) whereas maternal separation in early life increased METH self-administration (Lewis et al. 2015). Another PTSD model, single prolonged stress, enhanced locomotor sensitization to cocaine but did not alter cocaine SA (Eagle et al. 2015) and had mixed effects on METH- or amphetamine-induced behavioral sensitization (Eagle and Perrine 2013; Toledano et al. 2013). Predator odors, such as 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), are ecologically relevant stressors that reliably induce hyperarousal, a long-lasting avoidance of trauma-related cues, and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Fendt et al. 2005; Thomas et al. 2006). Repeated TMT exposure produces anxiety-like behaviors and long-lasting avoidance of trauma-related cues (Ojo et al. 2014). However, the effects of prior predator odor exposure on drug SA and reinstatement of drug-seeking has not been investigated previously. Thus, in this study, we investigated whether repeated TMT exposure in one environment (activity chambers) altered the response of rats to (1) METH SA and (2) reinstatement induced by re-experiencing the predator odor in the operant chambers previously associated with METH.
Because the suppressive effects of OXT on the reinstatement of METH-seeking induced by various stimuli have been established (Carson et al. 2010; Cox et al. 2013; Hicks et al. 2014), first we examined the effects of an acute dose of systemic OXT on conditioned cue-induced reinstatement followed by TMT-induced reinstatement of METH-seeking in rats with or without a history of TMT pre-exposure. Second, we examined whether daily, systemic OXT injections immediately after TMT exposure and before METH SA would prevent subsequent susceptibility to TMT-induced drug-seeking. The latter OXT paradigm is based on evidence that 10 days of daily OXT injections in adolescent rats suppressed alcohol consumption (Bowen et al. 2011) or drug-seeking induced by a METH prime (Hicks et al., 2014) weeks after the end of OXT administration.
Experimental Procedures
Animals
Adult male Sprague-Dawley rats (N=64; Charles River Laboratories, Wilmington, MA) weighing 300-325g at the start of the experiment were housed individually on a reverse light/dark cycle (6am-6pm). All animal use protocols were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and the USAMRMC ACURO. All experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (2010). Animals were divided into two cohorts (n=16 per cohort) in each of two experiments and weighed daily. One rat in Experiment 1 died prior to the start of METH self-administration (SA) and one rat in Experiment 2 died during METH SA and both were eliminated from the dataset.
IV Catheter Surgery
Intravenous catheters were implanted into the right jugular vein as described previously (Cox et al. 2013). Briefly, rats were anesthetized with ketamine (66 mg/kg, ip) and xylazine (1.33 mg/kg, ip), followed by equithesin and ketorolac (2.0 mg/kg, ip.). The catheters were attached to cannulae that were secured to a back mount cannula connector pedestal (Plastics One, Roanoke, VA). During the 3-5 d surgical recovery, rats were infused with 10 mg/0.1 ml of cefazolin and 0.05 ml of Taurolidine-Citrate Catheter Solution (TCS; Access Technologies, Skokie, IL) iv. daily.
Predator Odor Exposure and Locomotor Activity Testing
Baseline open field behavior was assessed during the dark cycle under red light conditions 24 hours prior to i.v. catheterization. Rats were placed individually in a Digiscan Animal Activity Monitor (Accuscan Instruments, Inc., Columbus, OH) for 15 min. during which locomotor activity was recorded and quantified by Versamax software. Then after 3-5 d of recovery from surgery, rats were individually exposed to either 10 ul of 2,5-dihydro-2,4,5-trimethylthiazonline (TMT; Phero Tech Inc., Delta, BC, Canada) diluted in saline or the saline vehicle on a filter paper in a weigh boat in the center of the activity chamber for 15 min per day for 5 days (between 0900 and 1030 hours). The saline-exposed group was tested and removed from the testing room each day before the second group was exposed to TMT to minimize the potential for unintentional exposure to the predator odor. Locomotor activity was recorded as described above for 15 min immediately following removal of the TMT or saline. The chambers were cleaned thoroughly with 70% ethanol and air-dried after each animal was removed to eliminate scent cues. On day 1 and day 5 of the protocol, each rat was removed from the chamber at the end of the 15 min exposure and 0.2 ml of blood was immediately collected via their i.v. catheter into tubes containing 100 U heparin for determination of corticosterone (CORT) levels. Blood samples were taken 2-4 hours after the start of the dark cycle.
Corticosterone Measurements
Blood was collected in tubes containing 100U heparin. Samples were centrifuged at 2000xg for 20 min, plasma collected, and samples stored at −20°C before analysis using a I125Corticosterone radioimmunoassay Kit (MP Biomedicals, Santa Ana, CA).
Experimental Design
All rats (n=32) underwent similar experimental conditions prior to being separated into the two experiments described below.
Experiment 1: Administration of OXT 30 min prior to Reinstatement Testing
Figure 1 details the design for Experiment 1. Two days after the last TMT exposure, METH self-administration (SA) was initiated as described previously (Cox et al. 2013). Each rat was placed into a self-administration chamber (Med Associates, St. Albans, VT) and allowed to lever press for 0.02 ug methamphetamine HCl in 50 ul sterile saline (Sigma-Aldrich Co., St. Louis, MO) on a fixed ratio 1 schedule for 120 min for 14 consecutive days (Roth and Carroll 2004; Rocha and Kalivas 2010). Each infusion was paired with a compound conditioned stimulus complex (light plus tone) that consisted of illumination of a white stimulus light directly above the active lever and a tone (2 kHz, 15 dB above ambient noise). Immediately following the last SA session, rats underwent 2h daily extinction training sessions in which responding on the previously active and inactive levers was recorded but had no programmed consequences. No light and tone cues were activated during extinction. Extinction criteria consisted of two consecutive days of less than 20 presses on the previously active lever (achieved within 6-7 days).
Fig. 1.
Experimental timeline. For experiment 1, the experimental groups were as follows: saline-pre-exposure (PE) + saline (saline-PE sal; n=8), saline-PE + oxytocin (OXT) (saline-PE OXT; n=7), TMT-PE + saline (TMT-PE sal; n=8), TMT-PE + OXT (TMT-PE OXT; n=8). For experiment 2, the experimental groups were as follows: saline-PE + 10 days saline (saline-PE 10d sal; n=7), saline-PE + 10 days OXT (saline-PE 10d OXT; n=8), TMT-PE + 10 days saline (TMT-PE 10d sal; n=8), TMT-PE + 10 days OXT (TMT-PE 10d OXT; n=8).
One day after extinction training, all rats were injected with either 1 mg/kg, ip. OXT, a dose that decreases METH-seeking (Carson et al. 2010; Cox et al. 2013), or saline. Thirty min later, at a time when the locomotor suppressive effects of OXT had diminished (Zhou et al. 2015), the rats were re-exposed to the operant chamber for a 2h cue-induced reinstatement test in which the compound light and tone stimuli were presented with each active lever press. Then the rats were re-extinguished as described above followed by testing for TMT-induced reinstatement of METH-seeking. Rats were injected with either 1 mg/kg, i.p. OXT or saline 30 min before exposure to 1% TMT for the first 15 min of the 2h reinstatement test. The groups were not counter-balanced to provide a group of pure saline controls for future molecular analyses. The complex conditioned stimulus was not presented during extinction or the TMT-induced reinstatement test. Active (right) and inactive (left) lever presses were recorded during the entire 2h of the reinstatement tests but had no programmed consequences. The rats were decapitated without anesthesia, the brains were extracted, and trunk blood was collected immediately after the TMT-induced reinstatement test.
Experiment 2: Repeated administration of OXT prior to METH SA
Figure 1 details the design for Experiment 2. One day after the last TMT exposure, the rats were injected with 1 mg/kg i.p. OXT or saline daily for 10 consecutive days. The day after the last injection, rats initiated METH SA followed by extinction training to the criteria described above (achieved in 6 days). The day after the end of extinction training, all rats were re-exposed to the operant chamber for a 2h reinstatement test in which they were exposed to 1% TMT for the first 15 min of the 2h test as described above. The rats were decapitated without anesthesia, the brains were extracted, and trunk blood was collected immediately after the TMT-induced reinstatement test.
Statistical analysis
All data were analyzed with two-way ANOVAs with or without repeated measures using Graph Pad Prism. Data yielding a significant interaction were further analyzed with Bonferroni-corrected tests. Unpaired t-tests were used post hoc to compare the ratio of OXT-induced changes in Sal-PE vs. TMT-PE groups in Experiment 1.
Results
TMT produces avoidance and dysregulation of the HPA axis
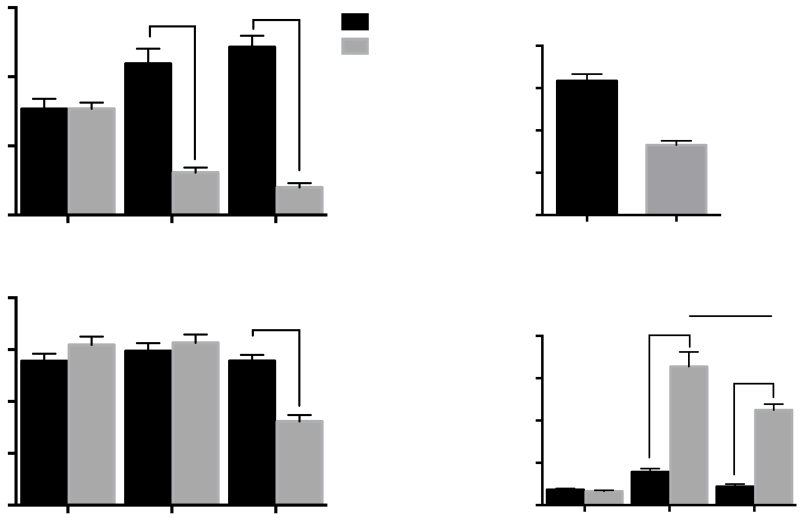
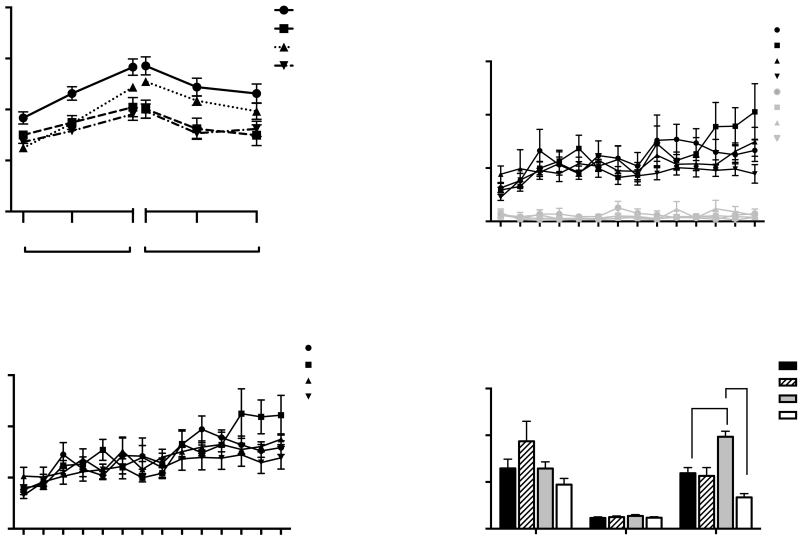
Qualitative observations indicated that rats exposed to 1% TMT for five days moved from corner to corner of the activity chamber (thigmotaxis) and did not freeze in the presence of the predator odor. Locomotor activity for 15 min after the TMT or saline was removed and CORT measurements from blood collected immediately after locomotor testing for rats in experiments 1 and 2 did not differ between groups so the data were pooled. Figure 2 illustrates the open field behavior (2A), total activity (2B), weight gain (2C) and CORT levels (2D) for all rats before and after TMT pre-exposure (PE). A 2-way RM ANOVA of the open field data revealed a significant day x TMT pre-exposure interaction [F(2,122)=49.6, p<0.0001]. No significant differences in baseline anxiety-like behavior were observed between groups as measured by performance in the open field (Fig 2A). However, TMT-PE rats spent significantly less time in the center of the open field than the saline-PE rats on both day 1 and day 5 (p<0.0001-Fig 2A) and they demonstrated significantly less overall activity (total distance traveled) on day 5 [Interaction F(2,122)=27.05, p<0.0001; Bonferroni p<0.0001-Fig 2B]. Although the average weight of the rats randomly selected for the saline-PE (342.3+/−2.3, n=31) and TMT-PE (349.8+/−3.8, n=32) groups did not differ on baseline day 1 (Supplemental Fig 1A), TMT exposure for 5 days resulted in significantly less weight gain for TMT-PE rats than for saline-PE rats [Fig. 2C; t(1,61)=8.058, p<0.0001]. With regard to CORT levels, a 2-way RM ANOVA revealed a significant day x TMT pre-exposure interaction [Fig. 2D; F(2,120)=34.88, p<0.0001; 2 samples lost]. Bonferroni-adjusted comparisons showed that while there were no significant differences in baseline CORT levels, TMT-PE rats had significantly higher CORT levels than Saline-PE rats immediately following exposure on day 1 (p<0.0001) and day 5 (p=0.0001). Although TMT-induced CORT levels decreased between day 1 and day 5 (p<0.001), CORT levels on both days were significantly higher than baseline. Further, the ratio between TMT-PE and SAL-PE groups on day 1 is 4.16 whereas the ratio on day 5 is 5.13, suggesting that there was an overall habituation of both groups to the environment and testing procedures.
Fig 2.
Open field activity, weight gain, and corticosterone levels before and during repeated stress exposure for all rats. (A) Percent time in open field on day 0, (Baseline), day 1, and day 5 of TMT or saline exposure (****p<0.0001). (B) Total distance moved in an open field on day 0, (Baseline), day 1, and day 5 of TMT or saline exposure (****p<0.0001). (C) Weight gain of TMT-PE and Saline-PE rats on day 5 of TMT or saline exposure (****p<0.0001). (D) Corticosterone levels of TMT-PE and Saline-PE rats on day 0, (Baseline), day 1, and day 5. (****p<0.0001, ###p<0.001). Mean +/− SEM; n=32 rats per group)
Experiment 1: A single dose of systemic OXT blocks reinstatement of METH-seeking regardless of stress pre-exposure
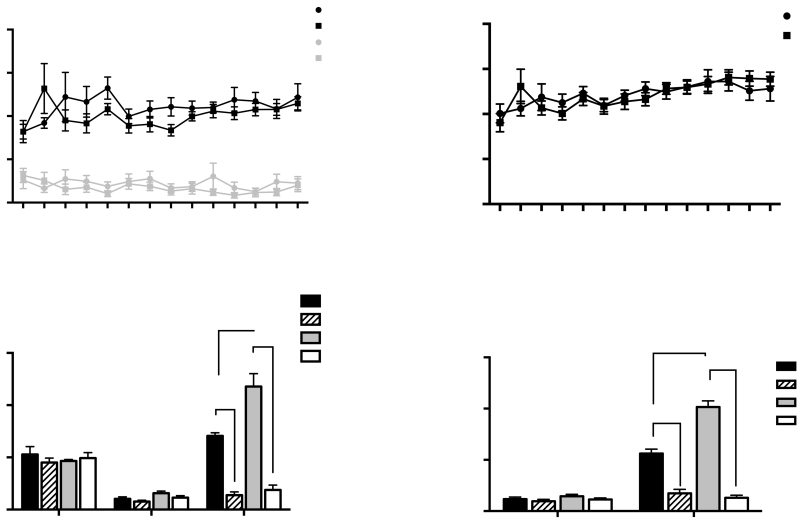
Despite the reduced weight gain seen in TMT-PE rats during stress exposure, there were no significant differences in body weight between Saline-PE rats and TMT-PE rats during METH SA (Supplemental Fig. 1B). TMT pre-exposure had no significant effect on active or inactive lever presses (Fig. 3A) or infusions (Fig. 3B) during METH SA. Further, there were no significant differences in the average number of lever presses between groups during the last 2 days of extinction (Fig. 3C) or the number of days to reach extinction criterion (Supplemental Fig 2). In contrast, A 2-way ANOVA of the effects of OXT on cue-induced reinstatement revealed that there was a significant drug treatment x stress pre-exposure interaction (F(1,12)=9.134, p<0.05-the data from one cohort was lost due to experimental error, leaving n=4 per group). A Bonferroni–adjusted comparison showed that TMT-PE rats pressed the active levers significantly more during the cue-induced reinstatement test than Saline-PE rats when rats were injected with saline 30 min before testing (p<0.01-Fig. 3C). Administration of OXT 30 min before testing blocked the cue-induced reinstatement of drug-seeking in both TMT-PE (p<0.0001) and Saline-PE groups (p<0.001-Fig. 3C). However, there was no difference in the amplitude of OXT-induced suppression in Sal-PE vs. TMT-PE rats (t(6)=0.9; p=0.4). The rats underwent further extinction training to criterion following the cue-induced reinstatement test. There were no significant differences in the number of lever presses between groups during the last 2 days of extinction in animals with a history of METH (Fig. 3D). Further, a 2-way ANOVA revealed a significant drug treatment x stress pre-exposure interaction [F(1,27)=31.10; p<0.05-n=8 per group] for the TMT-induced reinstatement data. Bonferroni-adjusted comparisons revealed significantly greater TMT-induced reinstatement in the TMT-PE group than in the Saline-PE group (p<0.0001-Fig. 3D). Injection of OXT 30 min prior to testing blocked the ability of TMT to elicit reinstatement in both TMT-PE rats and Saline-PE rats (p<0.0001-Fig. 3D). Further, there was a significant difference in the amplitude of OXT-induced suppression in Sal-PE vs. TMT-PE rats (t(12)=2.2; p=0.05).
Fig 3.
METH self-administration, extinction, and TMT-induced reinstatement in TMT-PE and Saline-PE rats. (A) METH self-administration over 14 days: active lever presses (black) and inactive lever presses (grey). (B) Average number of infusions received during METH SA for saline-PE and TMT-PE rats. (C) Average number of previously active lever presses during the last 2 days of extinction (left) and cue-induced reinstatement for TMT-PE and Saline-PE rats injected with saline or OXT 30 min prior to reinstatement testing (right). (D) Average number of previously active lever presses during the last 2 days of extinction (left) and TMT-induced reinstatement for TMT-PE and Saline-PE rats injected with saline or OXT 30 min prior to reinstatement testing (right). Mean +/− SEM ; ****p<0.0001, n=7-8 per group
A 2-way ANOVA revealed a significant interaction between stress pre-exposure and drug treatment for plasma CORT levels derived from trunk blood collected 2 hr after TMT exposure [F(1,27)=4.8. p<0.05]. A Bonferonni-adjusted comparison revealed that plasma CORT levels in Sal-PE or TMT-PE rats injected with saline before the reinstatement test were not different from each other. However, plasma CORT levels in TMT-PE rats injected with OXT were significantly greater than those of TMT-PE rats injected with saline (***p<0.001) and SAL-PE rats injected with OXT (#p<0.05-Fig 4).
Fig 4.
Plasma levels of corticosterone derived from trunk blood immediately following 2-hr TMT-induced reinstatement test in Experiment 1. #p<0.05 OXT Saline-PE vs. OXT TMT-PE; ***p<0.001 Saline TMT-PE vs. OXT TMT-PE (n=8 per group).
Experiment 2: Repeated systemic OXT selectively blocks reinstatement of METH-seeking in rats with a history of stress exposure
In Experiment 2, one day after the last TMT exposure, rats received an injection of either 1 mg/kg, i.p. OXT or saline for 10 days. Two-way repeated measures ANOVA for body weight gain over time revealed an interaction for groups X time (F(39,351)=4.37, p<0.0001). The body weights of TMT-PE rats remained significantly lower than SAL-PE rats during the first 5 days of the 10 d saline injection period [Day 1, 5 TMT-PE 10 d Sal vs. Saline-PE 10d Sal-p<0.01]; however, their average weight was not significantly different than the SAL-PE rats by day 10 of the saline injections (Fig. 5A). Similarly, the body weights of these two groups did not differ during METH SA, although neither group gained weight. In contrast, regardless of TMT pre-exposure, OXT-injected rats gained significantly less weight during the 10 day OXT or Sal injection period [Fig. 5A; Saline-PE 10d OXT vs. Saline-PE 10d Sal Day 10-p<0.001; TMT-PE 10d OXT Day 10 vs. TMT-PE 10d Sal-p<0.05]. The effect of OXT on weight persisted throughout METH SA [Fig. 5A; Saline-PE 10d Sal vs. Saline-PE 10d OXT Day 1, 7, 14-p<0.001; TMT-PE 10 d Sal vs. TMT-PE 10d OXT on METH SA Day 1-p<0.05; TMT-PE 10 d Sal vs. TMT-PE 10d OXT on METH SA Day 7-p<0.01].
Fig 5.
Effects of 10 days of OXT injections on weight gain, METH self-administration, extinction, and TMT-induced reinstatement in TMT-PE and Saline-PE rats. (A) Body weight gain of TMT-PE and Saline-PE rats during the ten days of saline or OXT injections and during METH self-administration. For clarity, only data from days 1, 5 and 10 of the saline or OXT injection duration and days 1, 7 and 14 of METH SA are illustrated (*p<0.05,***p<0.001 Saline-PE 10d Sal vs. Sal-PE 10d OXT, ^p<0.05 Sal-PE 10d Sal vs. TMT-PE 10d Sal; #p<0.05, ##p<0.01 TMT-PE 10d Sal vs. TMT-PE 10d OXT). (B) Average number of active lever presses (black) or inactive lever presses (grey) during the 14 days of METH self-administration. (C) Average number of METH infusions /animal weight during self-administration. (D) Average number of active lever presses during the last 3 days of SA (left), during the last 2 days of extinction (middle), and during the TMT-induced reinstatement test. (**p<0.01 Sal-PE 10d Sal vs. TMT-PE 10d Sal, ****p<0.0001. TMT-PE 10d Sal vs. TMT-PE 10 d OXT). Mean +/−SEM; n=7-8 per group
Neither TMT pre-exposure or repeated OXT injections had a significant effect on the acquisition or maintenance of METH SA as no group differences were observed in active or inactive lever presses (Fig. 5B). In addition, despite the differences in weight gain seen in the OXT-injected animals, no significant differences were observed in total METH intake (not shown) or METH intake by body weight (Fig. 5C; calculated as the number of METH infusions multiplied by the individual rat’s body weight, divided by the dosage of METH per infusion).
There were no significant group differences in the number of days to reach extinction criterion (Supplemental Fig 3) or in the number of active lever presses during the last 2 days of extinction (Fig. 5D). A 2-way ANOVA revealed a significant drug treatment x stress pre-exposure interaction [F(1,27)=22.72, p<0.0001]. Bonferroni–adjusted comparisons revealed that reinstatement in TMT-PE rats was significantly greater than that of Saline-PE rats in groups injected with saline for 10d prior to METH SA (p<0.01-Fig. 5D). Injection with 1 mg/kg, i.p. OXT for 10d prior to METH SA selectively blocked the enhanced TMT-induced reinstatement (p<0.0001-Fig. 5D) and caused a non-significant reduction in reinstatement compared to Saline-PE 10d OXT rats (p=0.07).
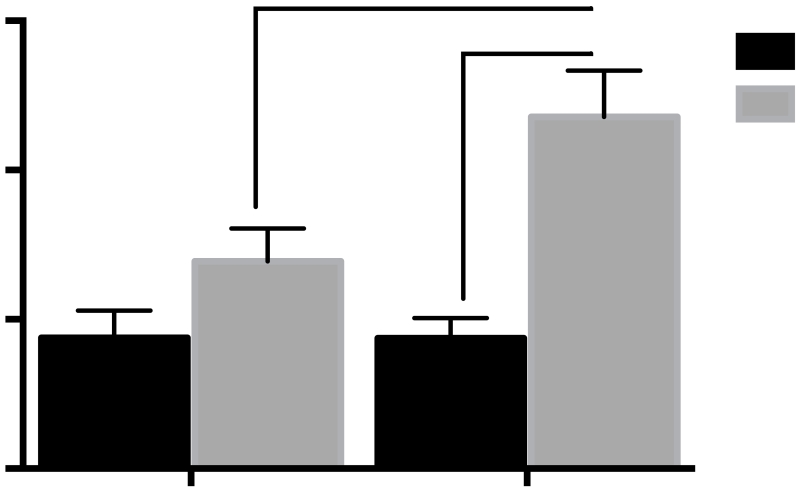
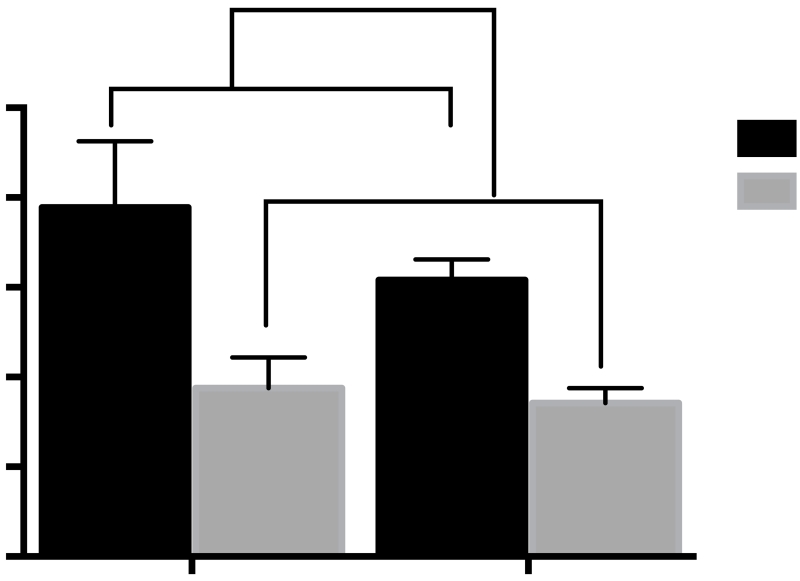
A 2-way ANOVA revealed a main effect of 10d drug treatment [F(1,27)=18. p<0.001] on plasma CORT levels derived from trunk blood collected 2 hr after TMT exposure in Sal-PE or TMT-PE rats. CORT levels in 10d OXT-injected rats were significantly lower than those in saline-injected rats regardless of TMT-pre-exposure (Fig 6).
Fig 6.
Plasma levels of corticosterone derived from trunk blood immediately following 2-hr TMT-induced reinstatement test in Experiment 2. 10d Saline vs. 10d OXT main effect ***p<0.001 (n=8 per group).
Discussion
This is the first study to demonstrate that peripheral administration of OXT suppresses drug-seeking exacerbated by pre-exposure to an aversive predator odor. Exposure to TMT caused rats to avoid the center of the open field on day 1 and day 5 after the TMT had been removed, suppressed weight gain, and caused increased plasma CORT levels, confirming its utility as a predator threat. Further, TMT itself induced reinstatement in rats whether or not they had been pre-exposed to TMT three (Experiment 1) or five weeks (Experiment 2) before. TMT pre-exposure exacerbated TMT-induced reinstatement of drug-seeking, suggesting that repeated exposure to a predator threat, has an enduring ability to exacerbate drug-seeking induced by the same threat but does not assume that the rats sustained a general exacerbation of stress responsivity. In Experiment 1, a single injection of OXT 30 min prior to reinstatement attenuated stress-induced METH-seeking regardless of TMT pre-exposure. In contrast, 10d of OXT injections suppressed reinstatement in TMT-PE rats only, suggesting that OXT can reduce drug-seeking by attenuating the enduring effects of repeated TMT exposure.
Pre-exposure to TMT had no effect on acquisition or maintenance of METH SA either when the exposure occurred immediately prior to acquisition of METH SA (Experiment 1) or following a delay of 10d (Experiment 2). This finding is consistent with a report that neither non-contingent electric footshock or injection of CORT immediately prior to initiating METH SA had an effect on acquisition (Moffett and Goeders 2005). In general, the effects of stress pre-exposure on the effects of abused drugs depend largely on the type of stressor, its severity, and the pattern and duration of exposure (see Introduction and Yap and Miczek 2008). Also a different self-administration paradigm that more precisely tests an animal’s motivation to self-administer METH (Hicks et al., 2014) may be more sensitive to TMT pre-exposure. In Experiment 2, there also was no effect of TMT pre-exposure or OXT injections on acquisition or maintenance of METH SA. Similarly, acquisition of METH SA on an FR1 schedule was not altered in female rats treated repeatedly as adolescents (12 weeks earlier) with 1 mg/kg OXT (Hicks et al. 2014); however, under a progressive ratio schedule of reinforcement, OXT pre-treatment reduced the motivation to self-administer METH and suppressed METH-primed reinstatement in that study.
The finding that repeated exposure to a predator odor potentiated reinstatement of drug-seeking suggests that sensitization developed in pre-stressed rats and persisted for 3-5 weeks. Sensitization to a variety of anxiety-producing stimuli is a hallmark of hyper-arousal in PTSD patients (Dykman et al. 1997) that is modeled in preclinical PTSD paradigms such as stress-enhanced fear learning (Rau et al. 2005). Behavioral sensitization has been shown to result from exposure to various stressors followed by an acute non-contingent psychostimulant challenge (Eagle and Perrine 2013; Toledano et al. 2013) or augmented drug-seeking on a progressive ratio reinforcement schedule (Covington and Miczek 2005).
The finding that an injection of OXT 30 min prior to testing blocked TMT-induced reinstatement confirms previous reports that this dose suppresses reinstatement induced by METH-associated cues, a METH-prime, or yohimbine (Carson et al. 2010; Cox et al. 2013). However, this is the first study to examine the effects of OXT on reinstatement of drug-seeking exacerbated by pre-exposure to a stressor. The fact that a single systemic dose of OXT decreased TMT-induced reinstatement in all rats in Experiment 1 reinforces the idea that mechanisms underlying both stress and drug-seeking are attenuated by OXT when it is injected on the day of reinstatement testing. Reinstatement of drug-seeking is thought to depend on disturbed glutamate transmission in the dorsomedial PFC-NAc pathway (Parsegian and See 2014). Thus, it is possible that administration of OXT acts via OXTRs in brain regions such as the PFC or NAc (Baracz et al. 2014) to suppress reinstatement of METH-seeking. In fact, OXTRs in the PFC of mice are expressed exclusively in GABA-somatostatin interneurons and bath application of OXT in PFC slices increases the firing of these neurons (Nakajima et al. 2014) that suppresses pyramidal neuron activity. Further, OXT infusion into the medial PFC blocks restraint stress-induced reinstatement of METH-induced conditioned place preference in a glutamate-dependent fashion (Carson et al. 2010; Han et al. 2014). Similarly, stress-induced behavioral cross-sensitization to stimulants is blocked by glutamate receptor antagonists (Yap et al. 2005).
OXT exerted significant effects on stress-induced reinstatement of drug-seeking only in rats with a history of TMT exposure in Experiment 2. This finding is consistent with other studies that have shown that repeated administration of OXT exerts long-lasting effects on drug taking and seeking (Bowen et al. 2011; Hicks et al. 2014) as well as behavioral (Slattery and Neumann 2010) and physiological measures (Petersson et al. 1996; Petersson et al. 1999) of anxiety. In this regard, there is evidence that repeated stimulation of the hypothalamic OXT neuronal system enhances its cellular and synaptic plasticity by inducing cellular hypertrophy, OXT and OXTR synthesis, and OXT release (Theodosis 2002). The duration of this functional augmentation depends on the intensity and duration of stimulation of the endogenous system. Importantly, repeated exogenous OXT administration stimulates OXT neuronal functionality (Petersson et al. 1999) which may lead to persistent changes in behavior that affect subsequent stress reactivity. Thus, it is possible that OXT administration after repeated stress exposure decreases vulnerability to stress-induced addictive drug-seeking and drug-taking for an extended period of time, promoting OXT as a therapeutic agent with significant implications for individuals with a dual diagnosis of PTSD and SUD.
Possible mechanisms underlying the ability of exogenous OXT to suppress interactions between stress and METH-seeking include both suppression of the HPA axis and direct CNS effects. However, it is not likely that peripheral CORT mediated the OXT effects in this study because OXT increased plasma CORT levels in TMT-PE rats in Experiment 1 and suppressed CORT levels in Experiment 2 regardless of TMT pre-exposure. Systemic injections of OXT have divergent effects on CORT levels depending on the timing; acute OXT increased basal CORT levels 30 min but not 2 hr after systemic injection whereas 5 days of OXT injections decreased CORT levels for an extended period of time-up to 10 days later (Petersson et al. 1999). Regarding direct effects of systemic OXT on the CNS, it is estimated that 0.2% of exogenous OXT enters the brain after systemic administration (Neumann et al. 2013) and presumably stimulates OXT receptors in the CNS (Neumann and Landgraf 2012). Oxytocin receptors are abundant in several brain regions that are associated with emotional triggers of drug-seeking as well as stress, including the prefrontal cortex, hypothalamus, and amygdala (Smeltzer et al. 2006) and chronic METH administration alters OXTR levels in several of these brain areas (Zanos et al. 2014). Future studies will address how peripheral administration of OXT affects both the HPA axis and the central OXTR system.
In conclusion, the present study demonstrates that pre-exposure to a ethologically-relevant stressor, TMT, exacerbated METH-seeking elicited by re-exposure to that stressor weeks later. Acute exposure to OXT administered prior to reinstatement prevented drug-seeking induced by the TMT stressor in all animals regardless of stress history. However chronic administration of OXT after the initial TMT exposure selectively prevented the exacerbated stress-induced drug-seeking only in animals with a history of TMT exposure. This evidence provides significant preclinical foundation for the use of OXT as a novel therapeutic in patients with comorbid METH- and stress-induced PTSD symptomatology.
Supplementary Material
Acknowledgments
The authors thank Amanda Kassab, Erica Herzig, and Shannon Ghee for technical support. This article was prepared while Chantelle Ferland was employed at The Medical University of South Carolina. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Funding Sources: This study was supported by DoD grant W81XWH-12-2-0048 Subaward 8A-293 and T32 DA007288.
Footnotes
Financial Disclosures: The authors report no biomedical financial disclosures, other than grants listed above, or potential conflicts of interest.
Conflict of interest The authors have no conflicts of interests.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th edn. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Back S, Dansky BS, Coffey SF, Saladin ME, Sonne S, Brady KT. Cocaine dependence with and without post-traumatic stress disorder: a comparison of substance use, trauma history and psychiatric comorbidity. The American Journal on Addictions. 2000;9:51–62. doi: 10.1080/10550490050172227. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, McGregor IS, Cornish JL. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addiction Biology. 2014 doi: 10.1111/adb.12198. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behavioural Brain Research. 2012;228:185–93. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PloS One. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT. Comorbid posttraumatic stress disorder and substance use disorders. Psychiatric Annals. 2001;31:313–319. [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Compton WM, 3rd, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. The American Journal of Psychiatry. 1992;149:664–70. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–40. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–53. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykman RA, Ackerman PT, Newton JE. Posttraumatic stress disorder: a sensitization reaction. Integrative Physiological and Behavioral Science. 1997;32:9–18. doi: 10.1007/BF02688609. [DOI] [PubMed] [Google Scholar]
- Eagle AL, Perrine SA. Methamphetamine-induced behavioral sensitization in a rodent model of posttraumatic stress disorder. Drug and alcohol dependence. 2013;131:36–43. doi: 10.1016/j.drugalcdep.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Singh R, Kohler RJ, Friedman AL, Liebowitz CP, Galloway MP, Enman NM, Jutkiewicz EM, Perrine SA. Single prolonged stress effects on sensitization to cocaine and cocaine self-administration in rats. Behavioural Brain Research. 2015;284:218–24. doi: 10.1016/j.bbr.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neuroscience and Biobehavioral Reviews. 2005;29:1145–56. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch SB, Nawijn L, Goslings JC, Luitse JS, Biesheuvel TH, Honig A, Bakker FC, Denys D, Veltman DJ, Olff M. Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: study protocol of a randomized controlled trial. BMC Psychiatry. 2014;14:92. doi: 10.1186/1471-244X-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M. Intranasal oxytocin affects amygdala functional connectivity after trauma script-driven imagery in distressed recently trauma-exposed individuals. Neuropsychopharmacology. 2015 Aug 31; doi: 10.1038/npp.2015.278. 2015. doi: 10.1038/npp.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Rodriguez-Sierra O, Cascardi M, Pare D. Animal models of post-traumatic stress disorder: face validity. Frontiers in Neuroscience. 2013;7:1–14. doi: 10.3389/fnins.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WY, Du P, Fu SY, Wang F, Song M, Wu CF, Yang JY. Oxytocin via its receptor affects restraint stress-induced methamphetamine CPP reinstatement in mice: Involvement of the medial prefrontal cortex and dorsal hippocampus glutamatergic system. Pharmacology, Biochemistry, and Behavior. 2014;119:80–7. doi: 10.1016/j.pbb.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Hien DA, Jiang H, Campbell ANC, Hu M-C, Miele GM, Cohen LR, Brigham GS, Capstick C, Kulaga A, Robinson J, Suarez-Morales L, Nunes EV. Do treatment improvements in PTSD severity afffect substance use outcomes? A secondary analysis froma randomized clinical trial in NIDA’s clinical trials network. Am J Psychiatry. 2010;167:95–101. doi: 10.1176/appi.ajp.2009.09091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Progress in Brain Research. 2008;170:337–50. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Hicks C, Cornish JL, Baracz SJ, Suraev A, McGregor IS. Adolescent pre-treatment with oxytocin protects against adult methamphetamine-seeking behavior in female rats. Addiction Biology. 2014 doi: 10.1111/adb.12197. doi:10.1111/adb.12197. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. The American Journal of Psychiatry. 2001;158:1184–90. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depression and Anxiety. 2010;27:1077–86. doi: 10.1002/da.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Scheck L, Olive MF. The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Frontiers in Psychiatry. 2015;4:55. doi: 10.3389/fpsyt.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Hormones and Behavior. 2012;61:331–9. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcoholism, Clinical and Experimental Research. 2013;37:566–74. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. Neither non-contingent electric footshock nor administered corticosterone facilitate the acquisition of methamphetamine self-administration. Pharmacology, Biochemistry, and Behavior. 2005;80:333–9. doi: 10.1016/j.pbb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Gorlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159:295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in Neurosciences. 2012;35:649–59. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–93. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Ojo JO, Greenberg MB, Leary P, Mouzon B, Bachmeier C, Mullan M, Diamond DM, Crawford F. Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Frontiers in Behavioral Neuroscience. 2014;8:213. doi: 10.3389/fnbeh.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:811–22. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Alster P, Lundeberg T, Uvnas-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiology & Behavior. 1996;60:1311–5. doi: 10.1016/s0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting AL, Uvnas-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neuroscience Letters. 1999;264:41–4. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmussen AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–65. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews. 2005;29:1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like effects of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. The European Journal of Neuroscience. 2010;31:903–9. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172:443–9. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z. Oxytocin as a potential mediator and modulator of drug addiction. Addiction Biology. 2011;16:199–201. doi: 10.1111/j.1369-1600.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58:56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neuroscience Letters. 2006;394:146–51. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Smith RC, Blumenthal H, Badour C, Feldner MT. An investigation of relations between crystal methamphetamine use and posttraumatic stress disorder. Addictive Behaviors. 2010;35:625–7. doi: 10.1016/j.addbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Theodosis DT. Oxytocin-secreting neurons: A physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Frontiers in Neuroendocrinology. 2002;23:101–35. doi: 10.1006/frne.2001.0226. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Experimental Neurology. 2006;201:308–15. doi: 10.1016/j.expneurol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Toledano D, Tassin JP, Gisquet-Verrier P. Traumatic stress in rats induces noradrenergic-dependent long-term behavioral sensitization: role of individual differences and similarities with dependence on drugs of abuse. Psychopharmacology. 2013;230:465–76. doi: 10.1007/s00213-013-3179-5. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–7. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Covington HE, 3rd, Gale MC, Datta R, Miczek KA. Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology. 2005;179:230–9. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Stress and Rodent Models of Drug Addiction: Role of VTA-Accumbens-PFC-Amygdala Circuit. Drug Discovery Today Disease Models. 2008;5:259–270. doi: 10.1016/j.ddmod.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Wright SR, Georgiou P, Yoo JH, Ledent C, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A. Chronic methamphetamine treatment induces oxytocin receptor up-regulation in the amygdala and hypothalamus via an adenosine A2A receptor-independent mechanism. Pharmacology, Biochemistry, and Behavior. 2014;119:72–9. doi: 10.1016/j.pbb.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behavioural Brain Research. 2015;283:184–90. doi: 10.1016/j.bbr.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.